Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array
Abstract
1. Introduction
1.1. The Studies of Stresses
1.2. Cellular Stress Responses
2. Experiments
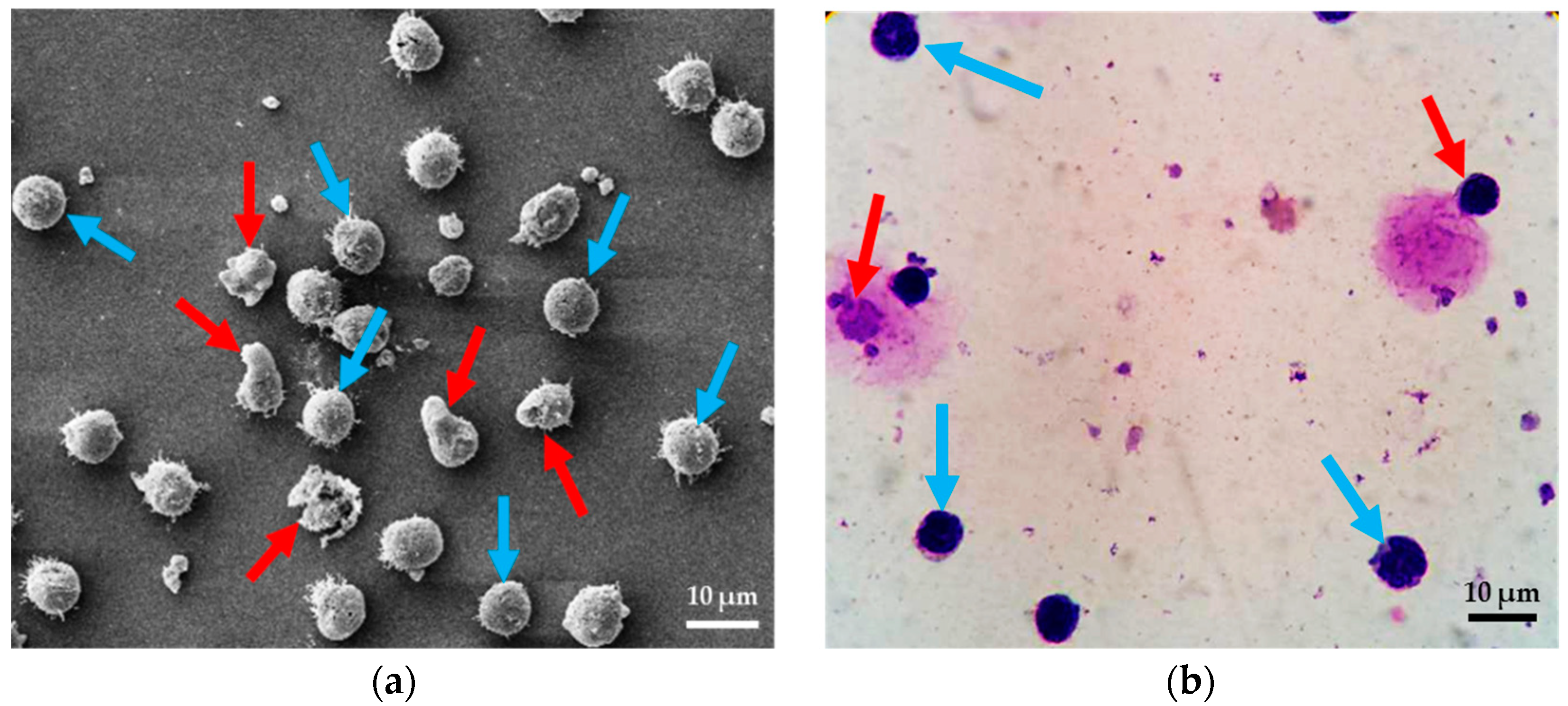
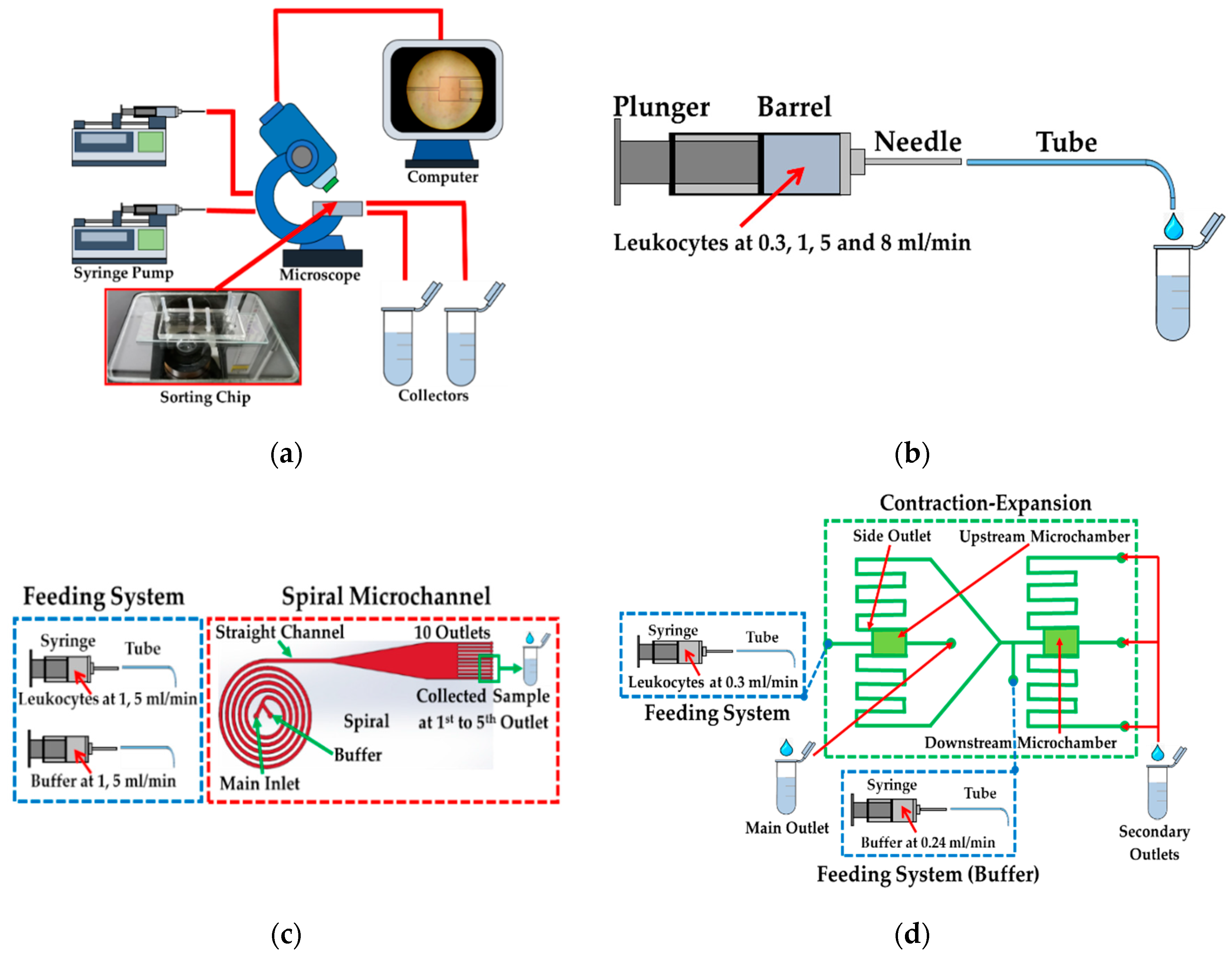
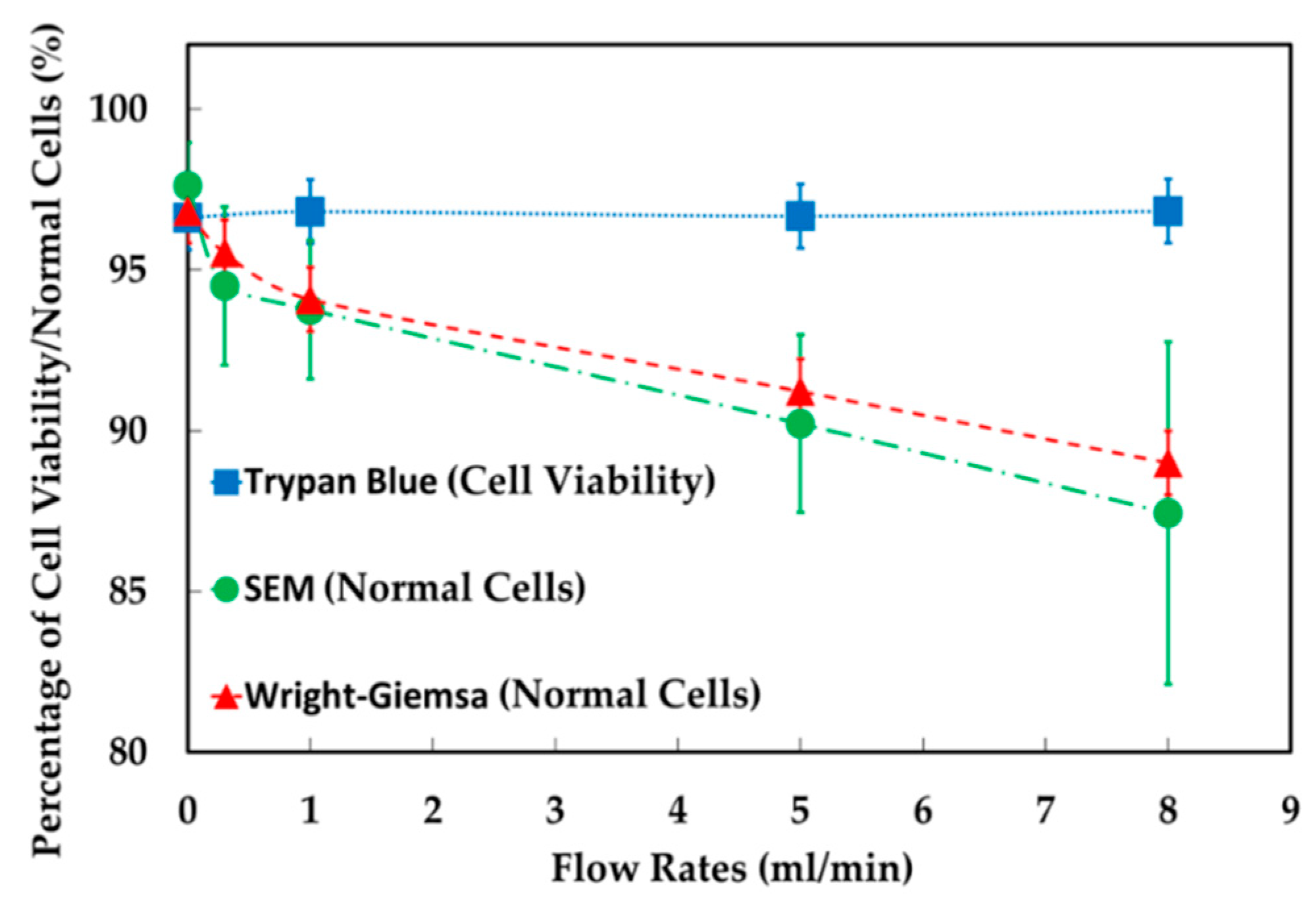
2.1. Test Methods
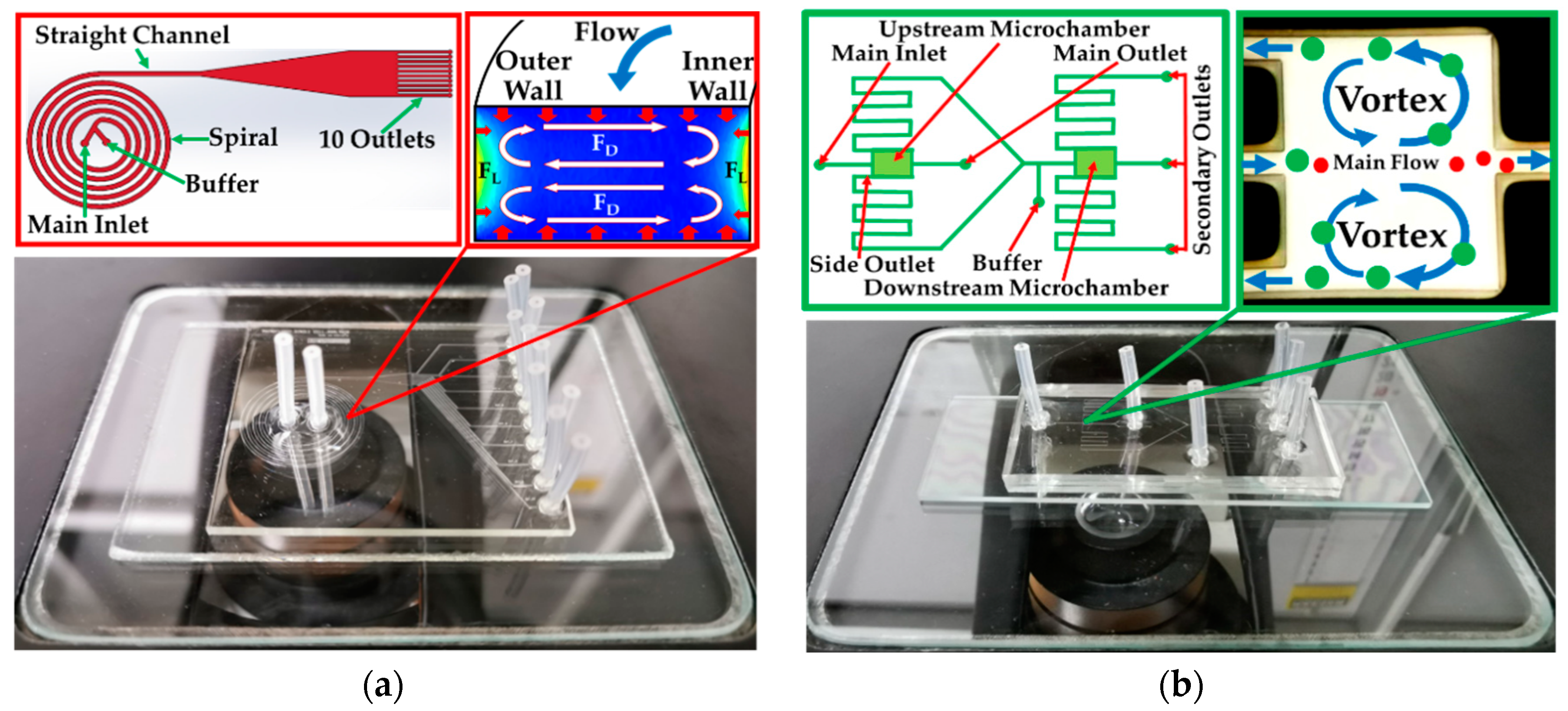
2.2. System Fabrication
2.3. Feeding System and Test Conditions
2.4. Spiral/Contraction-Expansion Array (CEA) Systems and Test Conditions
3. Simulation Study
3.1. Geometry and Boundary Conditions of the Feeding System
3.2. Geometry and Boundary Conditions of Spiral Microchannel
3.3. Geometry and Boundary Conditions of the Straight Channel and Outlets
3.4. Geometry and Boundary Conditions of CEA
4. Results
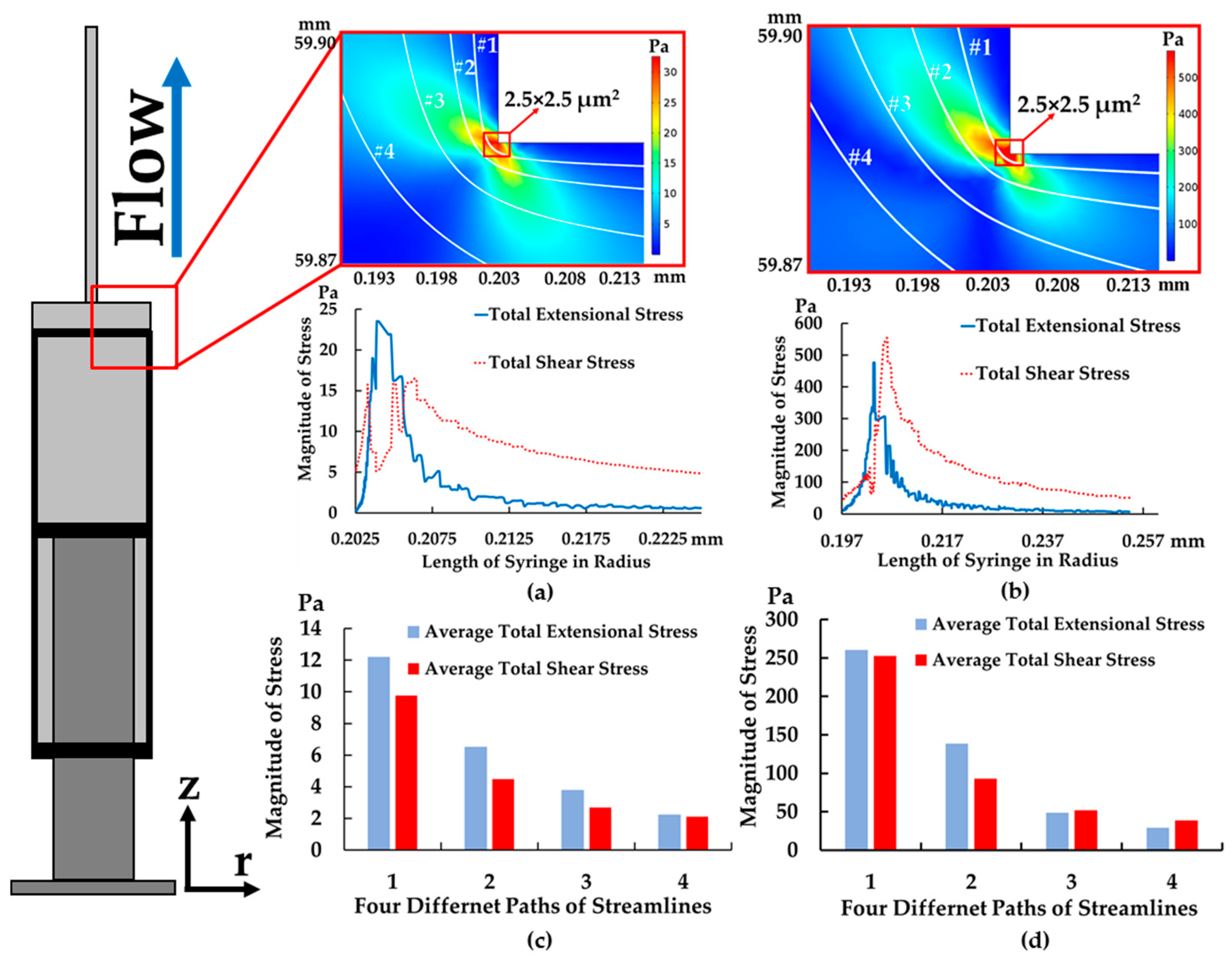
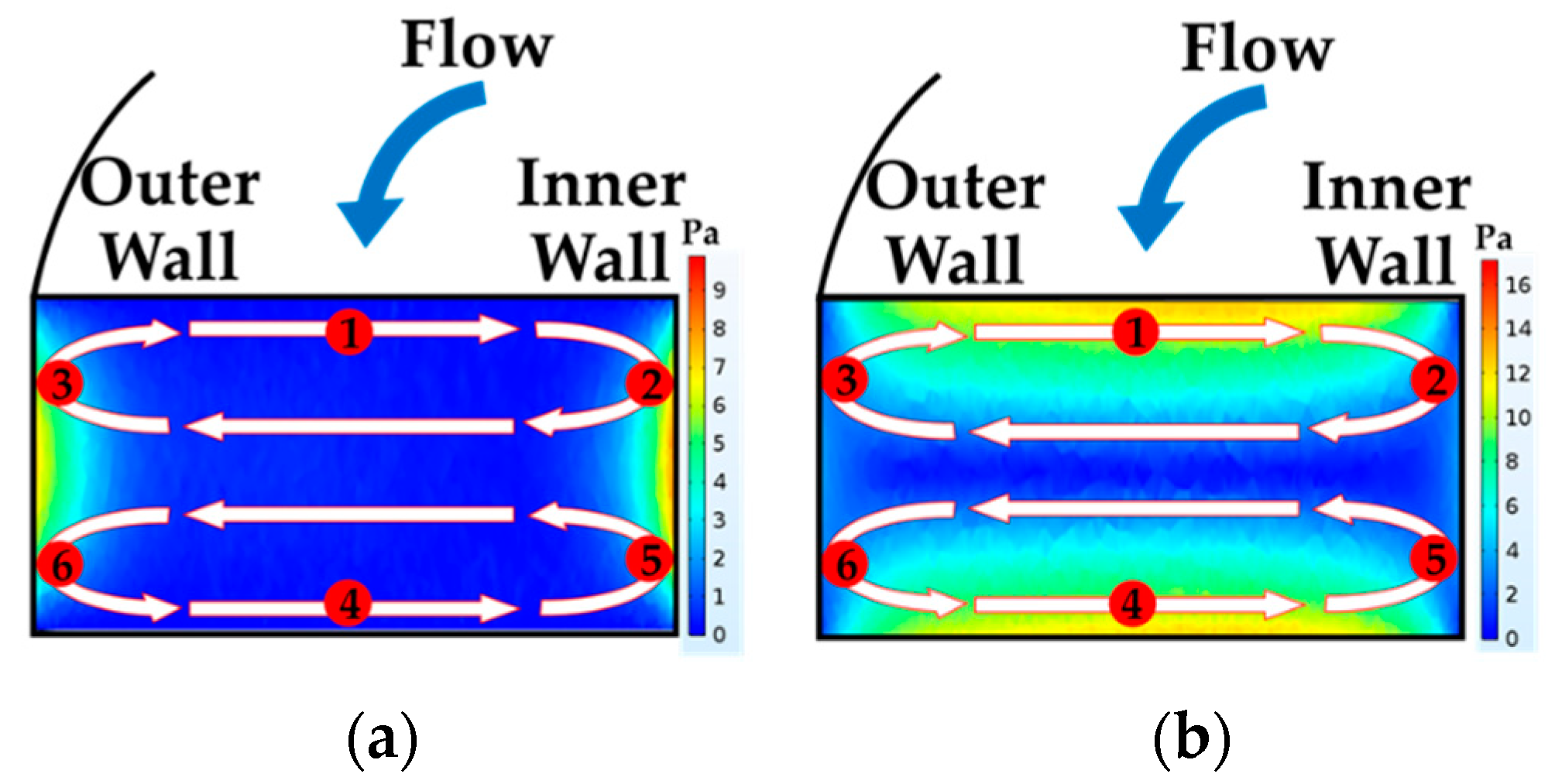
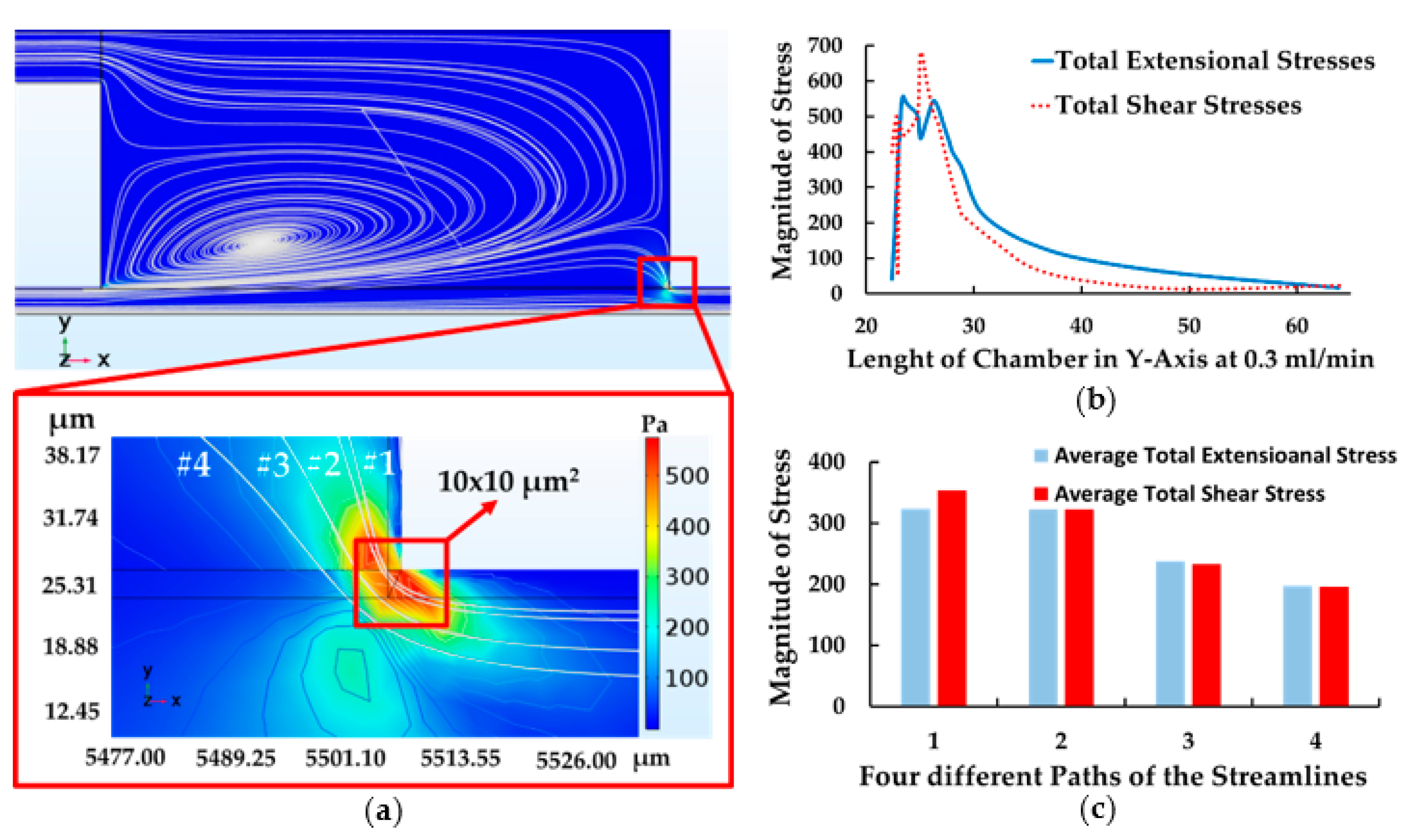
4.1. The Results of Feeding System
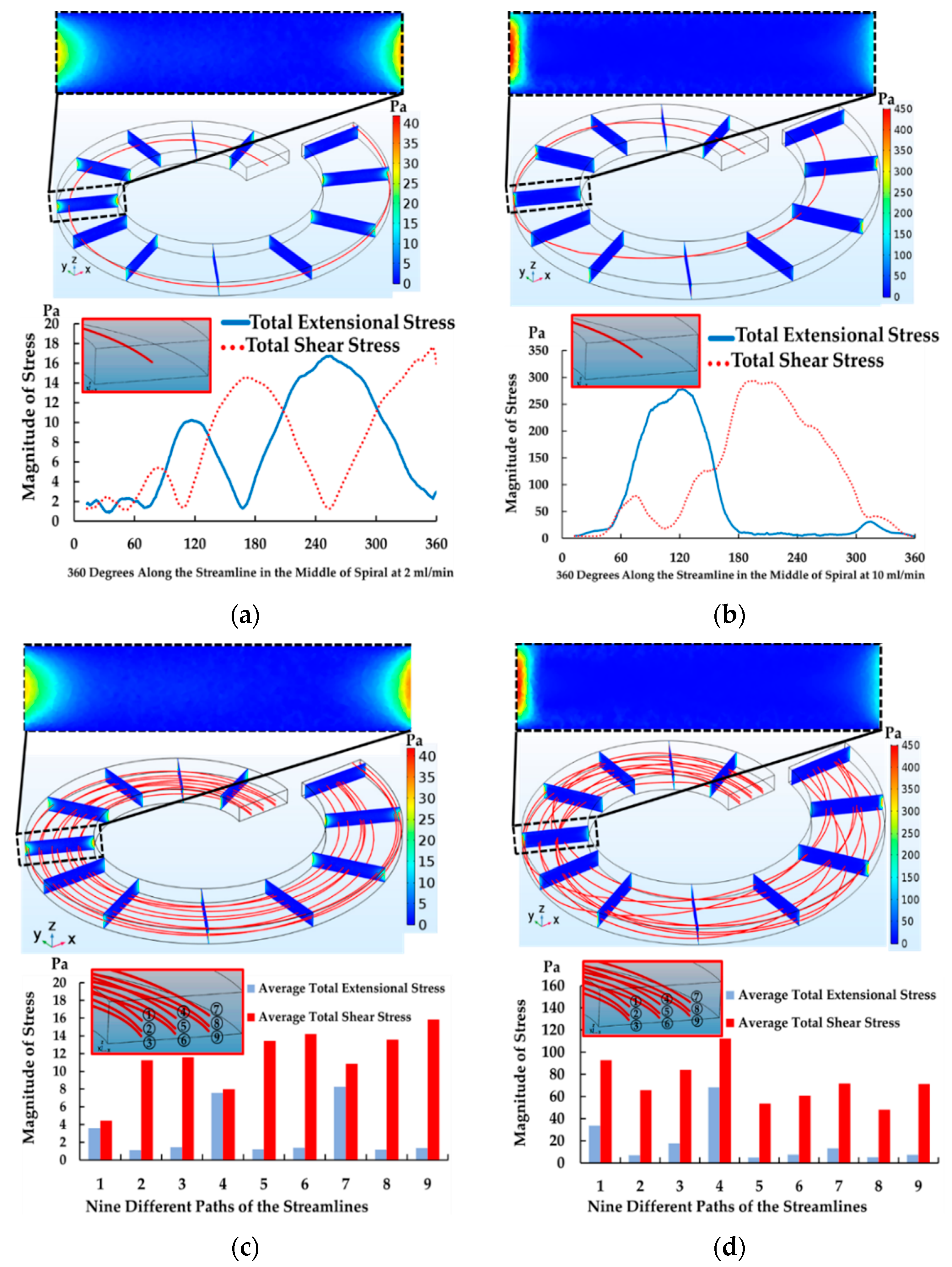
4.2. The Results of Spiral Microchannel
4.3. The Results of Contraction-Expansion Array
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Luo, T.; Fan, L.; Zhu, R.; Sun, D. Microfluidic single-cell manipulation and analysis: Methods and applications. Micromachines 2019, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, J.; Sun, Y. Microfluidic approaches for cancer cell detection, characterization, and separation. Lab Chip 2012, 12, 1753–1767. [Google Scholar] [CrossRef] [PubMed]
