Wettability Manipulation by Interface-Localized Liquid Dielectrophoresis: Fundamentals and Applications
Abstract
1. Introduction
2. Liquid Dielectrophoresis
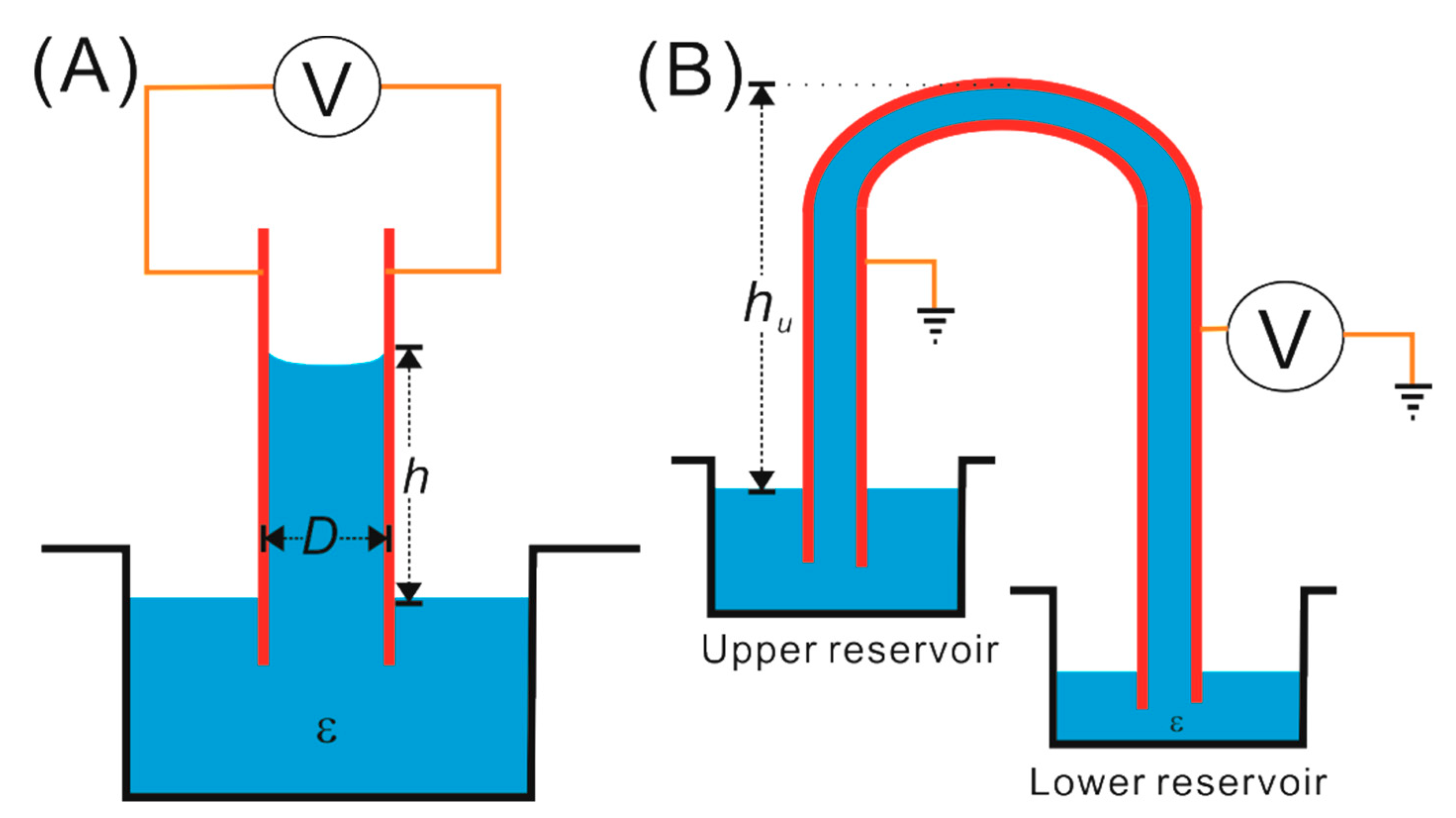
2.1. Basic Governing Force Analysis
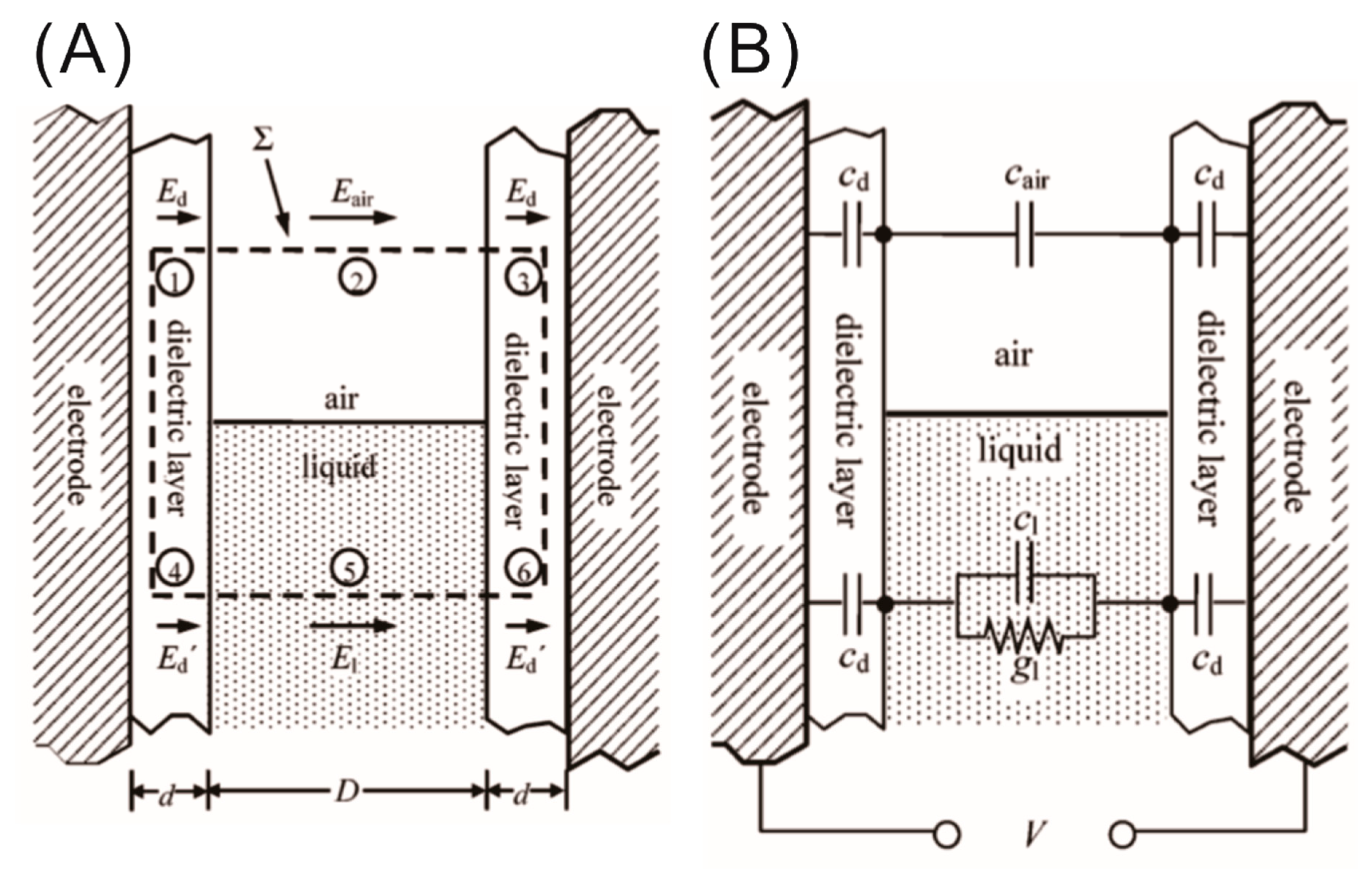
2.2. Equivalent Electrical Circuit Model
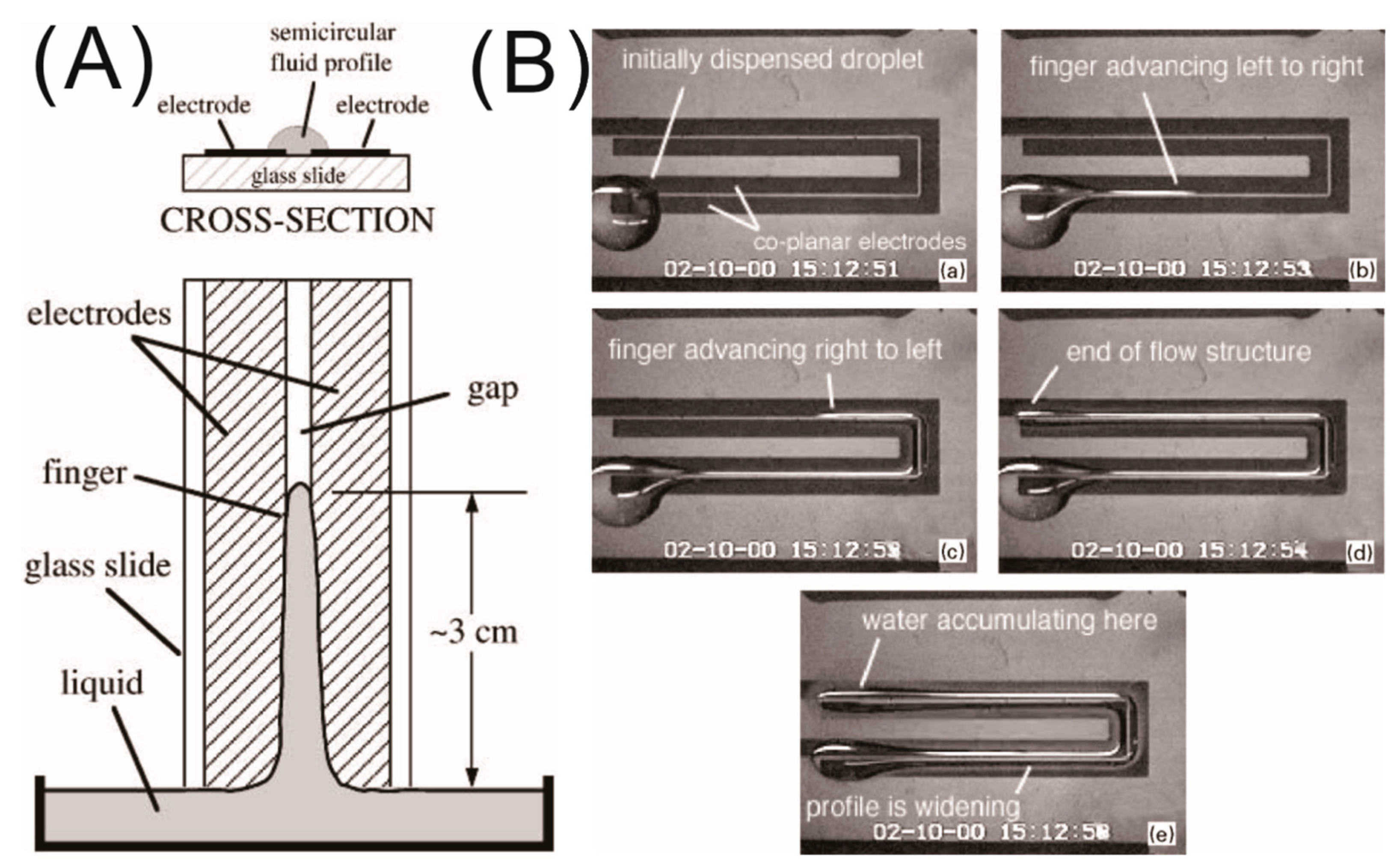
2.3. Liquid Manipulation in Microfluidics
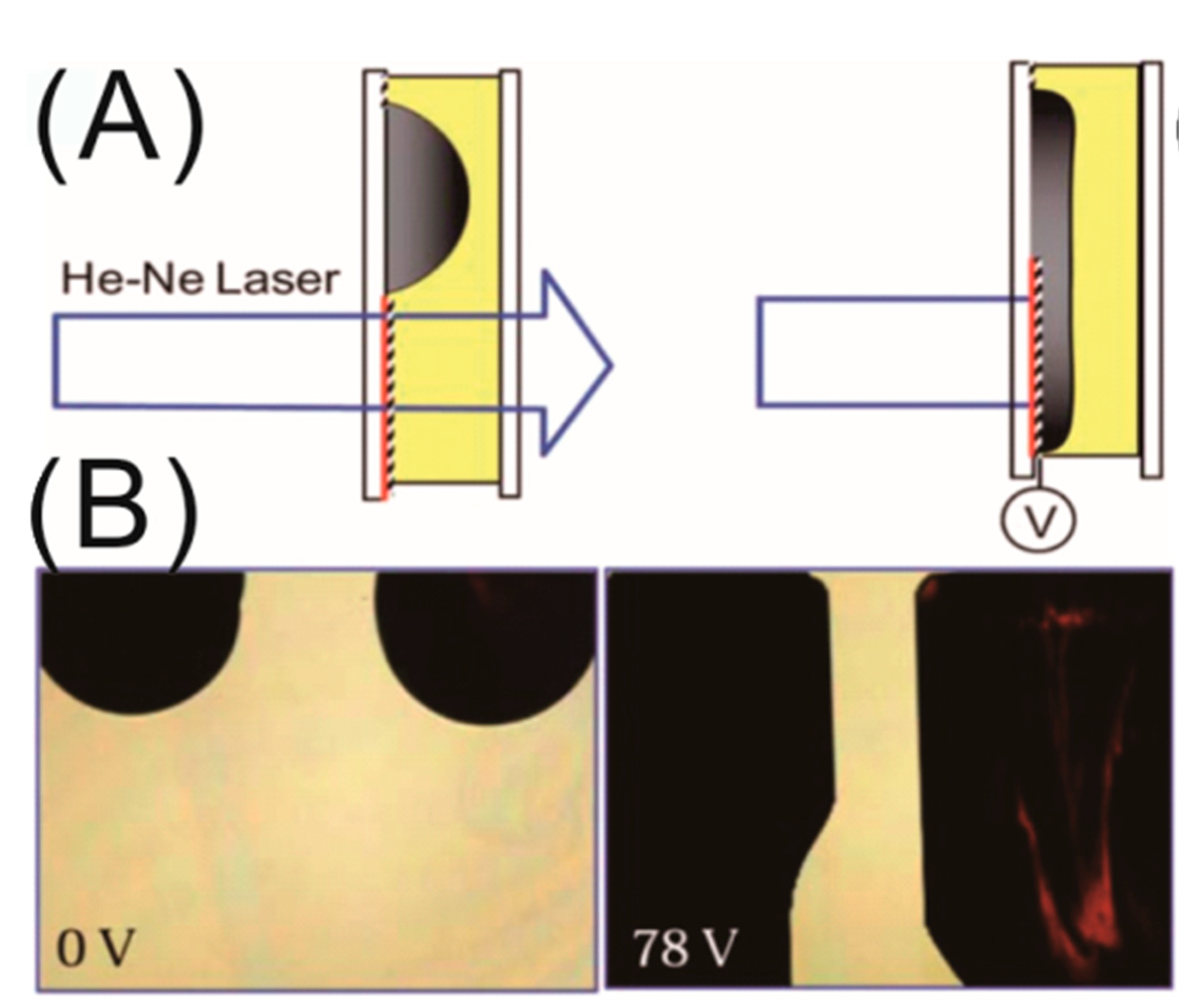
3. Interface-Localized Liquid Dielectrophoresis
3.1. Dielectrowetting
3.2. Wetting Manipulation in Two-Phase Liquids
3.3. Anisotropic Spreading
4. Recent Applications
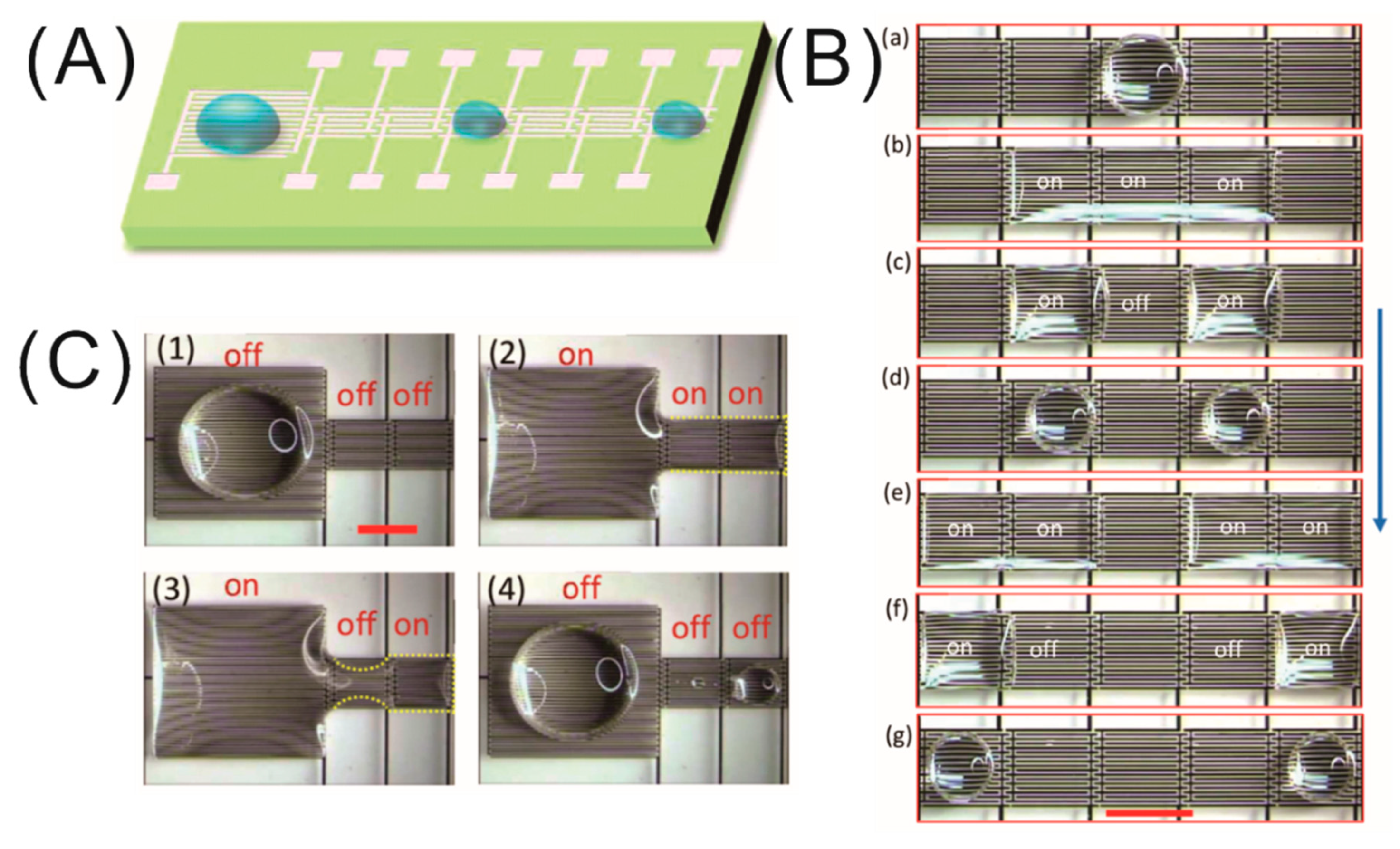
4.1. Digital Microfluidics Applications
4.2. Dielectrowetting-Based Liquid Lens
4.3. Dielectric Switch and Display Applications
4.4. Miscellaneous Applications
5. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Bonn, D.; Eggers, J.; Indekeu, J.; Meunier, J.; Rolley, E. Wetting and spreading. Rev. Mod. Phys. 2009, 81, 739–805. [Google Scholar] [CrossRef]
- Gao, L.; McCarthy, T.J. Contact Angle Hysteresis Explained. Langmuir 2006, 22, 6234–6237. [Google Scholar] [CrossRef]
- Guo, F.; Guo, Z. Inspired smart materials with external stimuli responsive wettability: A review. RSC Adv. 2016, 6, 36623–36641. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, K.; Xin, J.H. Stimuli-Responsive Bioinspired Materials for Controllable Liquid Manipulation: Principles, Fabrication, and Applications. Adv. Funct. Mater. 2018, 28, 1705128. [Google Scholar] [CrossRef]
- Zeng, J.; Korsmeyer, T. Principles of droplet electrohydrodynamics for lab-on-a-chip. Lab Chip 2004, 4, 265–277. [Google Scholar] [CrossRef]
- Byun, S.H.; Yuan, J.; Yoon, M.G.; Cho, S.K. Wirelessly powered electrowetting-on-dielectric (EWOD) by planar receiver coils. J. Micromech. Microeng. 2015, 25, 035019. [Google Scholar] [CrossRef]
- Zhao, Y.-P.; Wang, Y. Fundamentals and Applications of Electrowetting. Rev. Adhes. Adhes. 2013, 1, 114–174. [Google Scholar] [CrossRef]
