1. Introduction
Recently, microfluidic chips have attracted much attention from both academia and industry, which has led to their rapid development in the field of medical diagnostics. Microfluidics involve a volume of fluid in the range of microlitre to picolitre, and demonstrate many advantages such as rapid mass delivery and heat transfer, and diminished use of reagents and generation of waste. Miniaturized biological assays or processes on a chip have emerged as a prospective technology. A droplet-based microfluidic system produces a droplet of volume typically ranging from picolitre [
1,
2] to nanolitre [
3,
4]. There are two main methods to control the direction or motion of a droplet: one uses a pneumatic valve and the other one employs light waves of a specific wavelength to impinge on a droplet in the channel [
5,
6,
7,
8]. The particle-encapsulated droplet can be determined, while droplets absorb the reflected light of a particular spectral wavelength.
Droplet-based microfluidics are commonly conducted with a generating frequency > 10 Hz and a small volume of droplet (picolitre to nanolitre), but have the disadvantages of tedious fabrication, complicated experimental operation, and a requirement of expensive equipment. Embryos are statically cultured from zygote to blastocyst in a 5-μL human tubal fluid (HTF) medium or KSOM medium (Sigma-Aldrich, St. Louis, MO, USA) and covered with culture oil. In reality, the embryos grow dynamically in the oviduct of a female mammal for 5–6 days before implantation. The use of a culture medium as a dispersed phase droplet to encapsulate a mouse embryo flowing in a microchannel for a dynamic culturing condition is thus a highly suitable scheme.
Some methods to sort droplets use a pneumatic valve as a block to control the direction of motion of the droplet [
5,
6]. Some contemporary methods to sort droplets apply light waves of a specific wavelength to strike a droplet in the channel. Whether the droplet encapsulates a particle or not is then determined on absorbing reflected light at a particular spectral wavelength. If a droplet encapsulating a particle arrives, the spectrometer transmits a signal to the voltage amplifier that releases a voltage to the electrode. When it is necessary to inject liquid into a droplet, an electric field must be created by the electrode beside the droplet to invalidate temporarily the effect of the surfactant [
9,
10]. Although dozens of droplets could be sorted and injected in a brief interval, the tedious fabrication, complicated experimental operation, and necessity of many expensive pieces of equipment would be extremely inconvenient.
Many researchers have conducted fundamental studies on droplet microfluidics. Sarvothaman et al. developed a strategy that involved using fluoroalkyl polyethylene glycol copolymers to suppress protein adhesion, which causes a droplet movement to fail [
11]. Seiffert et al. proposed more rapid production of droplets by delayed addition of the surfactant to push droplet-based microfluidics to an industrially relevant scale [
12]. Pirbodaghi et al. developed an accurate approach that entailed using a bright-field microscope with illumination of white light and a standard high-speed camera to study the fluid dynamics of rapid processes within microfluidic devices [
13]. Musterd and coworkers calculated the volume of elongated droplets in microchannels from a top-view image in interpreting experiments on reaction kinetics and transport phenomena [
14].
In this work, we developed a microfluidic chip on a polydimethylsiloxane (PDMS) substrate to upgrade the volume of droplets from picolitre and nanolitre to microlitre for culturing mouse embryos. We also devised a convenient technique of operation to attain an effective and stable way for droplet sorting, liquid injection, and liquid exchange.
2. Materials and Methods
A microfluidic chip was fabricated using the traditional process. The microfluidic chip was designed to test four main functions of channel performed manually using microfluidic system. There are two main types of medium used for the experiment, the human tubal fluid medium (HTF or KSOM) and the cultural oil medium (OVOIL), which are used as dispersed phase liquid and as continuous phase liquid in the experiment. PDMS is used as main material in fabrication of the channel, due to its favorable mechanical properties like optical transparency, high biocompatibility, high transmittance, and ease of fabrication. Teflon was used after the bonding process to provide a hydrophobic surface coating on the chip [
15,
16], because the material has a superior biological property and high biocompatibility, which can provide better environmental conditions for embryo development.