- Damiati, S.; Kompella, U.B.; Damiati, S.A.; Kodzius, R. Microfluidic devices for drug delivery systems and drug screening. Genes 2018, 9, 103. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, H.; Tan, Z.; Quan, Y. Microfluidic cell trapping for single-cell analysis. Micromachines 2019, 10, 409. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.Y.; Han, J.I.; Park, J.K. Inertial microfluidics-based cell sorting. BioChip J. 2018, 12, 257–267. [Google Scholar] [CrossRef]
- Warkiani, M.E.; Tay, A.K.P.; Guan, G.; Han, J. Membrane-less microfiltration using inertial microfluidics. Sci. Rep. 2015, 5, 11018. [Google Scholar] [CrossRef] [PubMed]
- Matas, J.P.; Morris, J.F.; Guazzelli, E. Inertial migration of rigid spherical particles in Poiseuille flow. J. Fluid Mech. 2004, 515, 171–195. [Google Scholar] [CrossRef]
- Choi, Y.S.; Seo, K.W.; Lee, S.J. Lateral and cross-lateral focusing of spherical particles in a square microchannel. Lab Chip 2011, 11, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Chung, A.J.; Gossett, D.R.; Carlo, D.D. Three dimensional, sheathless, and high-throughput microparticle inertial focusing through geometry-induced secondary flows. Small 2013, 9, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Carlo, D.D.; Irimia, D.; Tompkins, R.G.; Toner, M. Continuous inertial focusing, ordering, and separation of particles in microchannels. Proc. Nalt. Acad. Sci. USA 2007, 104, 