- Pollack, M.G.; Fair, R.B.; Shenderov, A.D. Electrowetting-based actuation of liquid droplets for microfluidic applications. Appl. Phys. Lett. 2000, 77, 1725–1726. [Google Scholar] [CrossRef]
- Lee, J.; Moon, H.; Fowler, J.; Schoellhammer, T.; Kim, C.-J. Electrowetting and electrowetting-on-dielectric for microscale liquid handling. Sens. Actuators A 2002, 95, 259–268. [Google Scholar] [CrossRef]
- Hayes, R.A.; Feenstra, B.J. Video-speed electronic paper based on electrowetting. Nature 2003, 425, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Barman, J.; Swain, D.; Law, B.M.; Seemann, R.; Herminghaus, S.; Khare, K. Electrowetting Actuated Microfluidic Transport in Surface Grooves with Triangular Cross Section. Langmuir 2015, 31, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Banpurkar, A.G.; Nichols, K.P.; Mugele, F. Electrowetting-Based Microdrop Tensiometer. Langmuir 2008, 24, 10549–10551. [Google Scholar] [CrossRef]
- Zhao, Y.; Cho, S.K. Micro air bubble manipulation by electrowetting on dielectric (EWOD): Transporting, splitting, merging and eliminating of bubbles. Lab Chip 2007, 7, 273–280. [Google Scholar] [CrossRef]
- Pellat, H. Mesure de la force agissant sur les die´lectriques liquides non e´lectrise´s place´s dans un champ e´litrique. C. R. Acad. Sci. Paris 1895, 119, 691–694. [Google Scholar]
- Jones, T.B.; Perry, M.P.; Melcher, J.R. Dielectric Siphons. Science 1971, 174, 1232. [Google Scholar] [CrossRef]
- Jones, T.B.; Melcher, J.R. Dynamics of electromechanical flow structures. Phys. Fluids 1973, 16, 393–400. [Google Scholar] [CrossRef]
- Jones, T.B.; Gunji, M.; Washizu, M.; Feldman, M.J. Dielectrophoretic liquid actuation and nanodroplet formation. J. Appl. Phys. 2000, 89, 1441–1448. [Google Scholar] [CrossRef]
- Ahmed, R.; Jones, T.B. Dispensing picoliter droplets on substrates using dielectrophoresis. J. Electrostat. 2006, 64, 543–549. [Google Scholar] [CrossRef]