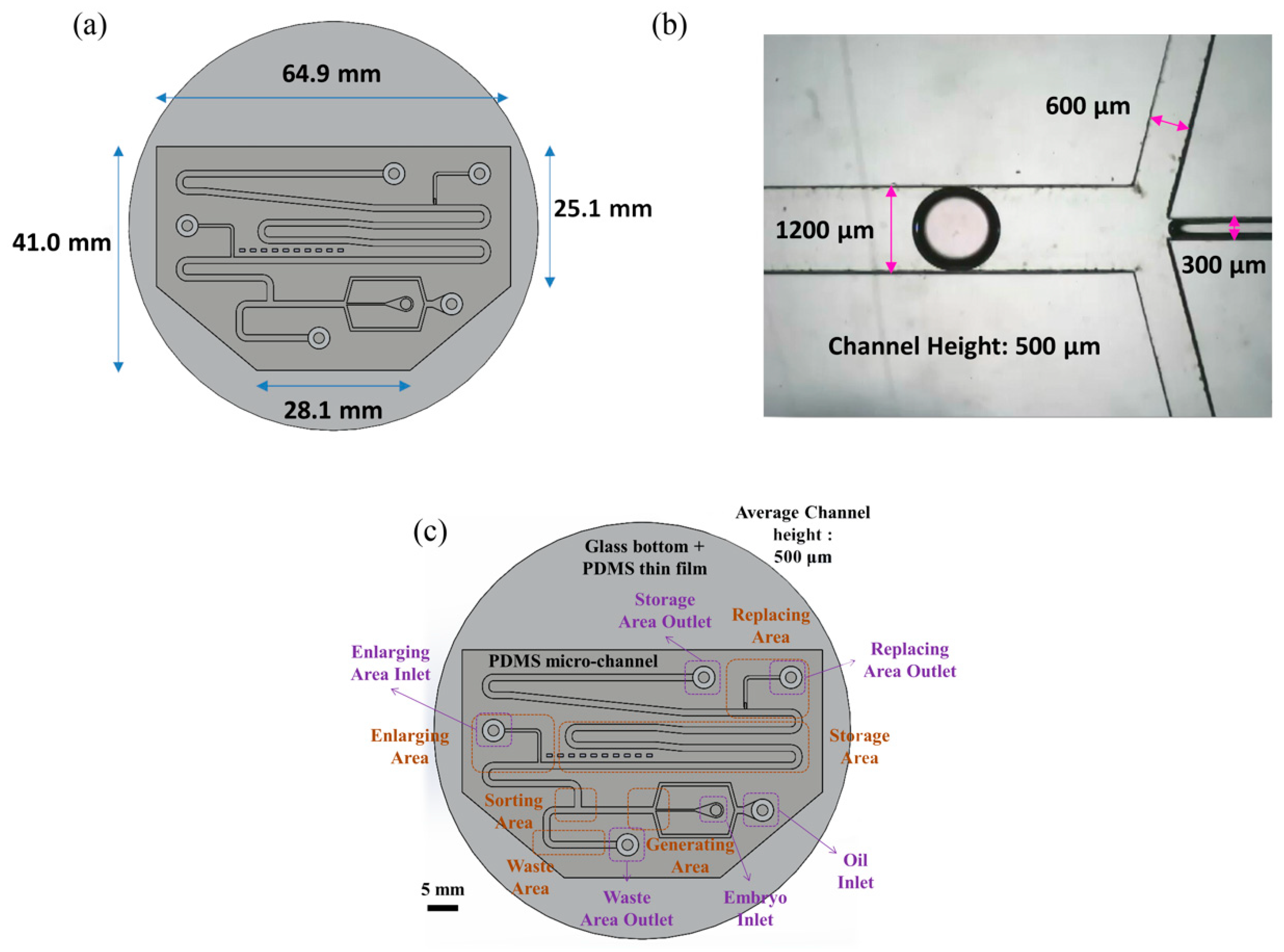
Figure 1 shows an overview image of the chip used in the experiment. As indicated in panel (a), the chip has a hexagonal structure with upper base length 64.9 mm, lower base length 28.1 mm, right-hand side length 25.1 mm, and left-hand side length 41.0 mm. As shown in panel (b), the main channel, continuous phase channel, and dispersed phase channel have widths of 1200, 600 and 300 μm, respectively. The channel height is 500 μm.
The chip includes four main segments—a generating area, a sorting area, an enlargement area and a replacement area, along with a storage area and a waste area (see
Figure 1c). The chip can use different inlets and outlets, and can also provide different flow rates according to the medium. We can apply multiple medium exchanges of droplets while performing the experiment, but in this experiment, the functions of the chip are set/secured. The mouse embryos are injected via pipette in embryo inlet, using microfluidic system droplets created in the generating area and sorted in the sorting area simultaneously. Later, the droplets without embryos were abandoned to the