Effects of UV Irradiation on the Sensing Properties of In2O3 for CO Detection at Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
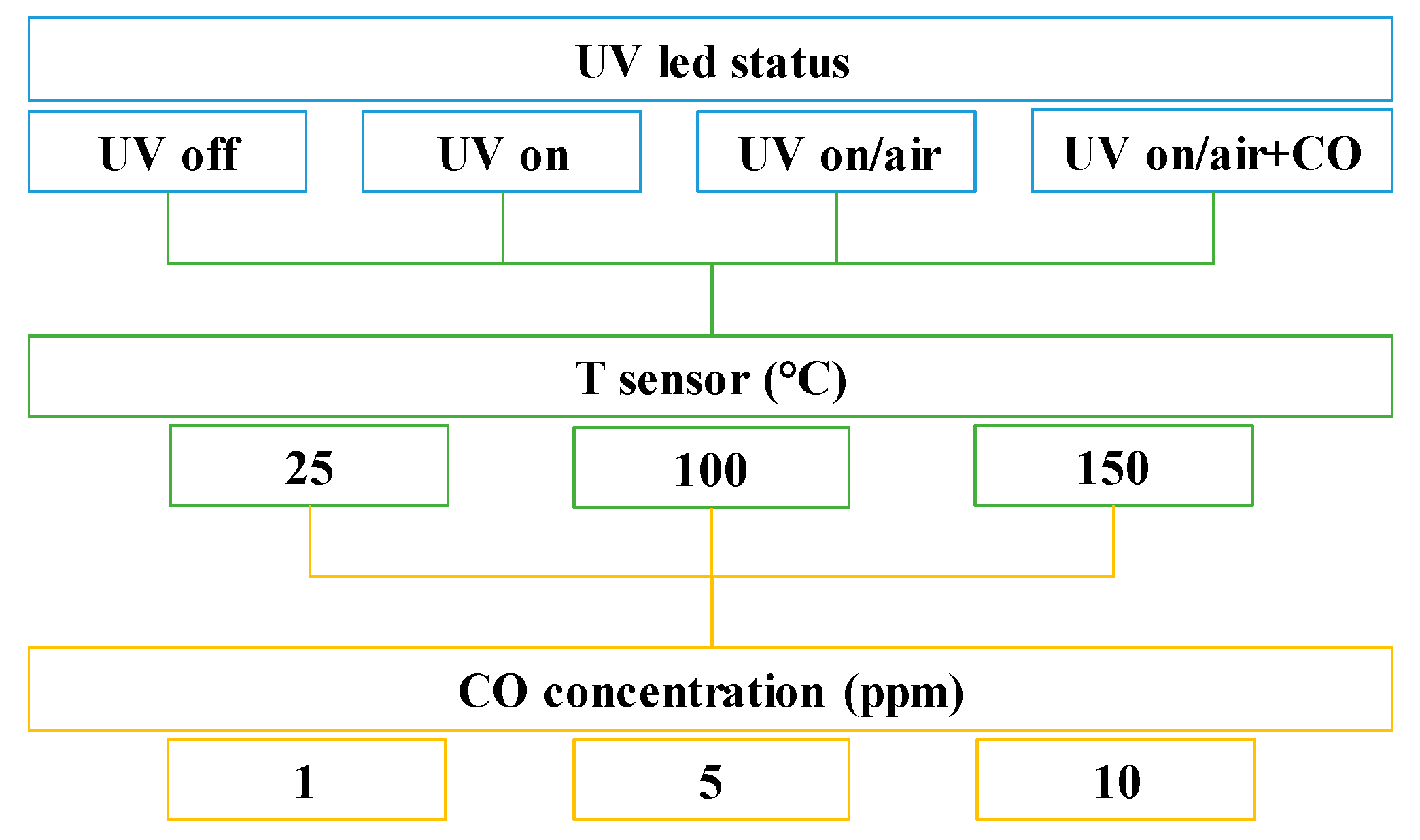
2.2. Sensor Preparation and Testing
3. Results and Discussion
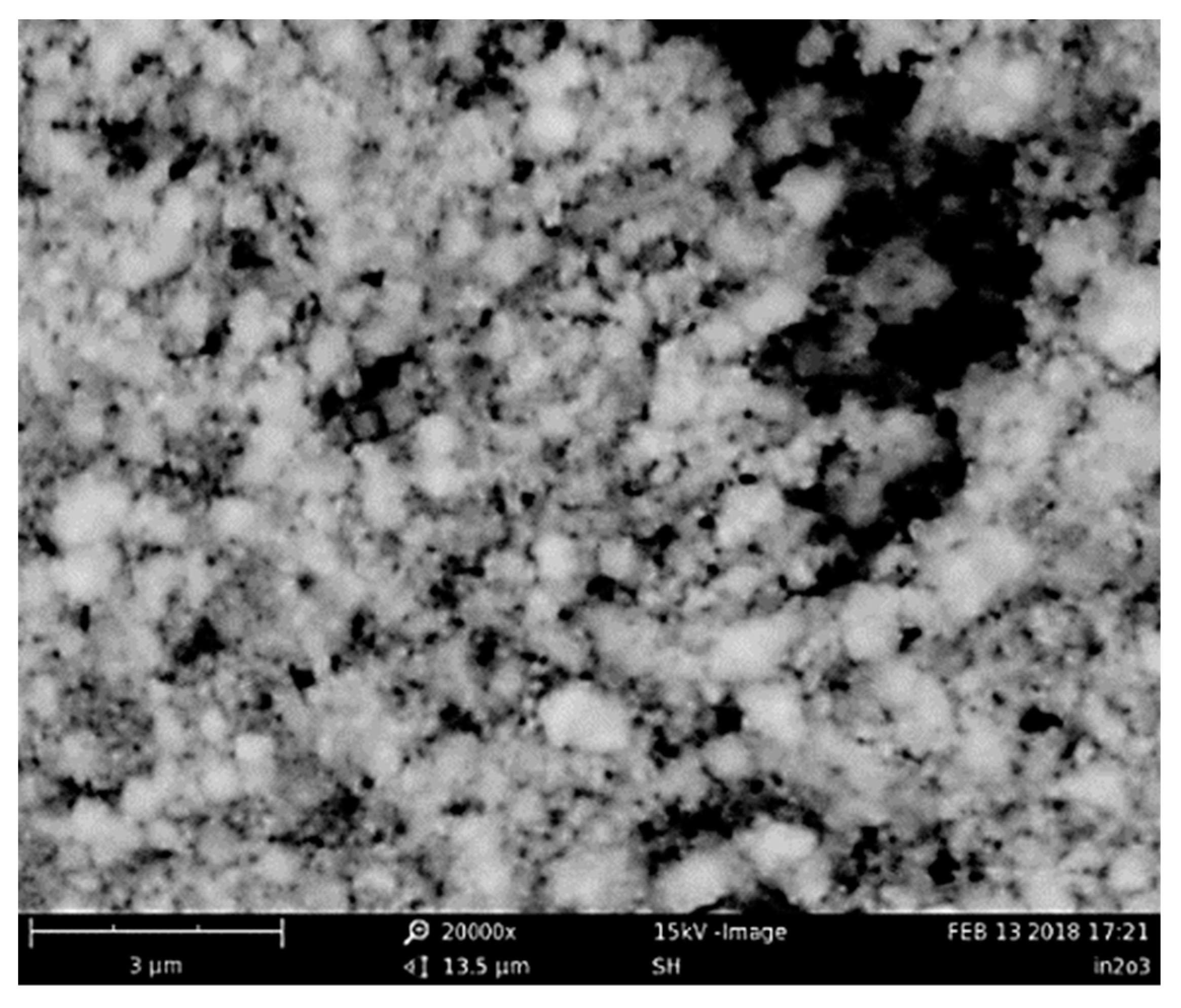
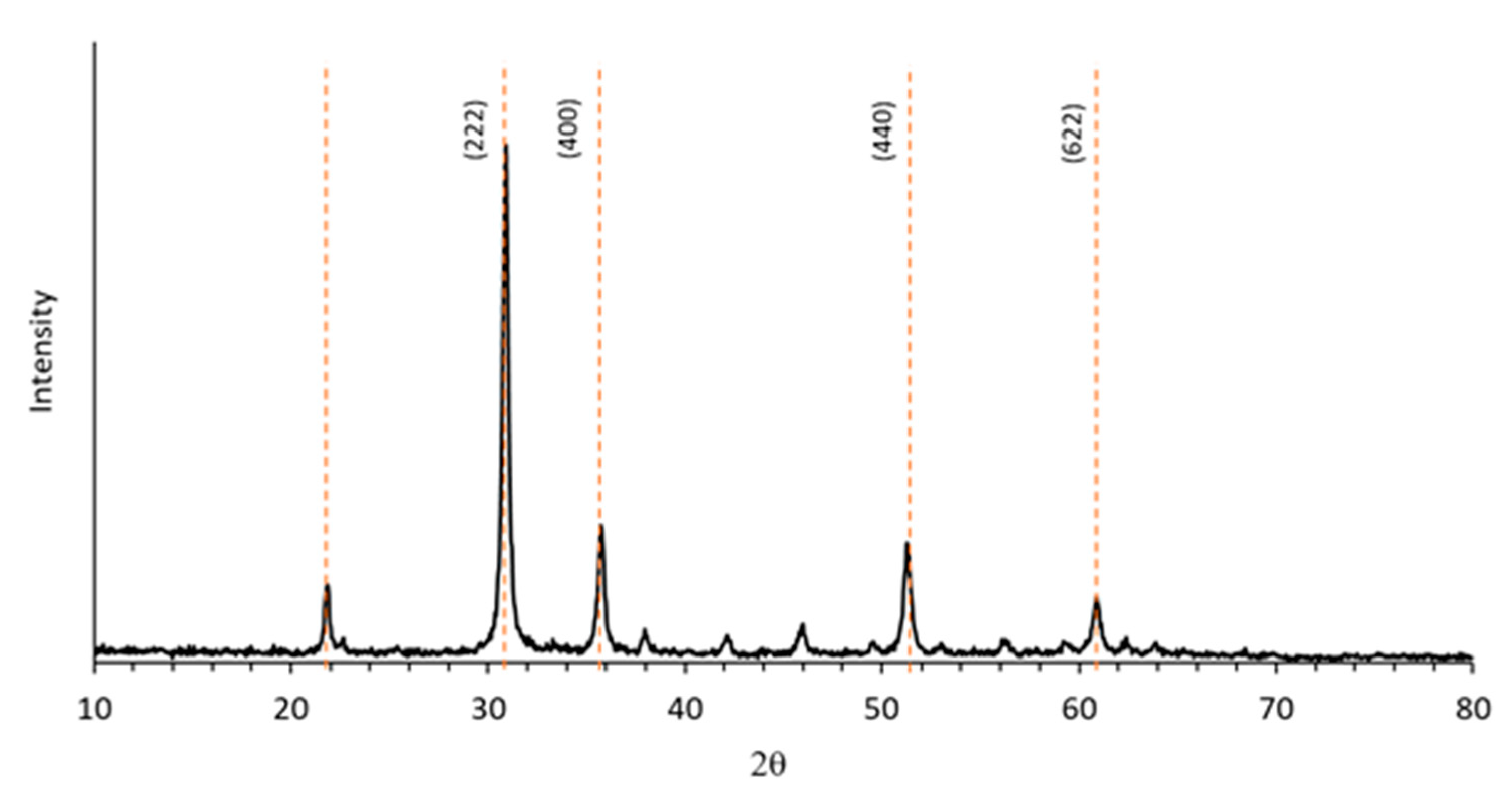
3.1. Morphological and Microstructural Characterization
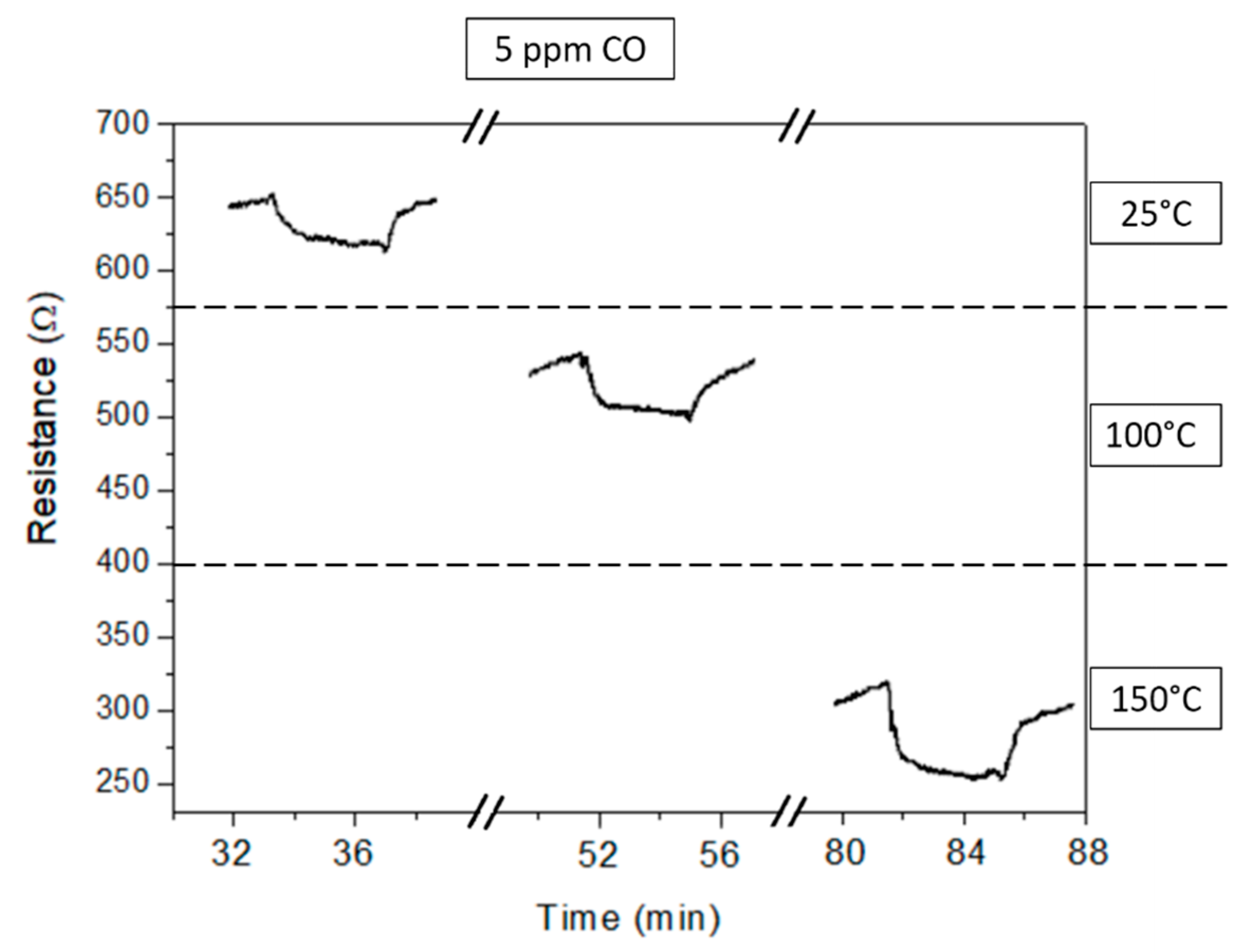
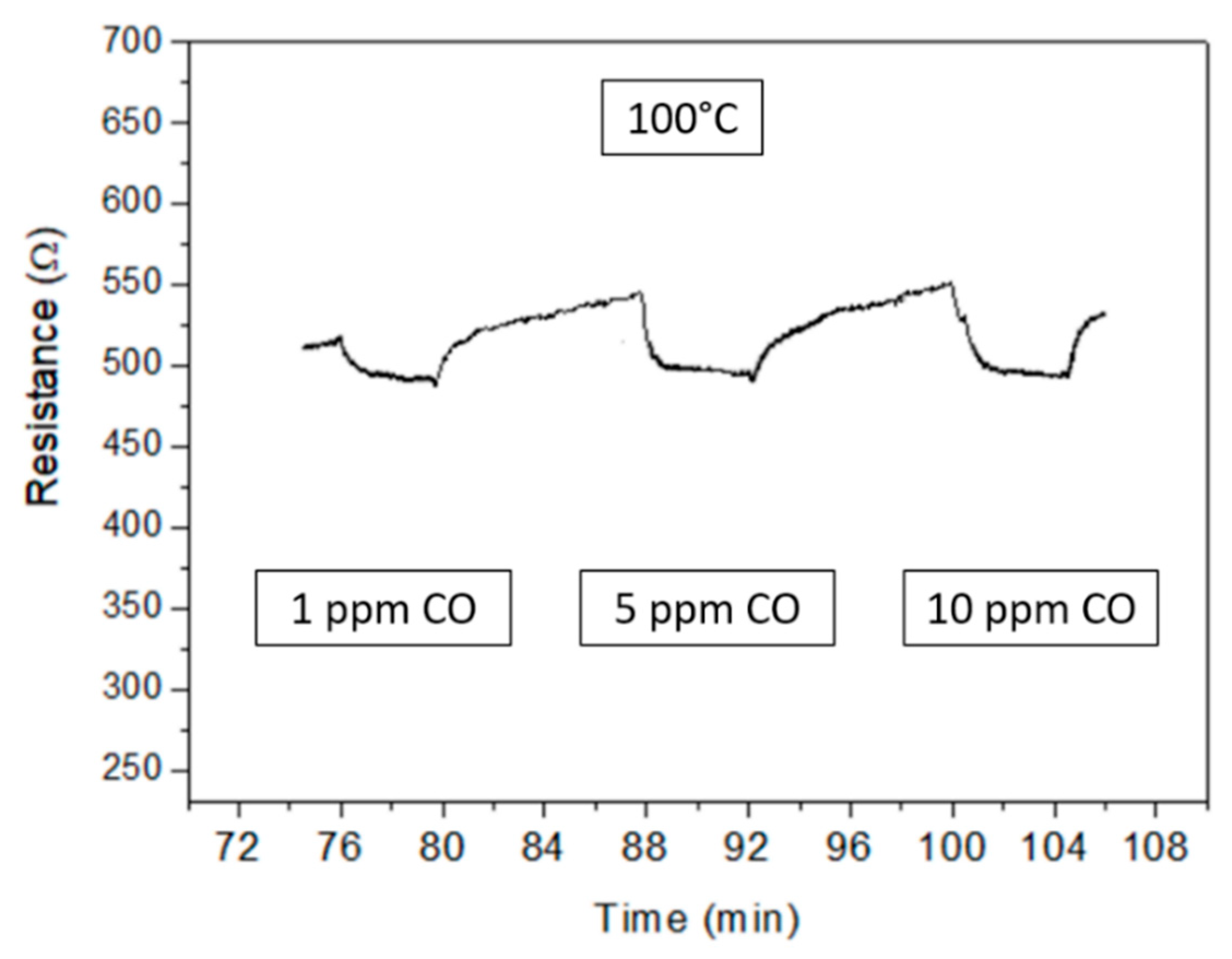
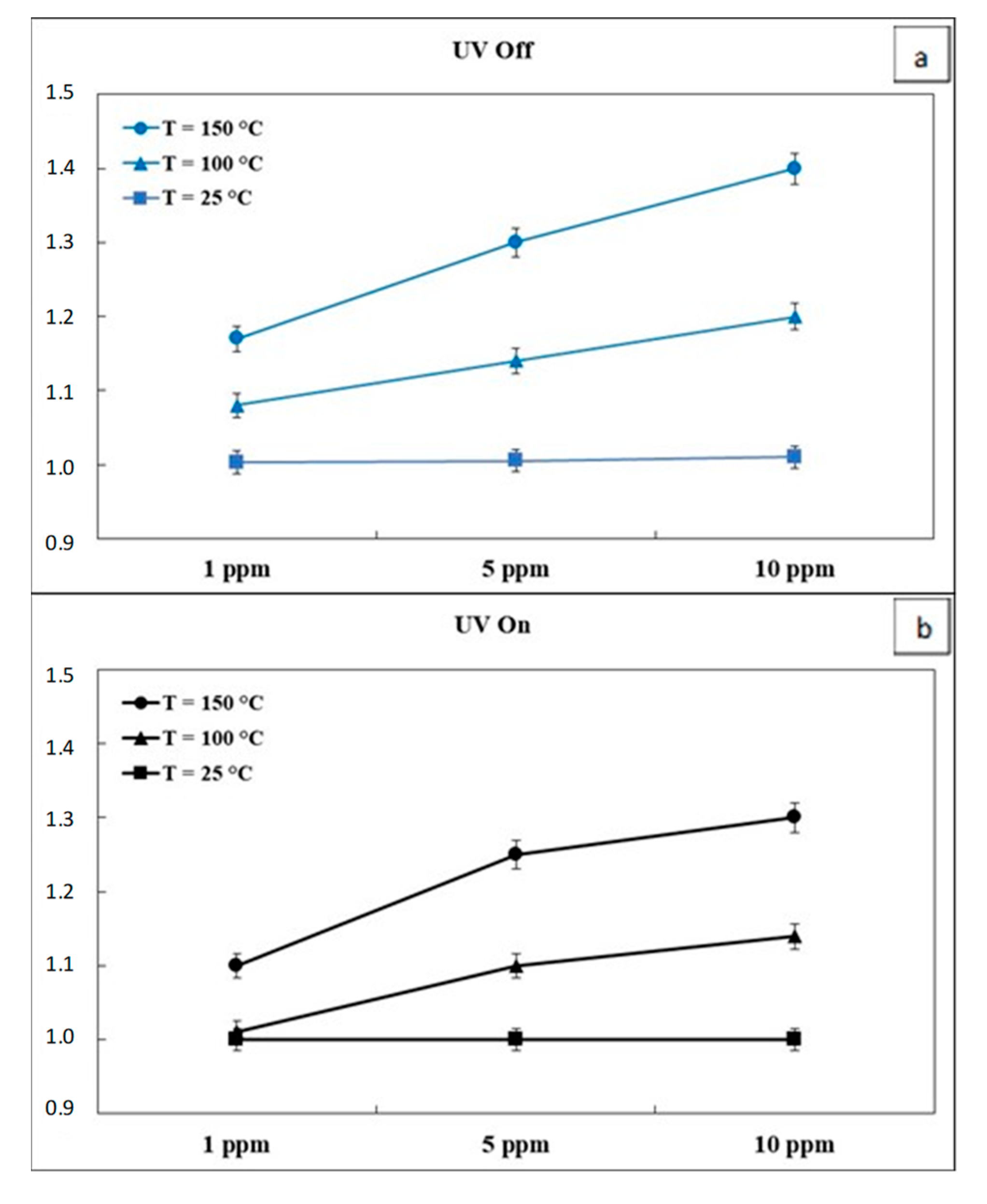
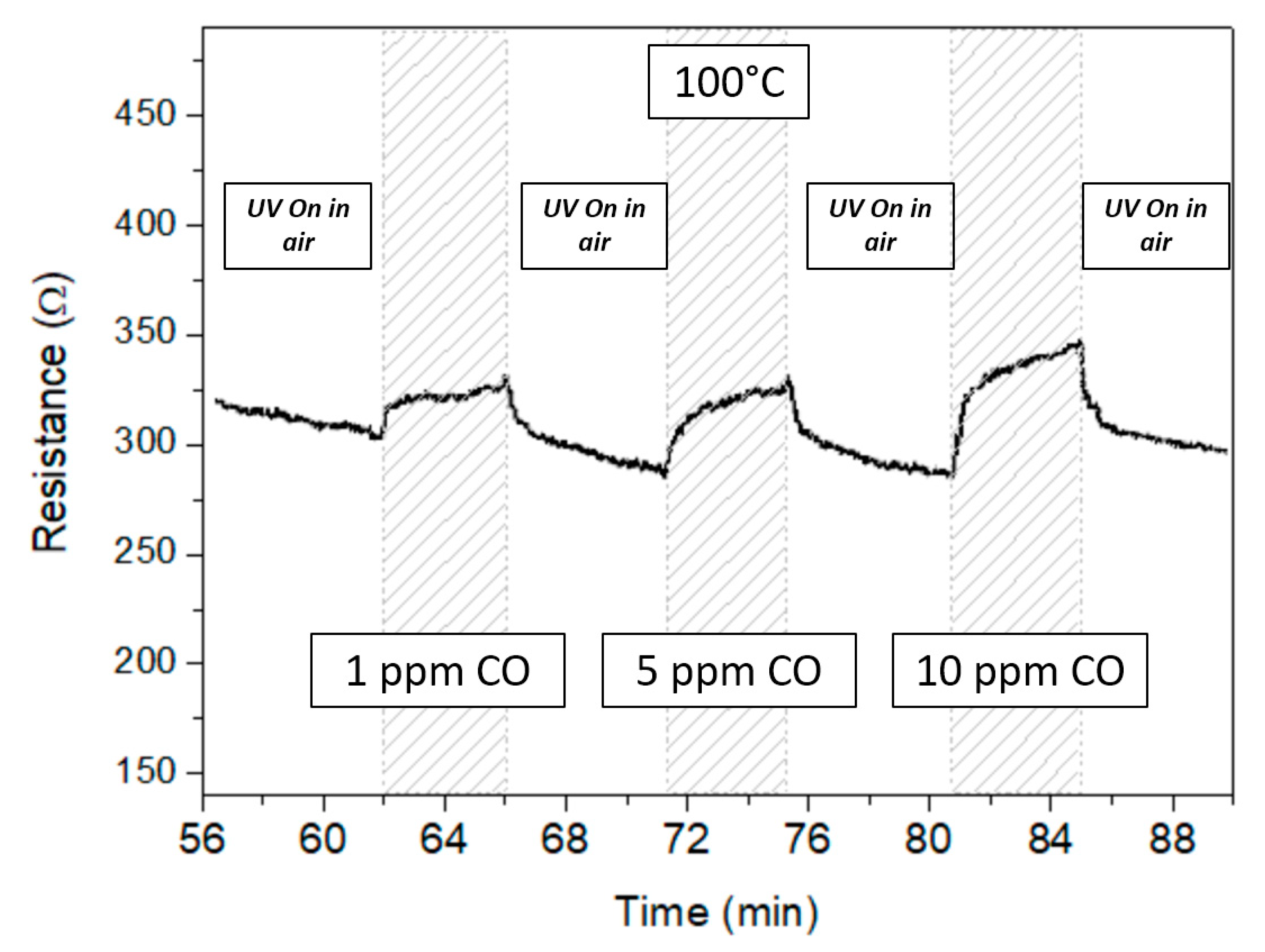
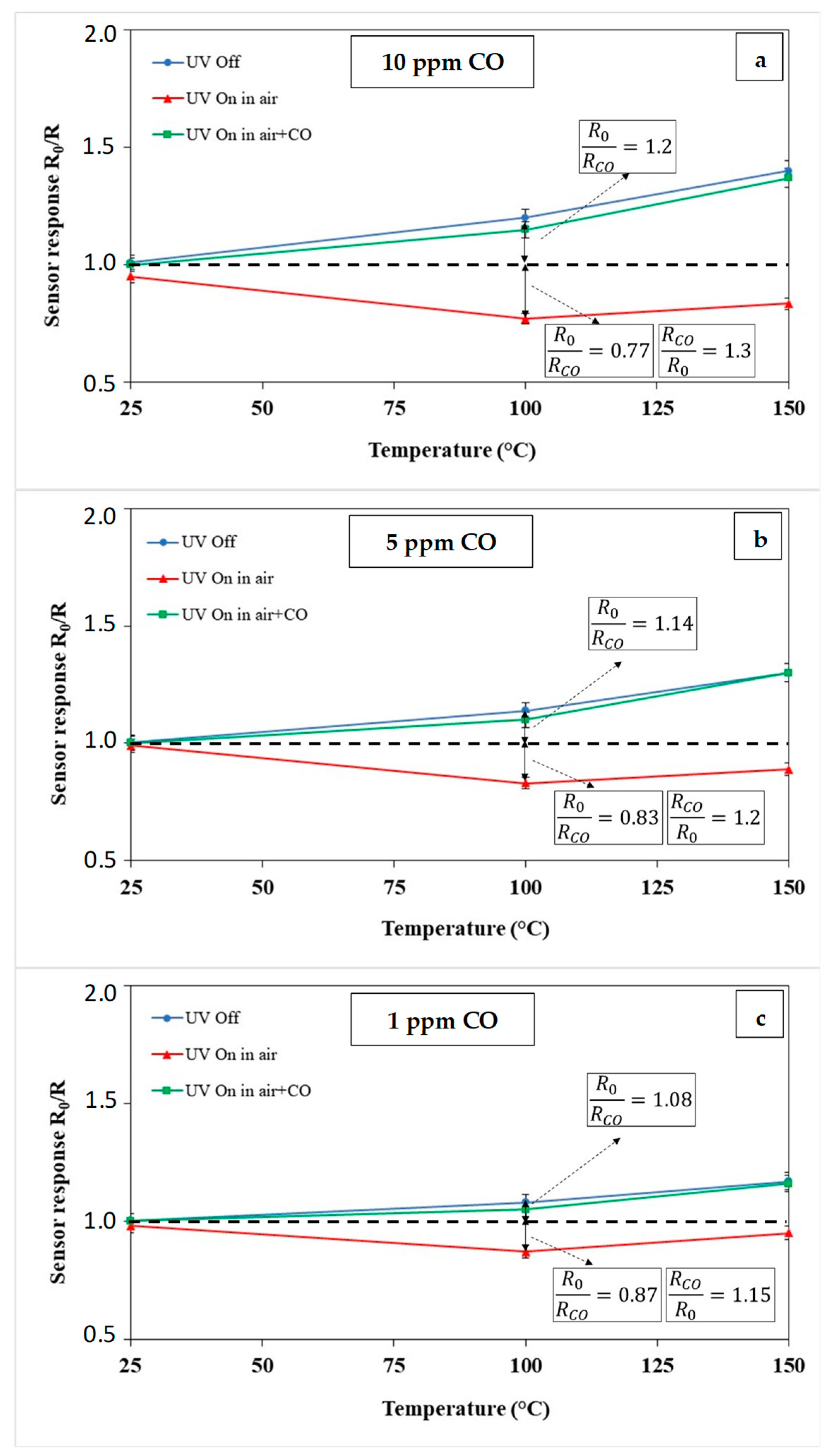
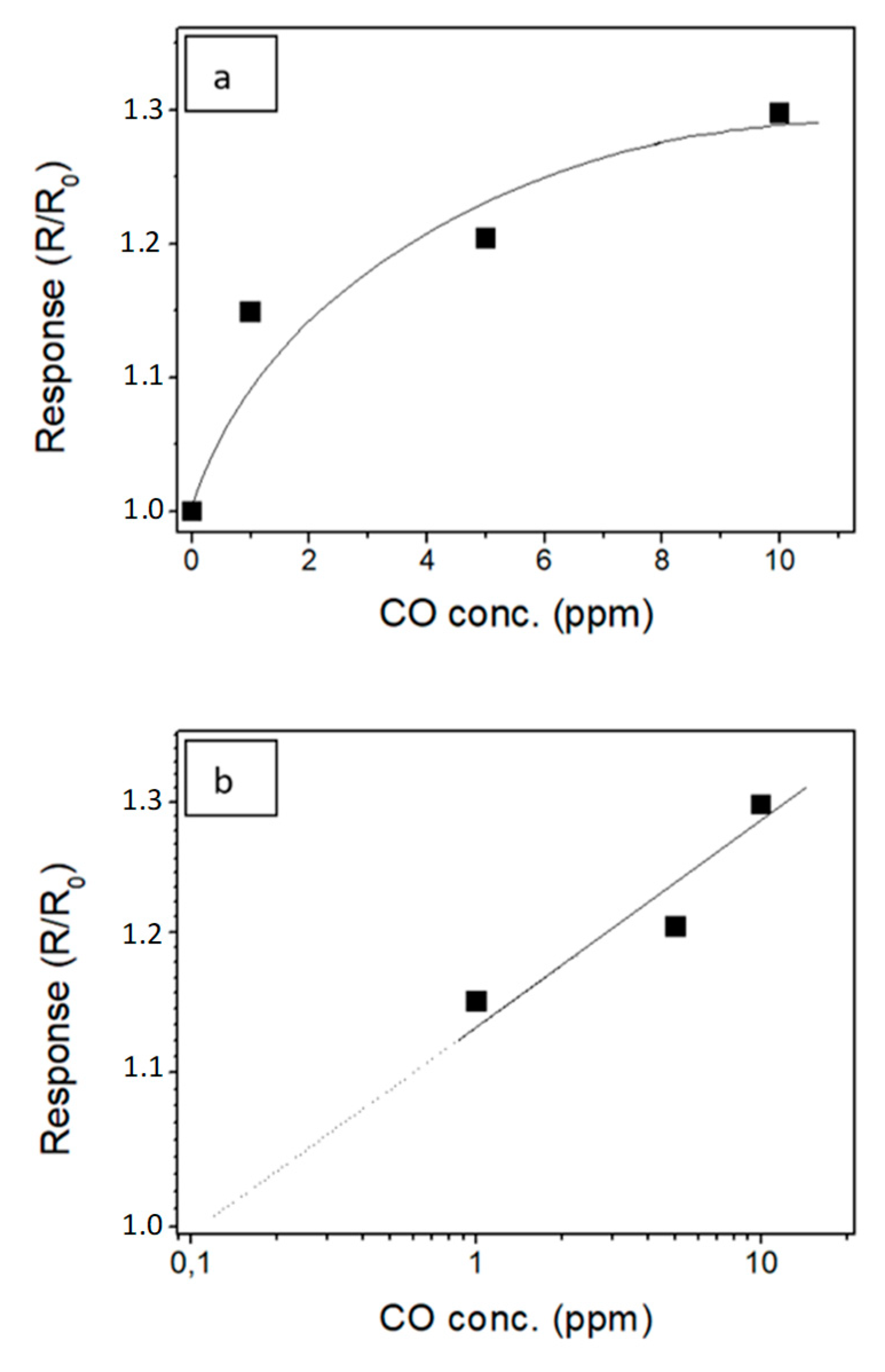
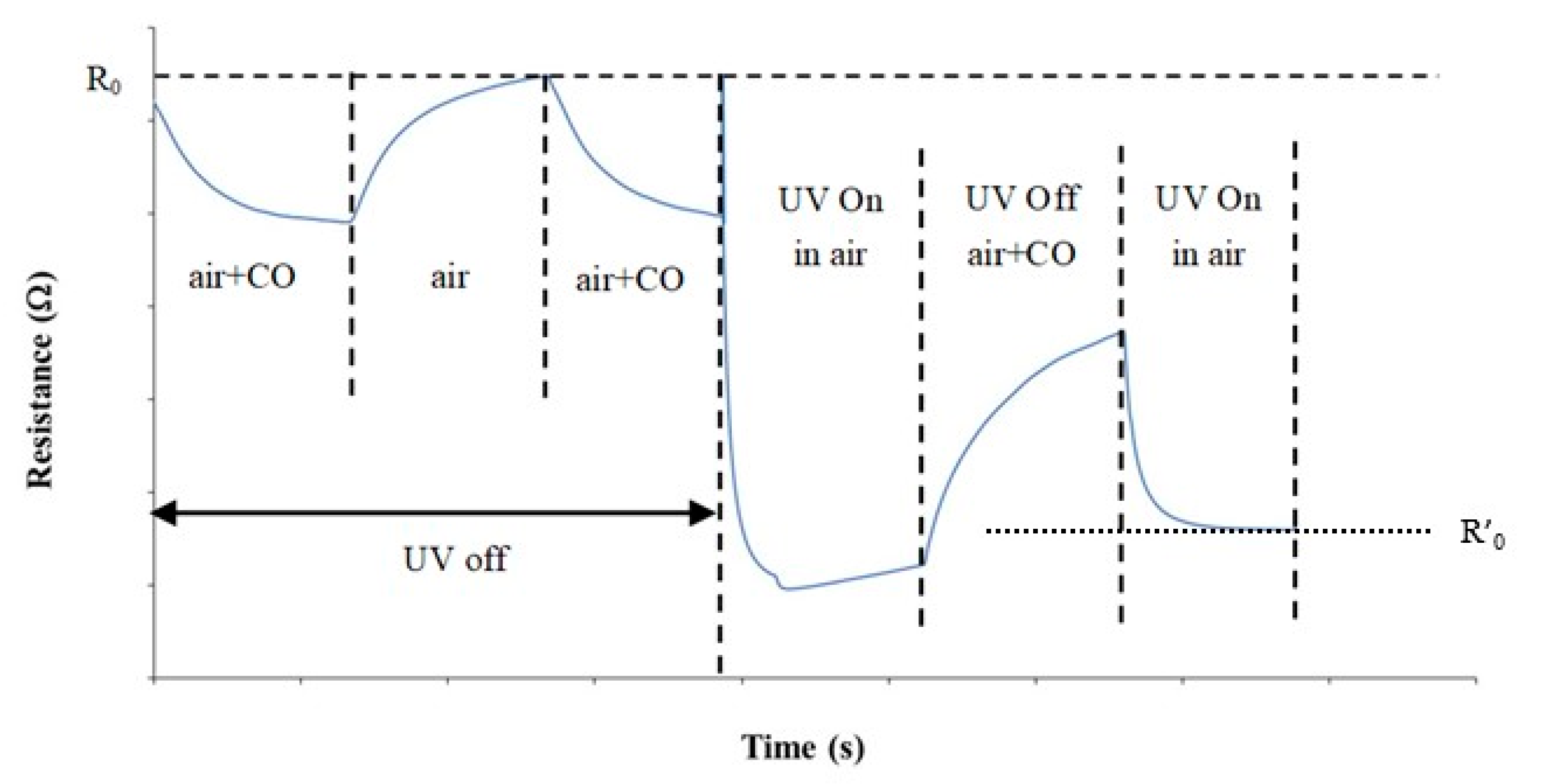
3.2. Gas Sensor Measurement
3.3. Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Forleo, A.; Francioso, L.; Epifani, M.; Capone, S.; Taurino, A.M.; Siciliano, P. NO2- gas-sensing properties of mixed In2O3–SnO2 thin films. Thin Solid Films 2005, 490, 68–73. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cerneavschi, A.; Ivanov, M.; Golovanov, V.; Cornet, A.; Morante, J.; Cabot, A.; Arbiol, J. The influence of film structure on In2O3 gas response. Thin Solid Films 2004, 460, 315–323. [Google Scholar] [CrossRef]
- Chung, W.Y.; Sakai, G.; Shimanoe, K.; Miura, N.; Lee, D.D.; Yamazoe, N. Preparation of indium oxide thin film by spin-coating method and its gas-sensing properties. Sens. Actuators B Chem. 1998, 46, 139–145. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Boris, I.; Cornet, A.; Rodriguez, J.; Cirera, A.; Golovanov, V.; Lychkovsky, Y.; Karkotsky, G. The influence of additives on gas sensing and structural properties of In2O3-based ceramics. Sens. Actuators B Chem. 2007, 120, 657–664. [Google Scholar] [CrossRef]
- Ivanovskaya, M.; Bogdanov, P.; Faglia, G.; Sberveglieri, G. The features of thin film and ceramic sensors at the detection of CO and NO2. Sens. Actuators B Chem. 2000, 68, 344–350. [Google Scholar] [CrossRef]
- Yamaura, H.; Jinkawa, T.; Tamaki, J.; Moriya, K.; Miura, N.; Yamazoe, N. Indium oxide-based gas sensor for selective detection of CO. Sens. Actuators B Chem. 1996, 35–36, 325–332. [Google Scholar] [CrossRef]
- Yamaura, H.; Moriya, K.; Miura, N.; Yamazoe, N. Mechanism of sensitivity promotion in CO sensor using indium oxide and cobalt oxide. Sens. Actuators B Chem. 2000, 65, 39–41. [Google Scholar] [CrossRef]