- Kanagasabapathi, T.T.; Kaler, K.V.I.S. Surface microfluidics—high-speed DEP liquid actuation on planar substrates and critical factors in reliable actuation. J. Micromech. Microeng. 2007, 17, 743–752. [Google Scholar] [CrossRef]
- Chugh, D.; Kaler, K.V.I.S. Leveraging liquid dielectrophoresis for microfluidic applications. Biomed. Mater. 2008, 3, 034009. [Google Scholar] [CrossRef] [PubMed]
- McHale, G.; Brown, C.V.; Newton, M.I.; Wells, G.G.; Sampara, N. Dielectrowetting Driven Spreading of Droplets. Phys. Rev. Lett. 2011, 107, 186101. [Google Scholar] [CrossRef]
- McHale, G.; Brown, C.V.; Newton, M.I.; Wells, G.G.; Sampara, N. Developing interface localized liquid dielectrophoresis for optical applications. SPIE 2012, 8557, 855703. [Google Scholar] [CrossRef]
- McHale, G.; Brown, C.V.; Sampara, N. Voltage-induced spreading and superspreading of liquids. Nat. Commun. 2013, 4, 1605. [Google Scholar] [CrossRef]
- Brown, C.V.; McHale, G.; Trabi, C.L. Dielectrophoresis-Driven Spreading of Immersed Liquid Droplets. Langmuir 2015, 31, 1011–1016. [Google Scholar] [CrossRef]
- Edwards, A.M.J.; Brown, C.V.; Newton, M.I.; McHale, G. Dielectrowetting: The past, present and future. Curr. Opin. Colloid Interface Sci. 2018, 36, 28–36. [Google Scholar] [CrossRef]
- Xu, S.; Ren, H.; Wu, S.-T. Dielectrophoretically tunable optofluidic devices. J. Phys. D Appl. Phys. 2013, 46, 483001. [Google Scholar] [CrossRef]
- Jones, T.B. Hydrostatics and steady dynamics of spatially varying electromechanical flow structures. J. Appl. Phys. 1974, 45, 1487–1491. [Google Scholar] [CrossRef]
- Jones, T.B. On the Relationship of Dielectrophoresis and Electrowetting. Langmuir 2002, 18, 4437–4443. [Google Scholar] [CrossRef]
- Jones, T.B.; Fowler, J.D.; Chang, Y.S.; Kim, C.-J. Frequency-Based Relationship of Electrowetting and Dielectrophoretic Liquid Microactuation. Langmuir 2003, 19, 7646–7651. [Google Scholar] [CrossRef]
- Jones, T.B.; Wang, K.L.; Yao, D.J. Frequency-Dependent Electromechanics of Aqueous Liquids: Electrowetting and Dielectrophoresis. Langmuir 2004, 20, 2813–2818. [Google Scholar] [CrossRef]
