Study Effects of Drug Treatment and Physiological Physical Stimulation on Surfactant Protein Expression of Lung Epithelial Cells Using a Biomimetic Microfluidic Cell Culture Device
Abstract
1. Introduction
2. Materials and Methods
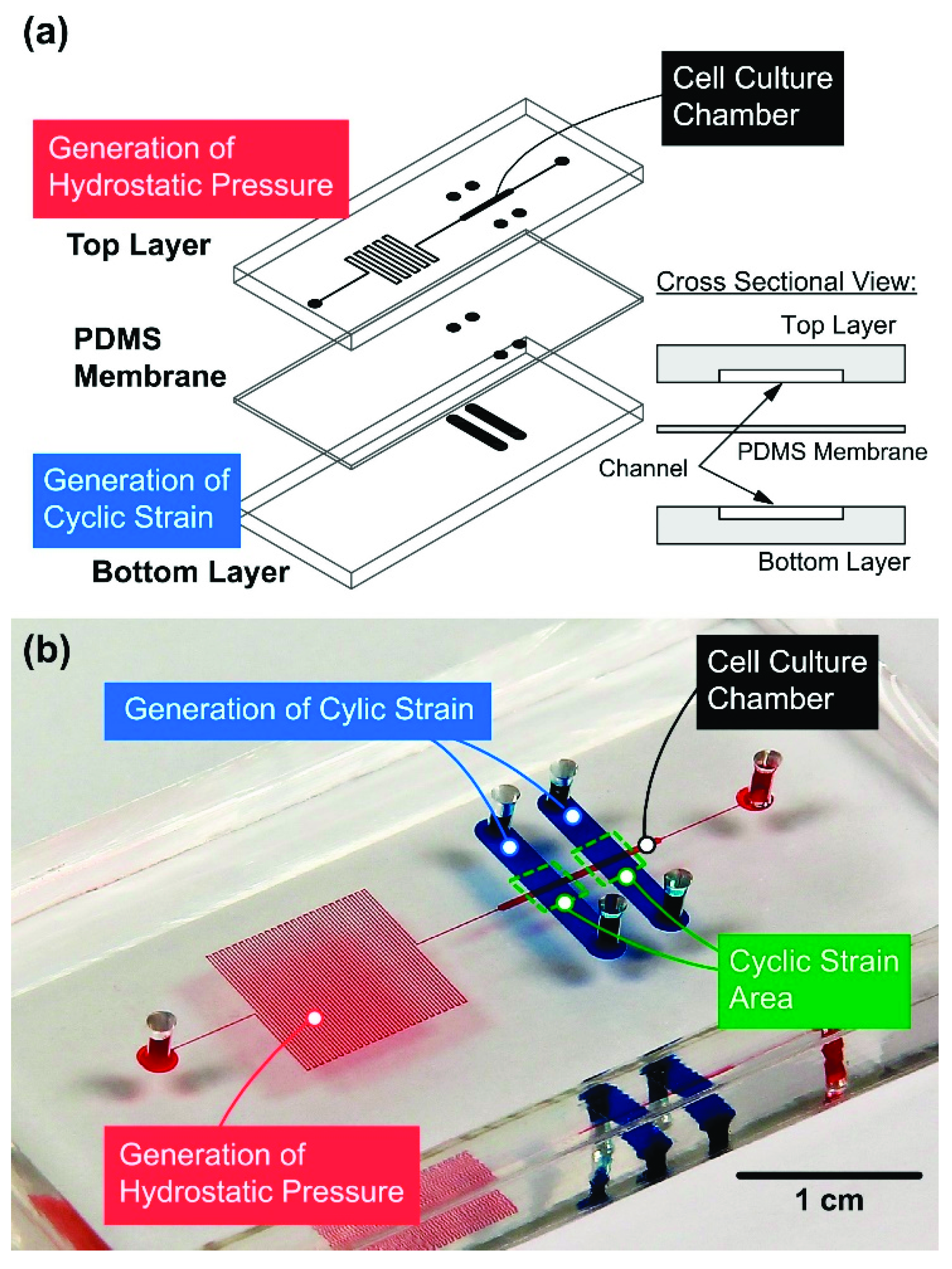
2.1. Device Design and Fabrication
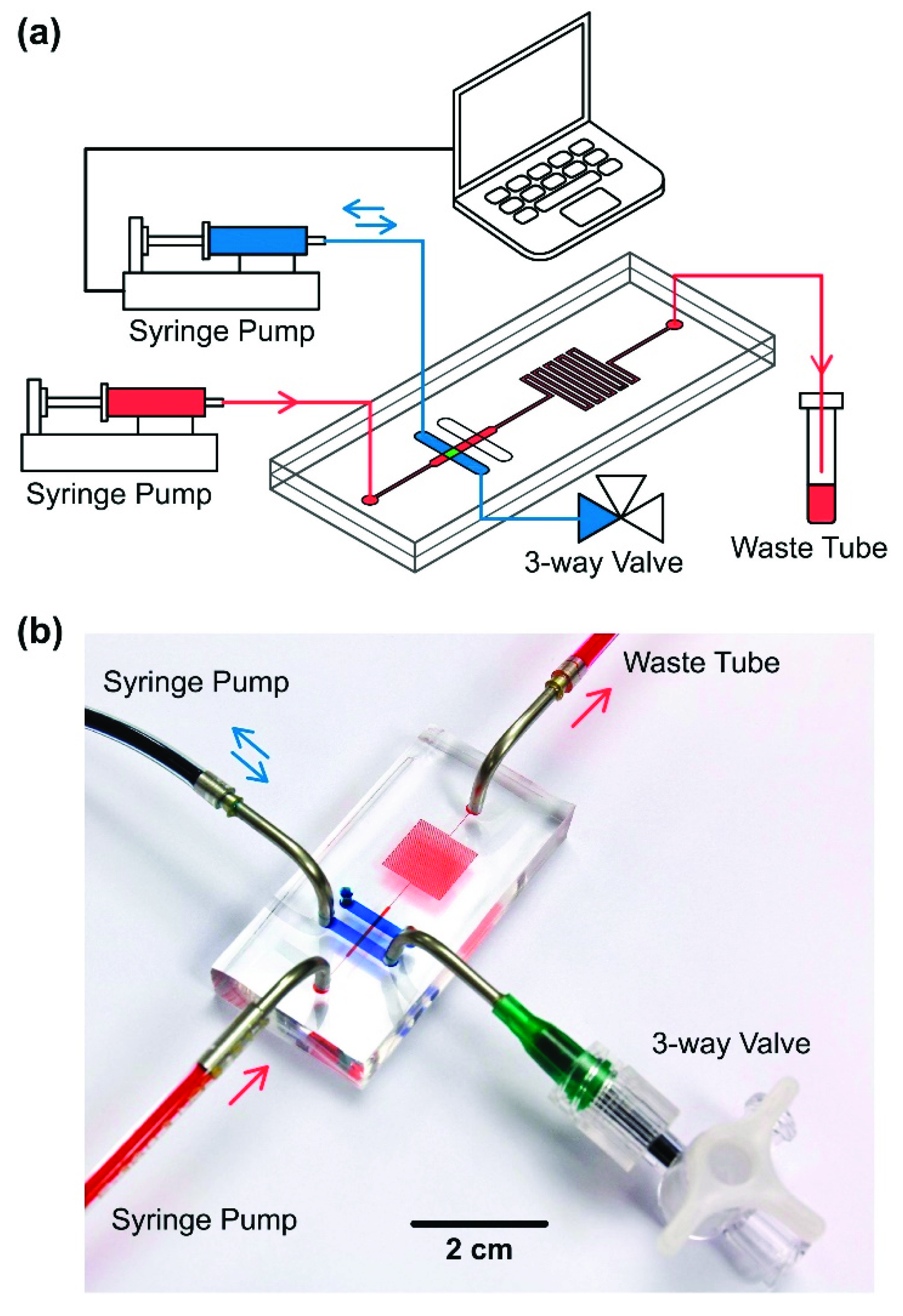
2.2. Experimental Setup
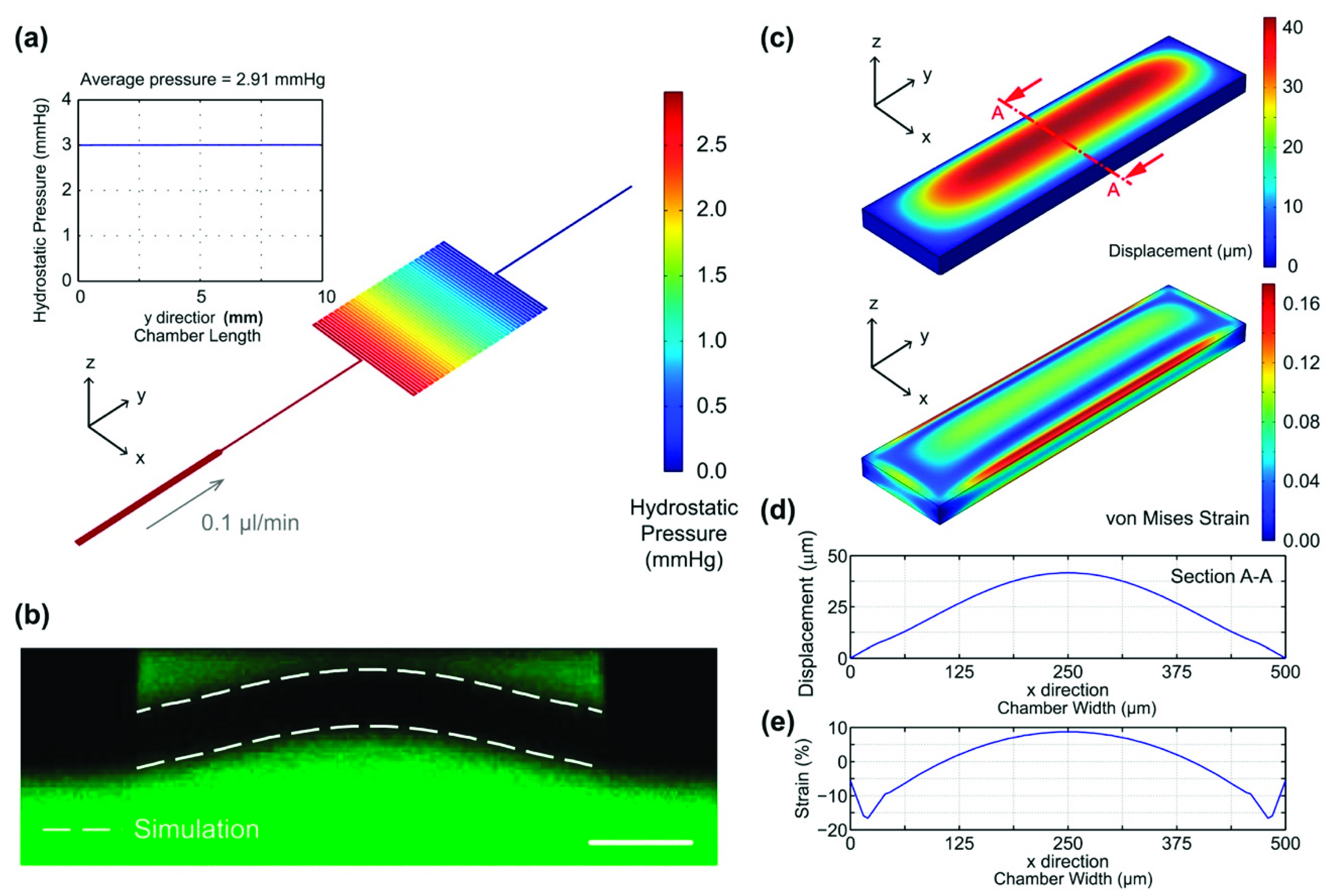
2.3. Device Simulation and Characterization
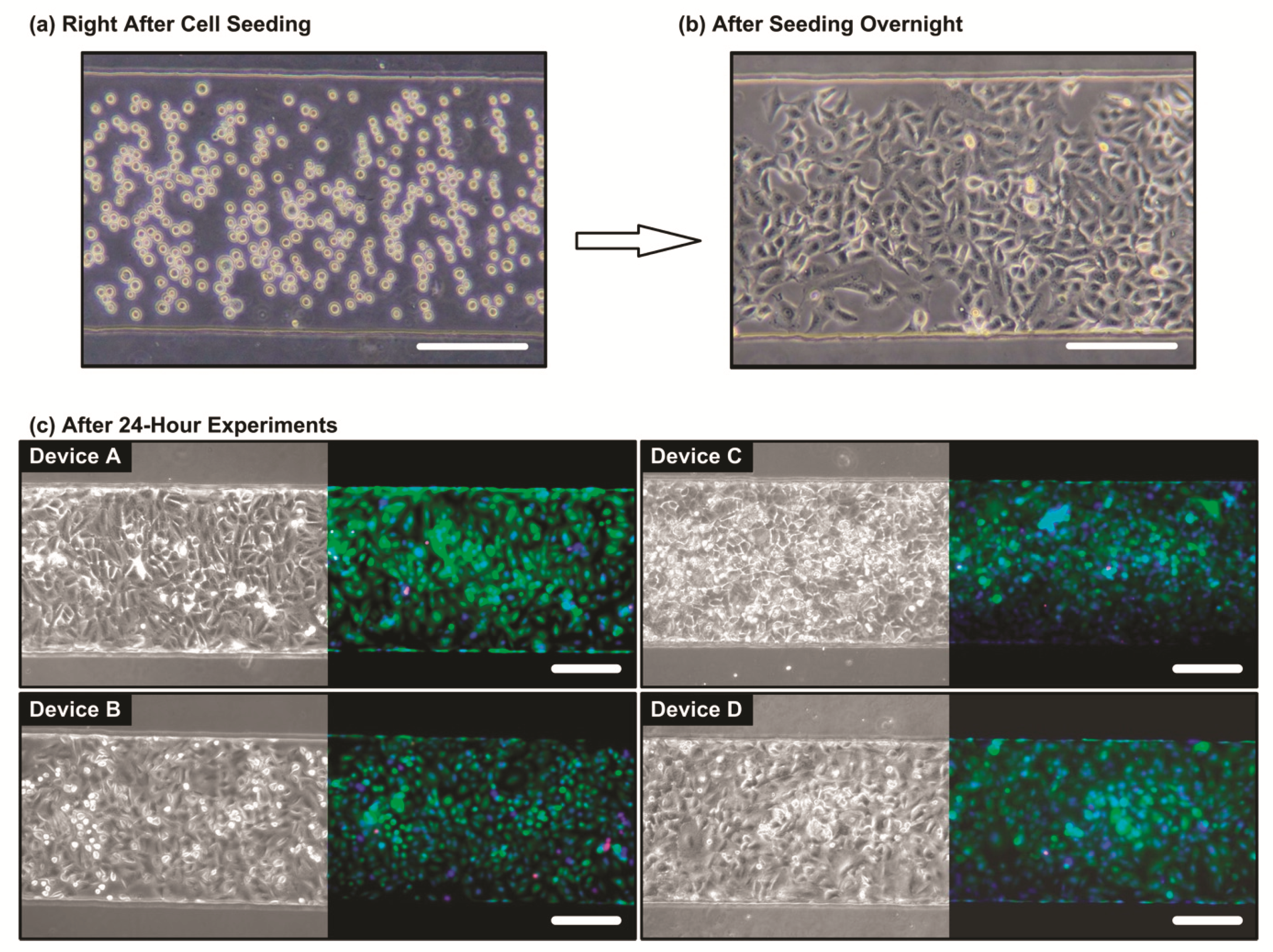
2.4. Cell Culture and Seeding
2.5. Analysis of Surfactant Protein Expression
3. Results and Discussion
3.1. Device Characterization
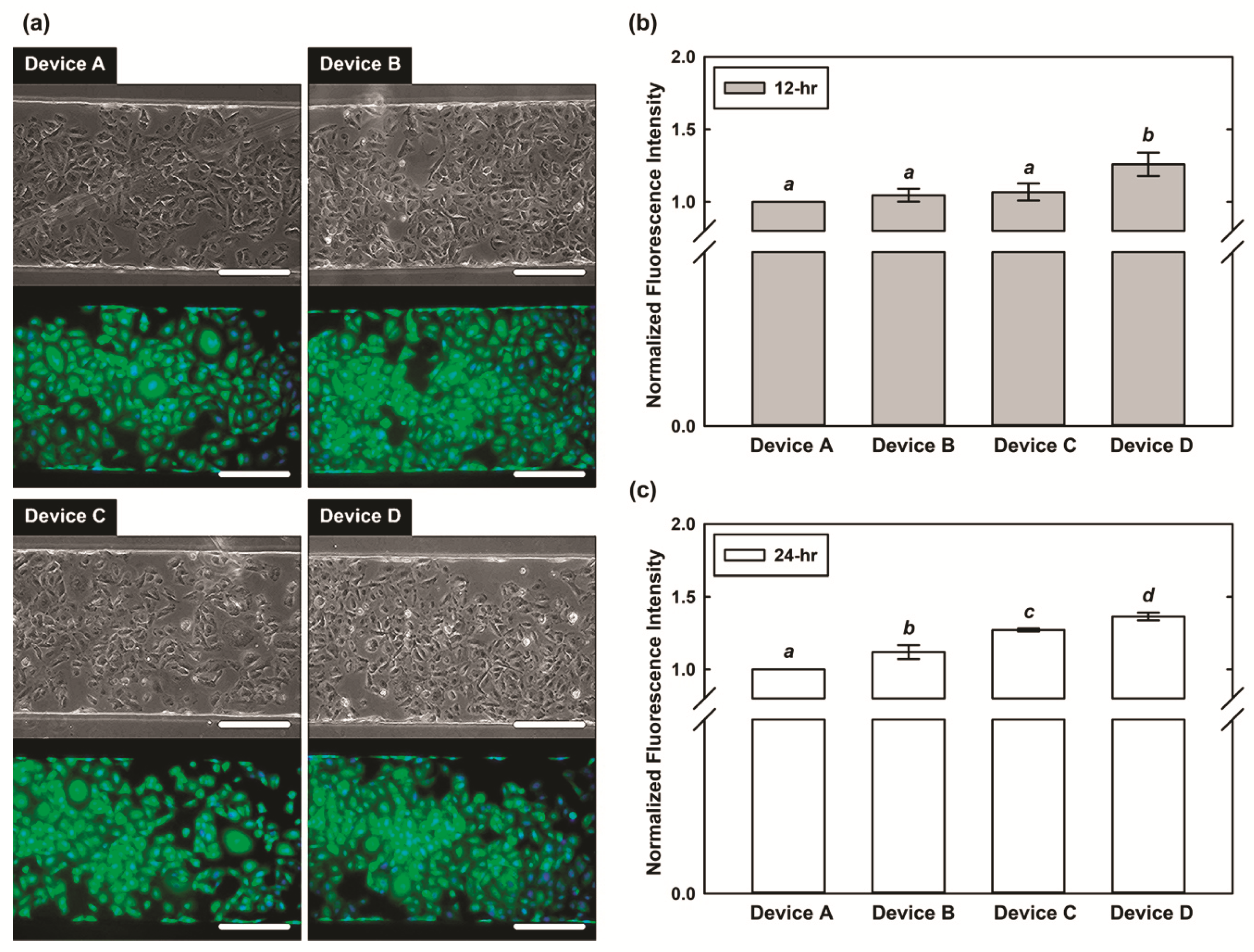
3.2. Cell Viability Characterization
3.3. Expression of SPC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tarbell, J.M. Shear Stress and the Endothelial Transport Barrier. Cardiovasc. Res. 2010, 87, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Shih, H.C.; Wu, J.G.; Weng, T.W.; Wu, C.Y.; Lu, J.C.; Tung, Y.C. Electrofluidic Pressure Sensor Embedded Microfluidic Device: A Study of Endothelial Cells under Hydrostatic