18892–18897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yan, S.; Sluyter, R.; Li, W.; Alici, G.; Nguyen, N.T. Inertial particle separation by differential equilibrium positions in a symmetrical serpentine micro-channel. Sci. Rep. 2014, 4, 4527. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Wu, L.; Bhagat, A.A.S.; Li, Z.; Chen, P.C.Y.; Chao, S.; Ong, C.J.; Han, J. Spiral microchannel with rectangular and trapezoidal cross-sections for size based particle separation. Sci. Rep. 2013, 3, 1475. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, M.; Liu, C.; Zhang, Y.; Liu, D.; Liu, W.; Hu, G.; Jiang, X. Double spiral microchannel for label-free tumor cell separation and enrichment. Lab Chip 2012, 12, 3952–3960. [Google Scholar] [CrossRef] [PubMed]
- Warkiani, M.E.; Khoo, B.L.; Tan, D.S.W.; Bhagat, A.A.S.; Lim, W.T.; Yap, Y.S.; Lee, S.C.; Soo, R.A.; Han, J.; Lim, C.T. An ultra-high-throughput spiral microfluidic biochip for the enrichment of circulating tumor cells. Analyst 2014, 139, 3245. [Google Scholar] [CrossRef] [PubMed]
- Chiu, D.T. Cellular manipulations in microvortices. Anal. Bioanal. Chem. 2007, 387, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Song, S.H.; Jung, H.I. Continuous focusing of microparticles using inertial lift force and vorticity via multi-orifice microfluidic channels. Lab Chip 2009, 9, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.S.; Kuntaegowdanahalli, S.S.; Papautsky, I. Continuous