- Jones, T.B. Liquid dielectrophoresis on the microscale. J. Electrost. 2001, 51, 290–299. [Google Scholar] [CrossRef]
- Prakash, R.; Paul, R.; Kaler, K.V.I.S. Liquid DEP actuation and precision dispensing of variable volume droplets. Lab Chip 2010, 10, 3094–3102. [Google Scholar] [CrossRef]
- Renaudot, R.; Agache, V.; Daunay, B.; Lambert, P.; Kumemura, M.; Fouillet, Y.; Collard, D.; Fujita, H. Optimization of Liquid DiElectroPhoresis (LDEP) Digital Microfluidic Transduction for Biomedical Applications. Micromachines 2011, 2, 258–273. [Google Scholar] [CrossRef]
- Renaudot, R.; Daunay, B.; Kumemura, M.; Agache, V.; Jalabert, L.; Collard, D.; Fujita, H. Optimized micro devices for liquid-dielectrophoresis (LDEP) actuation of conductive solutions. Sens. Actuators B 2013, 177, 620–626. [Google Scholar] [CrossRef]
- Fan, S.-K.; Hsieh, T.-H.; Lin, D.-Y. General digital microfluidic platform manipulating dielectric and conductive droplets by dielectrophoresis and electrowetting. Lab Chip 2009, 9, 1236–1242. [Google Scholar] [CrossRef]
- Kaler, K.V.I.S.; Prakash, R.; Chugh, D. Liquid dielectrophoresis and surface microfluidics. Biomicrofluidics 2010, 4, 022805. [Google Scholar] [CrossRef]
- Brown, C.V.; Wells, G.G.; Newton, M.I.; McHale, G. Voltage-programmable liquid optical interface. Nat. Photonics 2009, 3, 403. [Google Scholar] [CrossRef]
- Brown, C.V.; Al-Shabib, W.; Wells, G.G.; McHale, G.; Newton, M.I. Amplitude scaling of a static wrinkle at an oil-air interface created by dielectrophoresis forces. Appl. Phys. Lett. 2010, 97, 242904. [Google Scholar] [CrossRef]
- Brabcova, Z.; McHale, G.; Wells, G.G.; Brown, C.V.; Newton, M.I. Electric field induced reversible spreading of droplets into films on lubricant impregnated surfaces. Appl. Phys. Lett. 2017, 110, 121603. [Google Scholar] [CrossRef]
- Yang, C.-C.; Yang, L.; Gary Tsai, C.; Hongchang Jou, P.; Andrew Yeh, J. Fully developed contact angle change of a droplet in liquid actuated by dielectric force. Appl. Phys. Lett. 2012, 101, 182903. [Google Scholar] [CrossRef]