waste area outlet, whereas the droplets with embryos moved on to the enlargement area. The droplets in the enlargement area are then expanded. The medium from the droplet can eventually be absorbed in the replacement area.
The chip consists of the following regions—oil inlet, embryo inlet, waste area outlet, enlarge area inlet, replacement area outlet, and storage area outlet. The culture oil flows through the chip via the oil inlet; the culture medium with particles (mouse embryos or PS beads) flows into the chip through the embryo inlet. The abandoned droplets are discarded through the waste area outlet. The HTF or KSOM medium is injected into the droplet to enlarge its size to 5 μL through the enlargement area. The old media are carried away in the replacement area of the chip.
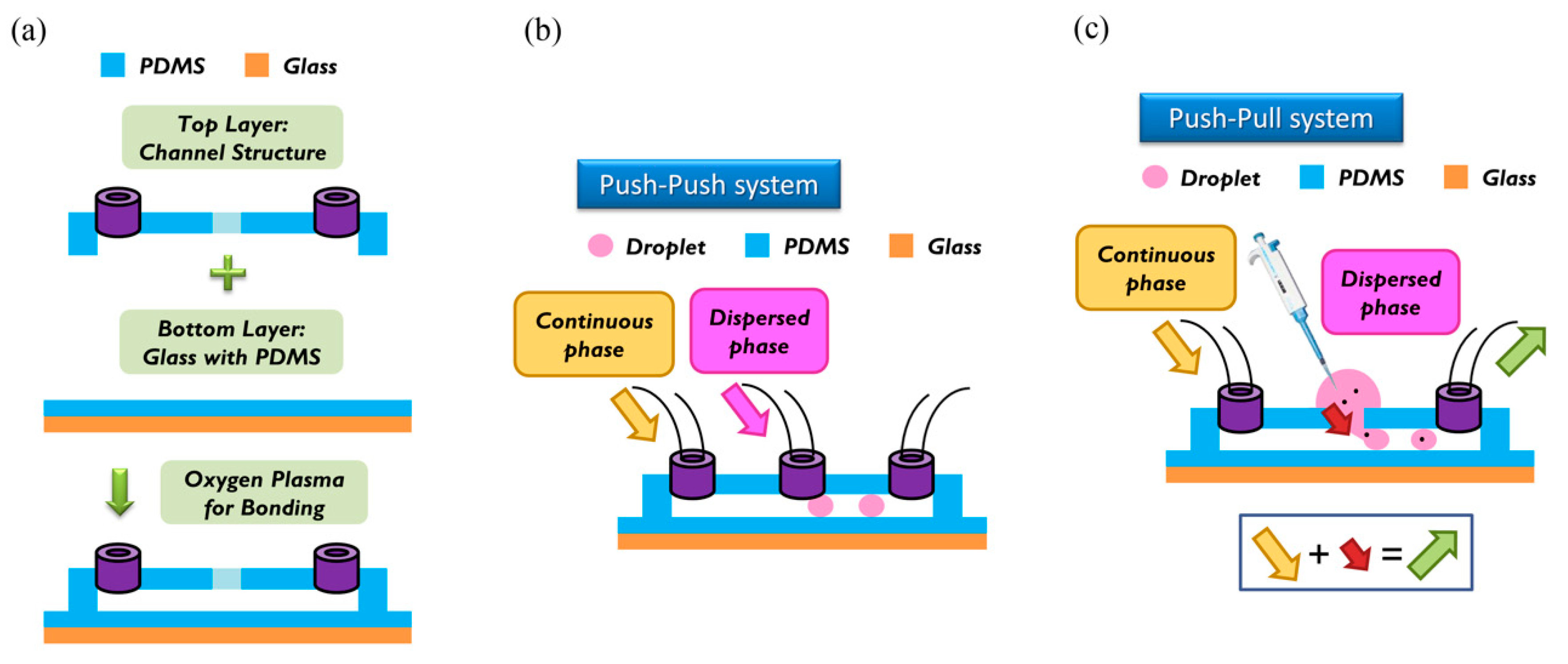
For the fabrication of the wafer, we used a soft lithographic process to fabricate the mold of the channel. Negative photoresist SU-8 (2150) was used on a silicon wafer of thickness less than 300 μm, as shown in the datasheet. We used spin speed of 500 rpm for 40 s. The average thickness of the channel was about 500 