Pressure and Shear Stress Combinations. Lab Chip 2013, 13, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Federico, V.; Bianchi, F.; Ahluwalia, B.; Domenici, C. Hydrostatic Pressure and Shear Stress Affect Endothelin-1 and Nitric Oxide Release by Endothelial Cells in Bioreactors. Biotechnol. J. 2013, 9, 146–154. [Google Scholar]
- Collingsworth, A.M.; Torgan, C.E.; Nagda, S.N.; Rajalingam, R.J.; Kraus, W.E.; Truskey, G.A. Orientation and Length of Mammalian Skeletal Myocytes in Response to a Unidirectional Stretch. Cell Tissue Res. 2000, 302, 243–251. [Google Scholar] [CrossRef]
- Kamotani, Y.; Bersano-Begey, T.; Kato, N.; Tung, Y.C.; Huh, D.; Song, J.W.; Takayama, S. Individually Programmable Cell Stretching Microwell Arrays Actuated by a Braille Display. Biomaterials 2008, 29, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, S. Lung Growth for Beginners. Paediatr. Respir. Rev. 2000, 1, 308–313. [Google Scholar] [CrossRef]
- Laudy, J.A.; Wladimiroff, J.W. The Fetal Lung. 1: Developmental Aspects. Ultrasound Obstet. Gynecol. 2000, 16, 284–290. [Google Scholar] [CrossRef]