- Geng, H.; Feng, J.; Stabryla, L.M.; Cho, S.K. Dielectrowetting manipulation for digital microfluidics: Creating, transporting, splitting, and merging of droplets. Lab Chip 2017, 17, 1060–1068. [Google Scholar] [CrossRef]
- Mannetje, D.; Ghosh, S.; Lagraauw, R.; Otten, S.; Pit, A.; Berendsen, C.; Zeegers, J.; van den Ende, D.; Mugele, F. Trapping of drops by wetting defects. Nat. Commun. 2014, 5, 3559. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Alex Chang, C.; Andrew Yeh, J. Variable focus dielectric liquid droplet lens. Opt. Express 2006, 14, 4101–4106. [Google Scholar] [CrossRef]
- Russell, A.C.; Hsieh, W.L.; Chen, K.C.; Heikenfeld, J. Experimental and Numerical Insights into Isotropic Spreading and Deterministic Dewetting of Dielectrowetted Films. Langmuir 2015, 31, 637–642. [Google Scholar] [CrossRef]
- Russell, A.; Kreit, E.; Heikenfeld, J. Scaling Dielectrowetting Optical Shutters to Higher Resolution: Microfluidic and Optical Implications. Langmuir 2014, 30, 5357–5362. [Google Scholar] [CrossRef]
- Brabcova, Z.; McHale, G.; Wells, G.G.; Brown, C.V.; Newton, M.I.; Edwards, A.M.J. Near Axisymmetric Partial Wetting Using Interface-Localized Liquid Dielectrophoresis. Langmuir 2016, 32, 10844–10850. [Google Scholar] [CrossRef]
- Xu, M.; Wang, X.; Ren, H. Tunable Focus Liquid Lens with Radial-Patterned Electrode. Micromachines 2015, 6, 1157–1165. [Google Scholar] [CrossRef]
- Srinivasan, V.; Pamula, V.K.; Fair, R.B. An integrated digital microfluidic lab-on-a-chip for clinical diagnostics on human physiological fluids. Lab Chip 2004, 4, 310–315. [Google Scholar] [CrossRef]
- Dittrich, P.S.; Manz, A. Lab-on-a-chip: Microfluidics in drug discovery. Nat. Rev. Drug Discovery 2006, 5, 210. [Google Scholar] [CrossRef]