- Lim, S.K.; Hwang, S.H.; Chang, D.; Kim, S. Preparation of mesoporous In2O3 nanofibers by electrospinning and their application as a CO gas sensor. Sens. Actuators B Chem. 2010, 149, 28–33. [Google Scholar] [CrossRef]
- Donato, N.; Neri, F.; Neri, G.; Latino, M.; Barreca, F.; Spadaro, S.; Pisagatti, I.; Currò, G. CO sensing devices based on indium oxide nanoparticles prepared by laser ablation in water. Thin Solid Films 2011, 520, 922–926. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Comini, E.; Cristalli, A.; Faglia, G.; Sberveglieri, G. Light enhanced gas sensing properties of indium oxide and tin dioxide sensors. Sens. Actuators B Chem. 2000, 65, 260–263. [Google Scholar] [CrossRef]
- Barquinha, P.; Pimentel, A.; Marques, A.; Pereira, L.; Martins, R.; Fortunato, E. Effect of UV and visible light radiation on the electrical performances of transparent TFTs based on amorphous indium zinc oxide. J. Non. Cryst. Solids 2006, 352, 1756–1760. [Google Scholar] [CrossRef]
- Wang, C.Y.; Becker, R.W.; Passow, T.; Pletschen, W.; Köhler, K.; Cimalla, V.; Ambacher, O. Photon stimulated sensor based on indium oxide nanoparticles I: Wide-concentration-range ozone monitoring in air. Sens. Actuators B Chem. 2011, 152, 235–240. [Google Scholar] [CrossRef]
- Wagner, T.; Kohl, C.D.; Malagù, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. UV light-enhanced NO2 sensing by mesoporous In2O3: Interpretation of results by a new sensing model. Sens. Actuators B Chem. 2013, 187, 488–494. [Google Scholar] [CrossRef]
- Espid, E.; Taghipour, F. Development of highly sensitive ZnO/In2O3 composite gas sensor activated by UV-LED. Sens. Actuators B Chem. 2017, 241, 828–839. [Google Scholar] [CrossRef]