- Cosmi, E.; Anceschi, M.M.; Piazze, J.; La Torre, R. Ultrasonographic Patterns of Fetal Breathing Movements in Normal Pregnancy. Int. J. Gynecol. Obstet. 2003, 80, 285–290. [Google Scholar] [CrossRef]
- Liu, M.; Skinner, S.J.M.; Xu, J.; Han, R.N.N.; Tanswell, A.K.; Post, M. Stimulation of Fetal Rat Lung Cell Proliferation in vitro by Mechanical Stretch. Am. J. Physiol. 1992, 263, L376–L383. [Google Scholar] [CrossRef]
- Nakamura, T.; Liu, M.; Mourgeon, E.; Slutsky, A.; Post, M. Mechancial Strain and Dexamethasone Selectively Increase Surfactant Protein C and Tropelastin Gene Expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L974–L980. [Google Scholar] [CrossRef]
- Sanchez-Esteban, J.; Wang, Y.; Cicchiello, L.A.; Rubin, L.P. Cyclic Mechanical Stretch Inhibits Cell Proliferation and Induces Apoptosis in Fetal Rat Lung Fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L448–L456. [Google Scholar] [CrossRef]
- Douville, N.J.; Zamankhan, P.; Tung, Y.C.; Li, R.; Vaughan, B.L.; Tai, C.F.; White, J.; Christensen, P.J.; Grotberg, J.B.; Takayama, S. Combination of Fluid and