μm. During soft baking, the viscosity of the photoresist increased, resulting in a problem of non-uniform thickness of the photoresist. To solve this problem, we rotated the wafer 90° per 3 min during soft baking to ensure uniformity. After spin coating, the ensuing procedure consisted of soft baking, exposure to UV light, and post-exposure baking. The last step involved putting the wafer into the SU-8 developer to wash away the excess photoresist. To prepare the top layer, the tubes were put at the entrance of the mold; then liquid polydimethylsiloxane (PDMS) (A:B = 10:1) was poured on the mold. The liquid PDMS was baked at 85 °C for 30 min until the PDMS became solid. The PDMS was removed from the mold to complete the top layer part of the channel. To enclose the whole channel with PDMS, we coated the glass with PDMS as a bottom layer at spin speed of 2000 rpm for 20 s. The thickness of the PDMS on the glass was about 50 μm [
17]. The top and bottom layers were bonded to each other with oxygen plasma to complete fabrication of the chip. We then baked the chip at 70 °C for 10 min to enhance the bonding force. The detailed procedure of fabrication and bonding is shown in
Figure 2a.
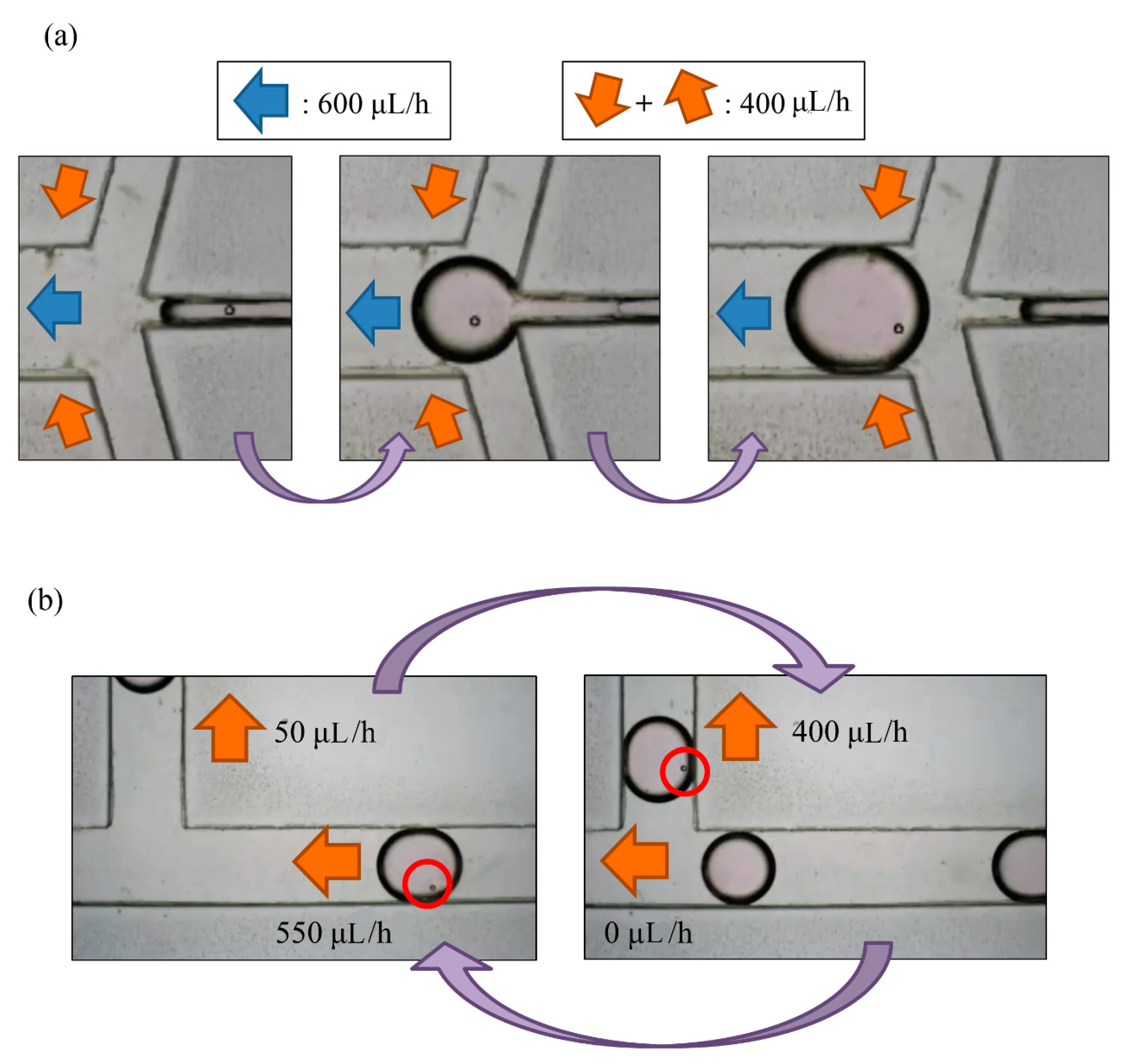
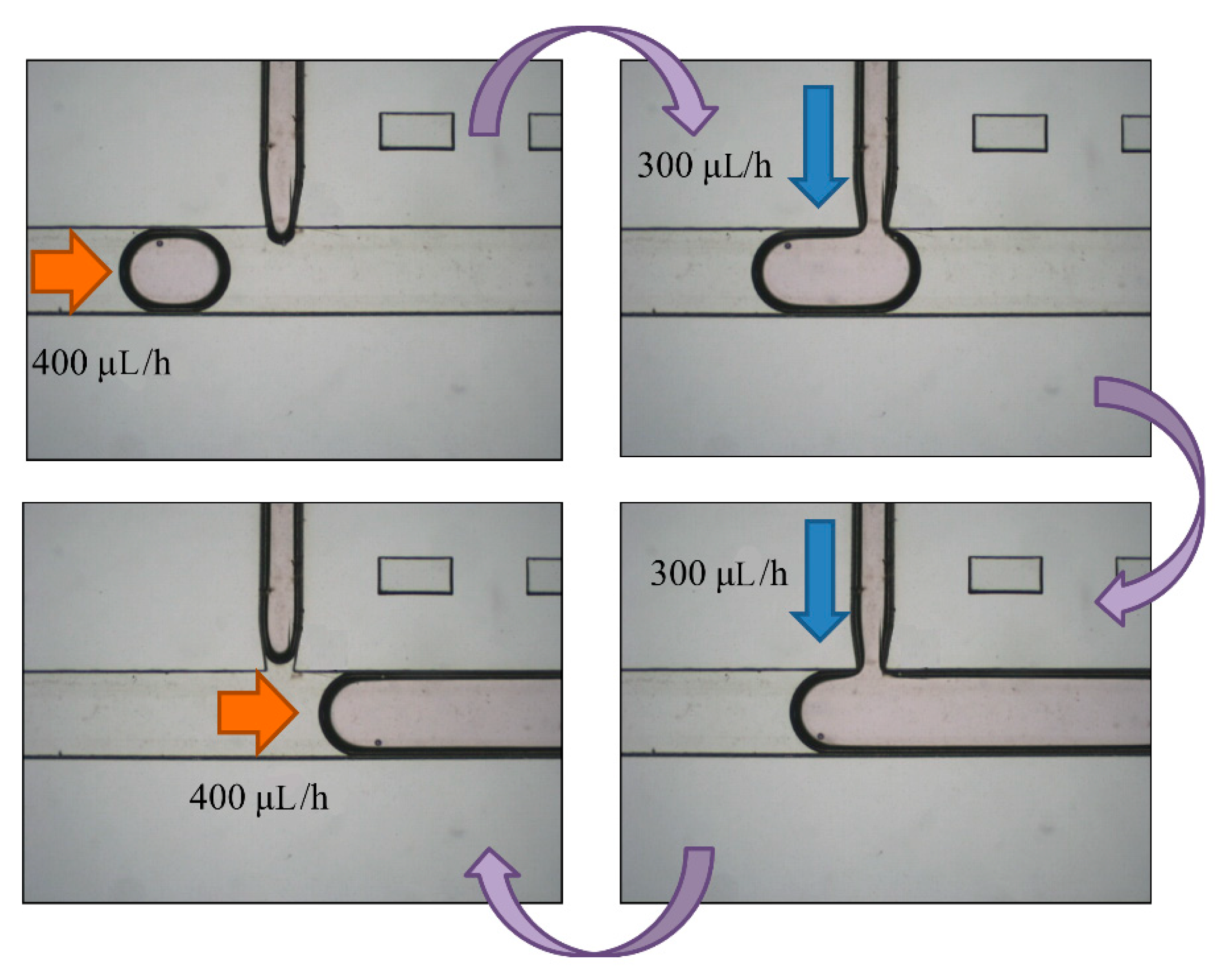
The breaking methods of the continuous phase liquid to cut off the dispersed phase liquid can be divided into two types—the push-push system and the push-pull system. In the former system, the continuous phase and dispersed phase liquids are both pushed with a forward force, whereas in the latter, the continuous phase liquid is pushed, while the dispersed phase liquid is pulled through the end of the channel (see
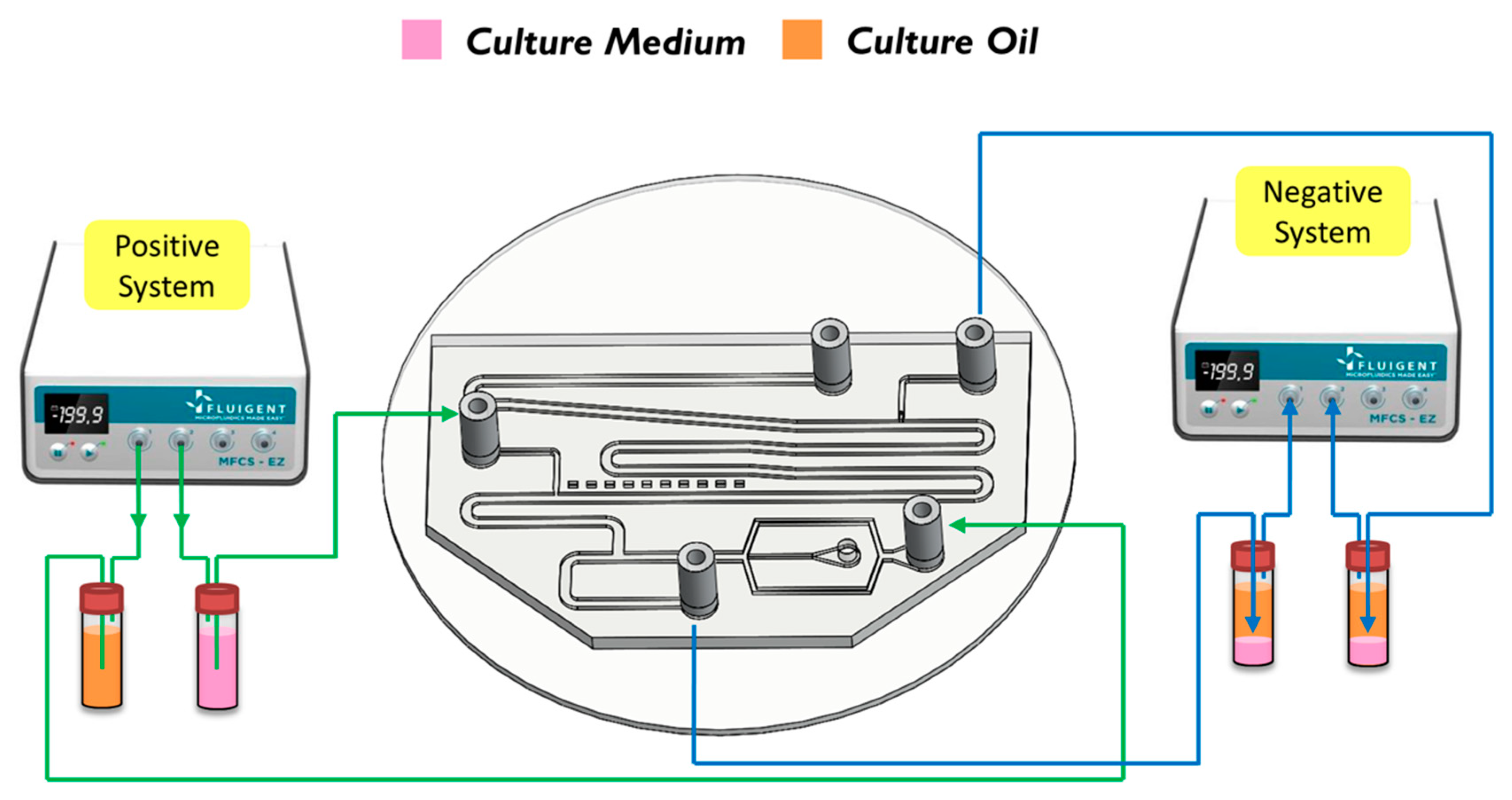
Figure 2b,c). We herein adopted the push-pull system to conduct the experiment because it allowed the embryos in the liquid to flow through the channel. The microfluidic flow control device served to perform the push-pull system. The positive system provides a pushing force from an air compressor with a positive pressure (0–345 mbar) to drive the oil and medium into the chip. In contrast, the negative system generates a pulling force from an air pump with a negative pressure (0 to −345 mbar) to extract the waste liquid from the chip (see
Figure 3).