- Abgrall, P.; Gué, A.M. Lab-on-chip technologies: Making a microfluidic network and coupling it into a complete microsystem—A review. J. Micromech. Microeng. 2007, 17, R15–R49. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Samiei, E.; Tabrizian, M.; Hoorfar, M. A review of digital microfluidics as portable platforms for lab-on a-chip applications. Lab Chip 2016, 16, 2376–2396. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Hejazian, M.; Ooi, C.H.; Kashaninejad, N. Recent Advances and Future Perspectives on Microfluidic Liquid Handling. Micromachines 2017, 8, 186. [Google Scholar] [CrossRef]
- Geng, H.; Cho, S.K. Dielectrowetting for Digital Microfluidics: Principle and Application. A Critical Review. Rev. Adhes. Adhes. 2017, 5, 268–302. [Google Scholar] [CrossRef]
- Sato, S. Liquid-Crystal Lens-Cells with Variable Focal Length. Jpn. J. Appl. Phys. 1979, 18, 1679–1684. [Google Scholar] [CrossRef]
- Ji, H.-S.; Kim, J.-H.; Kumar, S. Electrically controllable microlens array fabricated by anisotropic phase separation from liquid-crystal and polymer composite materials. Opt. Lett. 2003, 28, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.; Na, J.-H.; Lee, B.; Lee, S.-D. Liquid crystal-based square lens array with tunable focal length. Opt. Express 2014, 22, 3316–3324. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ye, M.; Sato, S. Experimental and Numerical Studies on Liquid Crystal Lens with Spherical Electrode. Mol. Cryst. Liq. Cryst. 2005, 433, 217–227. [Google Scholar] [CrossRef]
- Galstian, T.; Asatryan, K.; Presniakov, V.; Zohrabyan, A.; Tork, A.; Bagramyan, A.; Careau, S.; Thiboutot, M.; Cotovanu, M. High optical quality electrically variable liquid crystal lens using an additional floating electrode. Opt. Lett. 2016, 41, 3265–3268. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Wang, Y.-J.; Reshetnyak, V. Liquid crystal lenses with tunable focal length. Liq. Cryst. Rev. 2017, 5, 111–143. [Google Scholar] [CrossRef]
- Chiu, C.-P.; Chiang, T.-J.; Chen, J.-K.; Chang, F.-C.; Ko, F.-H.; Chu, C.-W.; Kuo, S.-W.; Fan, S.-K. Liquid Lenses and Driving Mechanisms: A Review. J. Adhes. Sci. Technol. 2012, 26, 1773–1788. [Google Scholar] [CrossRef]