Solid Mechanical Stresses Contribute to Cell Death and Detachment in a Microfluidic Alveolar Model. Lab Chip 2011, 11, 609–619. [Google Scholar] [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting Organ-Level Lung Functions on a Chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, U.; Fisk, N.M.; Rodeck, C.H.; Talbert, D.G.; Wigglesworth, J.S. Low Amniotic Pressure in Oligohydramnios—Is this the Cause of Pulmonary Hypoplasia? Am. J. Obstet. Gynecol. 1989, 161, 1098–1101. [Google Scholar] [CrossRef]
- Boyce, E.S.; Dawes, G.S.; Gough, J.D.; Poore, E.R. Doppler Ultrasound Method for Detecting Human Fetal Breathing in Utero. Br. Med. J. 1976, 2, 17–18. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meyer, K.C. Diagnosis and Management of Interstitial Lung Disease. Transl. Respir. Med. 2014, 2, 4. [Google Scholar] [CrossRef]
- Whitesides, G.M.; Ostuni, E.; Takayama, S.; Jiang, X.; Ingber, D.E. Soft Lithography in Biology and Biochemistry. Ann. Rev. Biomed. Eng. 2001, 3, 335–373. [Google Scholar] [CrossRef]
- Lu, J.C.; Liao, W.H.; Tung, Y.C. Magnet-assisted Device-level Alignment for the Fabrication of Membrane-sandwiched Polydimethylsiloxane Microfluidic Devices. J. Micromech. Microeng. 2012, 22, 006–075. [Google Scholar] [CrossRef]
- Chang, C.W.; Cheng, Y.J.; Tu, M.; Chen, Y.H.; Peng, C.C.; Liao, W.H.; Tung, Y.C. A Polydimethylsiloxane–polycarbonate Hybrid Microfluidic Device Capable of Generating Perpendicular Chemical and Oxygen Gradients for Cell Culture Studies. Lab Chip 2014, 14, 3762–3772. [Google Scholar] [CrossRef]
- Nardone, L.L.; Andrews, S.B. Cell Line A549 as a Model of the Type II Pneumocyte: Phospholipid Biosynthesis from Native and Organometallic Precursors. Biochim. Biophys. Acta Lipids Lipid Metab. 1979, 573, 276–295. [Google Scholar] [CrossRef]
- Kamath-Rayne, B.D.; DeFranco, E.A.; Marcotte, M.P. Antenatal Steroids for Treatment of Fetal Lung Immaturity After 34 Weeks of Gestation. Obstet. Gynecol. 2012, 119, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.C.; Orgeig, S. Dexamethasone and Epinephrine Stimulate Surfactant Secretion in Type II Cells of Embryonic Chickens. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001, 280, R770–R777. [Google Scholar] [CrossRef] [PubMed]