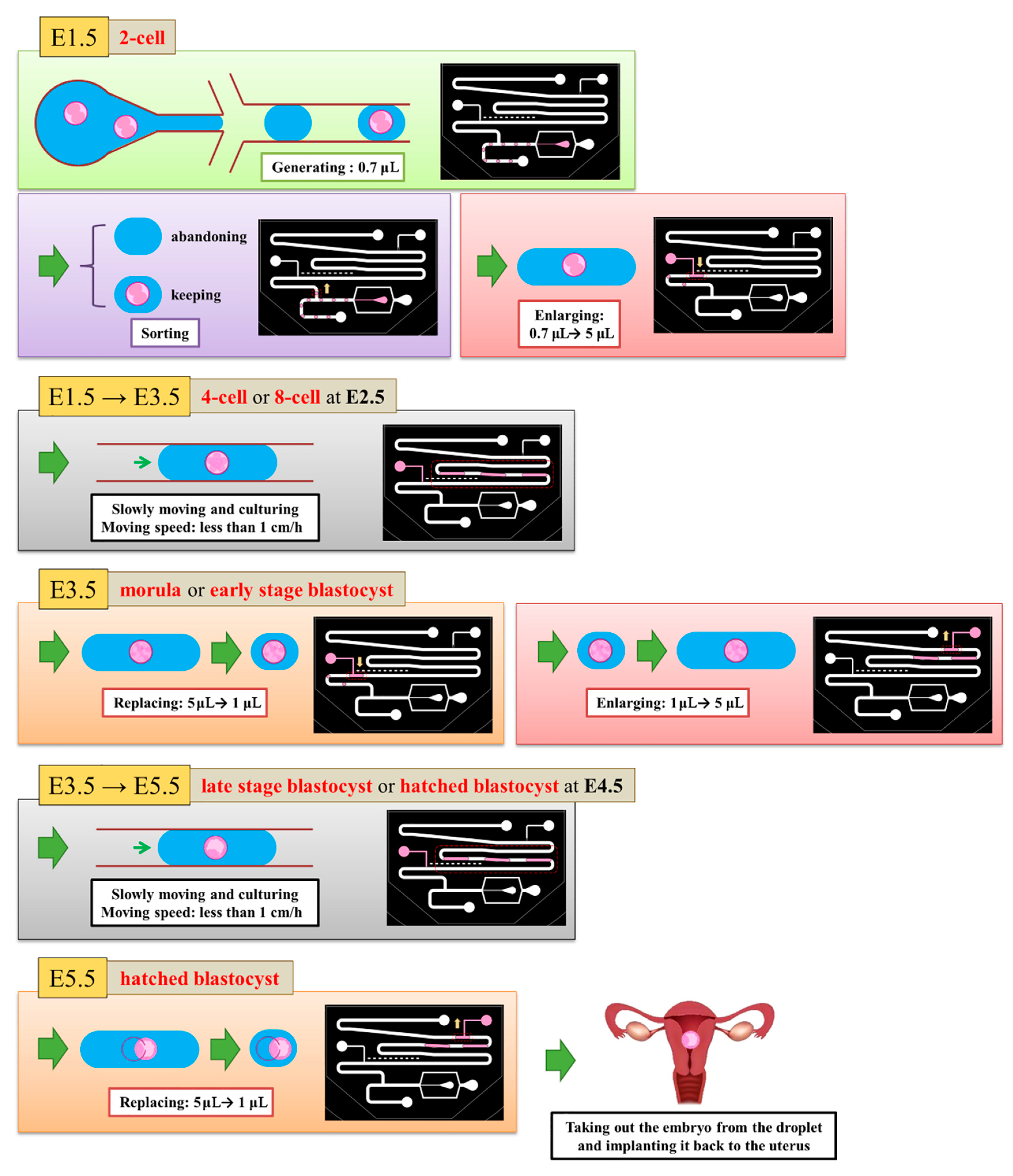
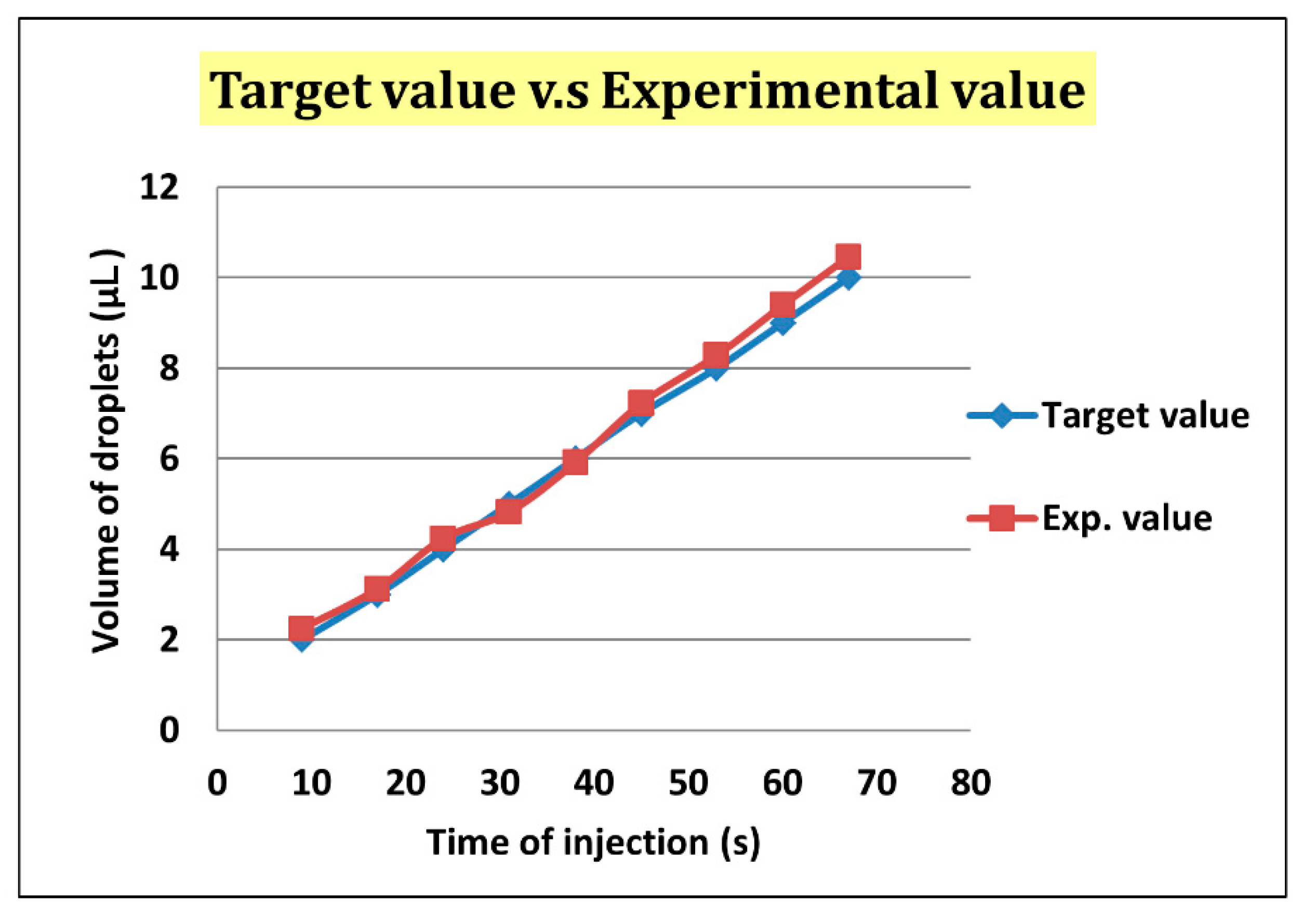
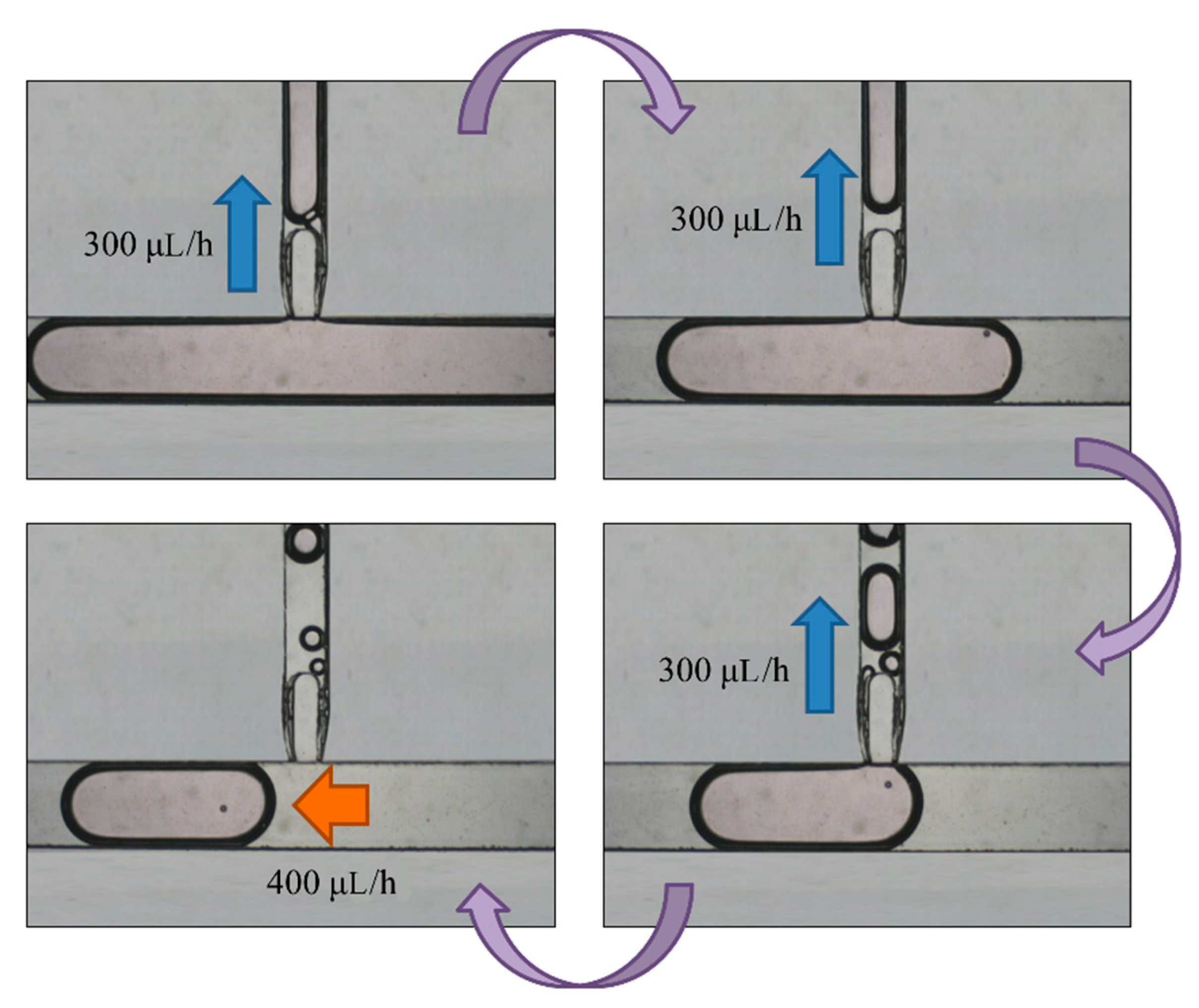
We defined the time when the embryo fertilized as Day 0. The two-cell stage embryo was used to inject into the channel as a start of the experiment on Day 1.5 because it is difficult to detect whether the zygote is fertilized or not. The medium (20 μL) was put with three two-cell embryos on top of the embryo inlet on Day 1.5. It was then pulled into the generating area and cut into small droplets of size about 0.7 μL. Some droplets encapsulated an embryo, but most did not. We retained a droplet with an embryo and abandoned a droplet without embryo in the sorting area. The volume of the selected droplets was enlarged to 5 μL in the enlargement area. From Day 1.5 to 3.5, the embryos were cultured in the droplets with a slow flow in the storage area. On Day 3.5, the medium of size 4 μL was extracted from each droplet through the replacement area and the extracted medium was recycled for another examination. The droplet was enlarged again to size 5 μL with the new medium. From Day 3.5 to 5.5, the embryos were cultured in the droplets again in the storage area. On Day 5.5, the 4-μL medium was extracted from the droplet. These steps are shown in
Figure 4.