particle separation in spiral microchannels using dean flows and differential migration. Lab Chip 2008, 8, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Samuel, R.; Gale, B.K.; Carrell, D.T.; Hotaling, J.M. Separation of sperm cells from samples containing high concentrations of white blood cells using a spiral channel. Biomicrofluidics 2017, 11, 054106. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Papautsky, I. Size-based microfluidic multimodal microparticle sorter. Lab Chip 2015, 15, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.C.; Mach, A.J.; Carlo, D.D. High-throughput size-based rare cell enrichment using microscale vortices. Biomicrofluidics 2011, 5, 022206. [Google Scholar] [CrossRef] [PubMed]
- Nevaril, C.G.; Lynch, E.C.; Alfrey, C.P.; Hellums, J.D. Erythrocyte damage and destruction induced by shearing stress. J. Lab. Clin. Med. 1968, 71, 784–790. [Google Scholar] [PubMed]
- Leverett, L.B.; Hellums, J.D.; Alfrey, C.P.; Lynch, E.C. Red blood cell damage by shear stress. Biophys. J. 1972, 12, 257–273. [Google Scholar] [CrossRef]
- Sutera, S.P.; Mehrjardi, M.H. Deformation and fragmentation of human red blood cells in turbulent shear flow. Biophys. J. 1975, 15, 1–10. [Google Scholar] [CrossRef]
- Greenfield, P.F.; Randerson, D.H. A technique for determining the shear sensitivity of maganlian cells in suspension culture. Biotechnol. Tech. 1987, 1, 39–44. [Google Scholar]