- Cheng, C.-C.; Andrew Yeh, J. Dielectrically actuated liquid lens. Opt. Express 2007, 15, 7140–7145. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Xu, D.; Ren, H.; Yoo, I.-S.; Wang, Q.-H. An adaptive liquid lens with radial interdigitated electrode. J. Opt. 2014, 16, 105601. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, H.; Xu, S.; Wu, S.-T. Temperature effects on dielectric liquid lenses. Opt. Express 2014, 22, 1930–1939. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-S.; Tu, H.; Xu, Y.; Jiang, H. Tunable dielectric liquid lens on flexible substrate. Appl. Phys. Lett. 2013, 103, 261113. [Google Scholar] [CrossRef]
- Ren, H.; Xu, S.; Wu, S.-T. Voltage-expandable liquid crystal surface. Lab Chip 2011, 11, 3426–3430. [Google Scholar] [CrossRef]
- Xu, S.; Ren, H.; Liu, Y.; Wu, S. Color Displays Based on Voltage-Stretchable Liquid Crystal Droplet. J. Disp. Technol. 2012, 8, 336–340. [Google Scholar] [CrossRef]
- Zhao, R.; Cumby, B.; Russell, A.; Heikenfeld, J. Large area and low power dielectrowetting optical shutter with local deterministic fluid film breakup. Appl. Phys. Lett. 2013, 103, 223510. [Google Scholar] [CrossRef]
- Luo, Z.; Xu, S.; Gao, Y.; Lee, Y.; Liu, Y.; Wu, S. Quantum Dots Enhanced Liquid Displays. J. Disp. Technol. 2014, 10, 987–990. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, G.; Ren, H. Large-Area Optical Switch Using Surface-Expandable Liquid Droplets. J. Disp. Technol. 2016, 12, 1565–1569. [Google Scholar] [CrossRef]
- Tsai, C.G.; Yeh, J.A. Circular dielectric liquid iris. Opt. Lett. 2010, 35, 2484–2486. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Ren, H.; Lin, Y.-H. Electrically actuated liquid iris. Opt. Lett. 2015, 40, 831–834. [Google Scholar] [CrossRef]
- Wells, G.G.; Sampara, N.; Kriezis, E.E.; Fyson, J.; Brown, C.V. Diffraction grating with suppressed zero order fabricated using dielectric forces. Opt. Lett. 2011, 36, 4404–4406. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-C.; Tsai, Y.-C.; Shih, W.-P. Flexible PDMS micro-lens array with programmable focus gradient fabricated by dielectrophoresis force. Microelectron. Eng. 2011, 88, 2748–2750. [Google Scholar] [CrossRef]