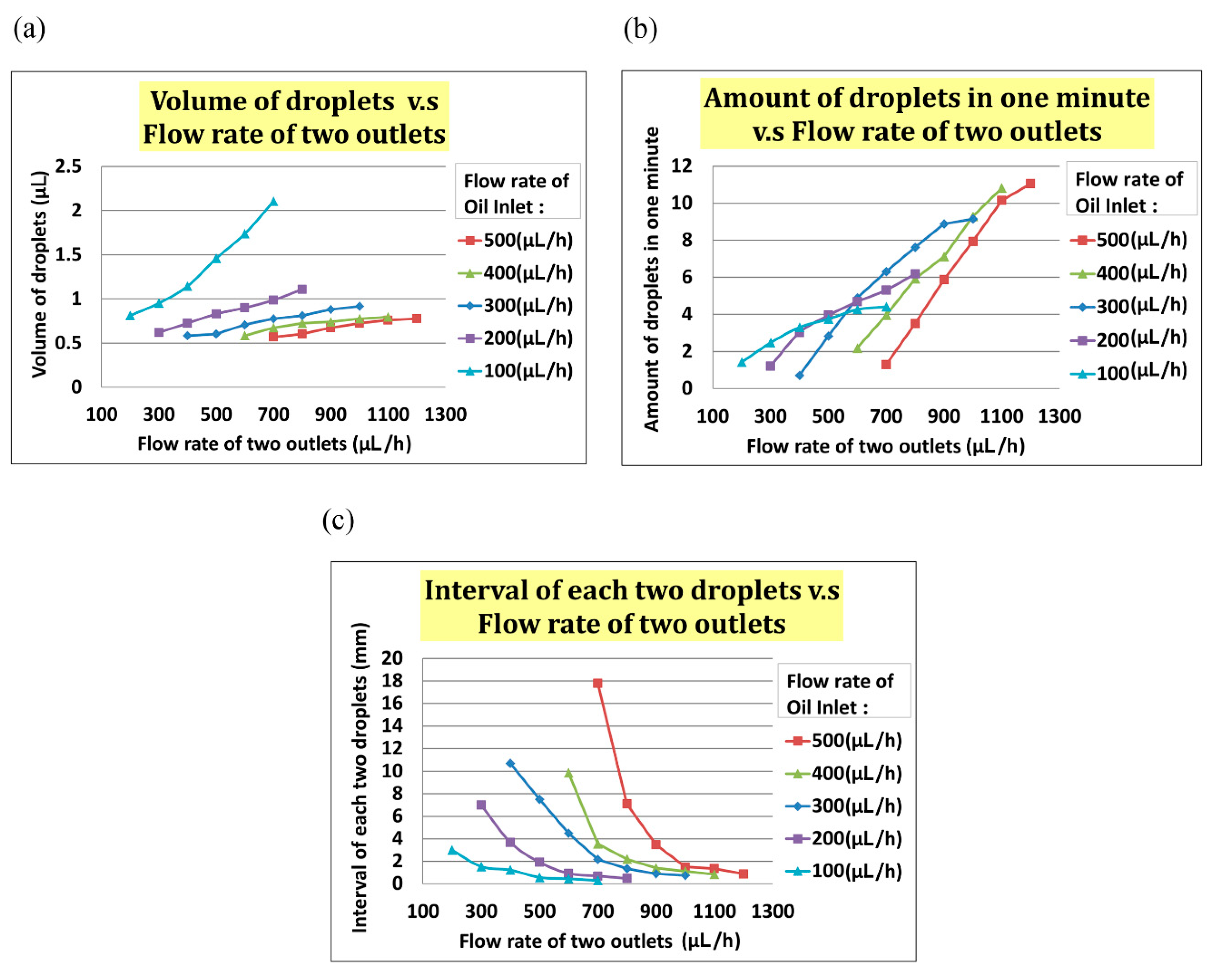
In the experiment, we set the flow rate of the oil inlet (i.e.,
Qoil inlet) from 100 µL/h to 500 µL/h and the total flow rate of two outlets (i.e.,
Qtwo outlets) from
Qoil inlet + 100 µL/h to
Qoil inlet + 500 µL/h. As indicated in
Figure 5a, the volume of the droplet increased with
Qtwo outlets when
Qoil inlet was fixed. In
Figure 5b, the interval between two droplets decreased when the flow rate of two outlets increased at any fixed
Qoil inlet. In
Figure 5, the frequency of droplet generation decreased when the flow rate of the two outlets increased at any fixed
Qoil inlet.
4. Conclusions
In our research, we achieved a technique wherein a chip could perform four main functions—droplet generation, sorting, droplet enlargement, and restoration—simultaneously. A droplet was generated with volume in range of microlitres, whereas in past studies, the droplet was picolitre or nanolitre in size. Second, we undertook sorting of the droplets. A droplet carries an embryo, proceeds towards the enlargement area, and another droplet without embryo is abandoned and directed towards the waste area outlet using a manual sorting method. The next step involves enlarging the size of the selected droplet from 1 μL to 5 μL by injecting HTF or KSOM medium into the selected droplet, whereas during droplet generation, the size of the droplet is approximately 0.7–1 μL, although the droplet size is not enough for embryo growth; therefore, the droplet size is increased to 5 μL in the enlarging area. In the last step of the experiment, we removed the old HTF or KSOM medium from the enlarged droplet without losing embryo particles of diameter 100 μm. In subsequent steps, an embryo culture experiment is conducted. In future, we aim to conduct embryo culture from Day 1 to Day 5 and from Day 3 to Day 5.