- Petersen, J.F.; Mclntire, L.V.; Papoutsakis, E.T. Shear sensitivity of cultured hybridoma cells (CRL-8018) depends on mode of growth, culture age and metabolite concentration. J. Biotechnol. 1988, 7, 229–246. [Google Scholar] [CrossRef]
- Schürch, U.; Kramer, H.; Einsele, A.; Widmer, F.; Eppenberger, H.M. Experimental evaluation of laminar shear stress on the behaviour of hybridoma mass cell cultures, producing monoclonal antibodies against mitochondrial creatine kinase. J. Biotechnol. 1988, 7, 179–184. [Google Scholar] [CrossRef]
- Abu-Reesh, I.; Kargi, F. Biological responses of hybridoma cells to defined hydrodynamic shear stress. J. Biotechnol. 1989, 9, 167–178. [Google Scholar] [CrossRef]
- Tanzeglock, T.; Soos, M.; Stephanopoulos, G.; Morbidelli, M. Induction of mammalian cell death by simple shear and extensional flows. Biotechnol. Bioeng. 2009, 104, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Down, L.A.; Papavassiliou, D.V.; O’rear, E.A. Significance of extensional stresses to red blood cell lysis in a shearing flow. Ann. Biomed. Eng. 2011, 39, 1632–1642. [Google Scholar] [CrossRef] [PubMed]
- Aguado, B.A.; Mulyasasmita, W.; Su, J.; Lampe, K.J.; Heilshorn, S.C. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng. Part A 2012, 18, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Chen, S.F.; Chern, M.K.; Lu, P.C. The effects of extensional stress on red blood cell hemolysis. Biomed. Eng. 2015, 27, 1550042. [Google Scholar] [CrossRef]