- Kalina, M.; Mason, R.J.; Shannon, J.M. Surfactant Protein C Is Expressed in Alveolar Type II Cells but Not in Clara Cells of Rat Lung. Am. J. Respir. Cell Mol. Biol. 1992, 6, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Quinn, T.P.; Schlueter, M.; Soifer, S.J.; Gutierrez, J.A. Cyclic Mechanical Stretch Induces VEGF and FGF-2 Expression in Pulmonary Vascular Smooth Muscle Cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002, 282, L897–L903. [Google Scholar] [CrossRef]
- Rucka, Z.; Vanhara, P.; Koutna, I.; Tesarova, L.; Potesilova, M.; Stejskal, S.; Simara, P.; Dolezel, J.; Zvonicek, V.; Coufal, O.; et al. Differential Effects of Insulin and Dexamethasone on Pulmonary Surfactant-Associated Genes and Proteins in A549 and H441 Cells and Lung Tissue. Int. J. Mol. Med. 2013, 32, 211–218. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-R.; Yeh, S.-L.; Peng, C.-C.; Liao, W.-H.; Tung, Y.-C. Study Effects of Drug Treatment and Physiological Physical Stimulation on Surfactant Protein Expression of Lung Epithelial Cells Using a Biomimetic Microfluidic Cell Culture Device. Micromachines 2019, 10, 400. https://doi.org/10.3390/mi10060400
Lin T-R, Yeh S-L, Peng C-C, Liao W-H, Tung Y-C. Study Effects of Drug Treatment and Physiological Physical Stimulation on Surfactant Protein Expression of Lung Epithelial Cells Using a Biomimetic Microfluidic Cell Culture Device. Micromachines. 2019; 10(6):400. https://doi.org/10.3390/mi10060400
Chicago/Turabian StyleLin, Ting-Ru, Sih-Ling Yeh, Chien-Chung Peng, Wei-Hao Liao, and Yi-Chung Tung. 2019. "Study Effects of Drug Treatment and Physiological Physical Stimulation on Surfactant Protein Expression of Lung Epithelial Cells Using a Biomimetic Microfluidic Cell Culture Device" Micromachines 10, no. 6: 400. https://doi.org/10.3390/mi10060400
APA StyleLin, T.-R., Yeh, S.-L., Peng, C.-C., Liao, W.-H., & Tung, Y.-C. (2019). Study Effects of Drug Treatment and Physiological Physical Stimulation on Surfactant Protein Expression of Lung Epithelial Cells Using a Biomimetic Microfluidic Cell Culture Device. Micromachines, 10(6), 400. https://doi.org/10.3390/mi10060400