- Renaudot, R.; Fouillet, Y.; Jalabert, L.; Kumemura, M.; Collard, D.; Fujita, H.; Agache, V. Programmable LDEP technology to fabricate versatile master molds for PDMS continuous-flow microfluidic applications. Microfluid. Nanofluid. 2014, 16, 701–710. [Google Scholar] [CrossRef]
- Edwards, A.M.J.; Ledesma-Aguilar, R.; Newton, M.I.; Brown, C.V.; McHale, G. Not spreading in reverse: The dewetting of a liquid film into a single drop. Sci. Adv. 2016, 2, e1600183. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Feng, J.; Cho, S.K. Cheerios Effect Controlled by Electrowetting. Langmuir 2015, 31, 8502–8511. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Cho, S.K. Active control of Cheerios effect for dielectric fluid. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 496–499. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barman, J.; Shao, W.; Tang, B.; Yuan, D.; Groenewold, J.; Zhou, G. Wettability Manipulation by Interface-Localized Liquid Dielectrophoresis: Fundamentals and Applications. Micromachines 2019, 10, 329. https://doi.org/10.3390/mi10050329
Barman J, Shao W, Tang B, Yuan D, Groenewold J, Zhou G. Wettability Manipulation by Interface-Localized Liquid Dielectrophoresis: Fundamentals and Applications. Micromachines. 2019; 10(5):329. https://doi.org/10.3390/mi10050329
Chicago/Turabian StyleBarman, Jitesh, Wan Shao, Biao Tang, Dong Yuan, Jan Groenewold, and Guofu Zhou. 2019. "Wettability Manipulation by Interface-Localized Liquid Dielectrophoresis: Fundamentals and Applications" Micromachines 10, no. 5: 329. https://doi.org/10.3390/mi10050329
APA StyleBarman, J., Shao, W., Tang, B., Yuan, D., Groenewold, J., & Zhou, G. (2019). Wettability Manipulation by Interface-Localized Liquid Dielectrophoresis: Fundamentals and Applications. Micromachines, 10(5), 329. https://doi.org/10.3390/mi10050329