- Bae, Y.B.; Jang, H.K.; Shin, T.H.; Phukan, G.; Tran, T.T.; Lee, G.; Hwang, W.R.; Kim, J.M. Microfluidic assessment of mechanical cell damage by extensional stress. Lab Chip 2016, 16, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Kieran, P.M.; Malone, D.M.; MacLoughlin, P.F. Effects of Hydrodynamic and Interfacial Forces on Plant Cell Suspension Systems. Adv. Biochem. Eng. Biotechnol. 2000, 67, 139–177. [Google Scholar] [PubMed]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell Biol. 2009, 2010, 23. [Google Scholar] [CrossRef] [PubMed]
- Stathopoulos, N.A.; Hellums, J.D. Shear stress effects on human embryonic kidney cells in vitro. Biotechnol. Bioeng. 1985, 27, 1021–1026. [Google Scholar] [CrossRef] [PubMed]
- Kristine, S.L.; Andre, C.S. Cell viability analysis using trypan blue: Manual and automated methods. Methods Mol. Biol. 2011, 740, 7–12. [Google Scholar]
- Ketpun, D.; Sailasuta, A.; Suwannaphan, T.; Bhanpattanakul, S.; Pimpin, A.; Srituravanich, W.; Sripumkhai, W.; Jeamsaksiri, W.; Piyaviriyakul, P. The viability of single cancer cells after exposure to hydrodynamic shear stresses in a spiral microchannel: A canine cutaneous mast cell tumor model. Micromachines 2018, 9, 9. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Sample | Method | Type of Stress | Magnitudes of Cell Damage (Pa) | Magnitudes of Cell Loss (Pa) | Time |