- Mishra, S.; Ghanshyam, C.; Ram, N.; Bajpai, R.P.; Bedi, R.K. Detection mechanism of metal oxide gas sensor under UV radiation. Sens. Actuators B Chem. 2004, 97, 387–390. [Google Scholar] [CrossRef]
- Wagner, T.; Kohl, C.D.; Morandi, S.; Malagù, C.; Donato, N.; Latino, M.; Neri, G.; Tiemann, M. Photoreduction of Mesoporous In2O3: Mechanistic Model and Utility in Gas Sensing. Chem. Eur. J. 2012, 18, 8216–8223. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Micali, G.; Rizzo, G.; Callone, E.; Carturan, G. Resistive CO gas sensors based on In2O3 and InSnOx nanopowders synthesized via starch-aided sol-gel process for automotive applications. Sens. Actuators B Chem. 2008, 132, 224–233. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Surv. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Bender, M.; Katsarakis, N.; Gagaoudakis, E.; Hourdakis, E.; Douloufakis, E.; Cimalla, V.; Kiriakidis, G. Dependence of the photoreduction and oxidation behavior of indium oxide films on substrate temperature and film thickness. J. Appl. Phys. 2001, 90, 5382. [Google Scholar] [CrossRef]
- Werner, R.L.; Ley, R.P. Optical Properties of Indium Oxide. J. Appl. Phys. 1966, 37, 299. [Google Scholar]
- De Lacy Costello, B.P.J.; Ewen, R.J.; Ratcliffe, N.M.; Richards, M. Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens. Actuators B Chem. 2008, 134, 945–995. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonaccorsi, L.; Malara, A.; Donato, A.; Donato, N.; Leonardi, S.G.; Neri, G. Effects of UV Irradiation on the Sensing Properties of In2O3 for CO Detection at Low Temperature. Micromachines 2019, 10, 338. https://doi.org/10.3390/mi10050338
Bonaccorsi L, Malara A, Donato A, Donato N, Leonardi SG, Neri G. Effects of UV Irradiation on the Sensing Properties of In2O3 for CO Detection at Low Temperature. Micromachines. 2019; 10(5):338. https://doi.org/10.3390/mi10050338
Chicago/Turabian StyleBonaccorsi, Lucio, Angela Malara, Andrea Donato, Nicola Donato, Salvatore Gianluca Leonardi, and Giovanni Neri. 2019. "Effects of UV Irradiation on the Sensing Properties of In2O3 for CO Detection at Low Temperature" Micromachines 10, no. 5: 338. https://doi.org/10.3390/mi10050338
APA StyleBonaccorsi, L., Malara, A., Donato, A., Donato, N., Leonardi, S. G., & Neri, G. (2019). Effects of UV Irradiation on the Sensing Properties of In2O3 for CO Detection at Low Temperature. Micromachines, 10(5), 338. https://doi.org/10.3390/mi10050338