|---|---|---|---|---|---|---|---|
| Nevaril et al. | 1968 | RBCs | Viscometer | Shear | 150–300 (Hemolysis) | — | 2 min |
| Sutera et al. | 1975 | RBCs | Viscometer | Shear | <250 (Deformation) >250 (Fragmentation) | — | 4 min |
| Smith et al. | 1987 | Hybridoma | Viscometer | Shear | — | 0.67 | 15 h |
| Petersen et al. | 1988 | Hybridoma | Viscometer | Shear | — | 5 | 10 min |
| Schürch et al. | 1988 | Hybridoma | Viscometer | Shear | — | <6 | — |
| Abu et al. | 1989 | Hybridoma | Viscometer | Shear | <5 (Respiration Activity) | >5 | 0.5–2 h |
| Tanzeglock et al. | 2009 | HEKs and CHOs | Rheometer and Syringe | Extensional | — | 500 | — |
| Down et al. | 2011 | RBCs | Capillary | Extensional | — | 3000 | Milliseconds |
| Aguado et al. | 2012 | — | Needle | Extensional | — | — | — |
| Yen et al. | 2015 | RBCs | Capillary | Extensional | — | 1000 | 0.06 ms |
| Bae et al. | 2016 | CHOs | Cross-Slot | Extensional | — | 250 | — |
| Component | Flow Rate (mL/min) | Cell Loss (%) | Cell Deformation (%) | Intracellular Damage (%) | Type of Stress | Maximum Stress (Pa) | Time |
|---|---|---|---|---|---|---|---|
| Feeding System | 0.3 | 0 | 3.1 | 1.2 | Extensional | N/A | N/A |
| 1 | 0 | 3.8 | 2.7 | 24 | 0.25 ms | ||
| 5 | 0 | 7.4 | 5.6 | N/A | N/A | ||
| 8 | 0 | 10.2 | 7.8 | 500 | 0.0125 ms | ||
| Spiral | 2 | 10.3 | 29.8 | 2.1 | Shear | 18 | 0.158 s |
| 10 | 7.1 | 28.5 | 8.7 | 300 | 0.032 s | ||
| Contraction-Expansion | 0.3 (Main Inlet) 0.24 (Buffer) | 6.9 (Main Outlet) 7.4 (Secondary Outlet) | 12.2 (Main Outlet) N/A (Secondary Outlet) | 14.2 (Main Outlet) N/A (Secondary Outlet) | Extensional and Shear | 550 (Extensional) 700 (Shear) | 0.0125 ms |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suwannaphan, T.; Srituravanich, W.; Sailasuta, A.; Piyaviriyakul, P.; Bhanpattanakul, S.; Jeamsaksiri, W.; Sripumkhai, W.; Pimpin, A. Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array. Micromachines 2019, 10, 772. https://doi.org/10.3390/mi10110772
Suwannaphan T, Srituravanich W, Sailasuta A, Piyaviriyakul P, Bhanpattanakul S, Jeamsaksiri W, Sripumkhai W, Pimpin A. Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array. Micromachines. 2019; 10(11):772. https://doi.org/10.3390/mi10110772
Chicago/Turabian StyleSuwannaphan, Thammawit, Werayut Srituravanich, Achariya Sailasuta, Prapruddee Piyaviriyakul, Suchaya Bhanpattanakul, Wutthinan Jeamsaksiri, Witsaroot Sripumkhai, and Alongkorn Pimpin. 2019. "Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array" Micromachines 10, no. 11: 772. https://doi.org/10.3390/mi10110772
APA StyleSuwannaphan, T., Srituravanich, W., Sailasuta, A., Piyaviriyakul, P., Bhanpattanakul, S., Jeamsaksiri, W., Sripumkhai, W., & Pimpin, A. (2019). Investigation of Leukocyte Viability and Damage in Spiral Microchannel and Contraction-Expansion Array. Micromachines, 10(11), 772. https://doi.org/10.3390/mi10110772





