Discrimination of Red Wines with a Gas-Sensor Array Based on a Surface-Acoustic-Wave Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Red Wine Samples
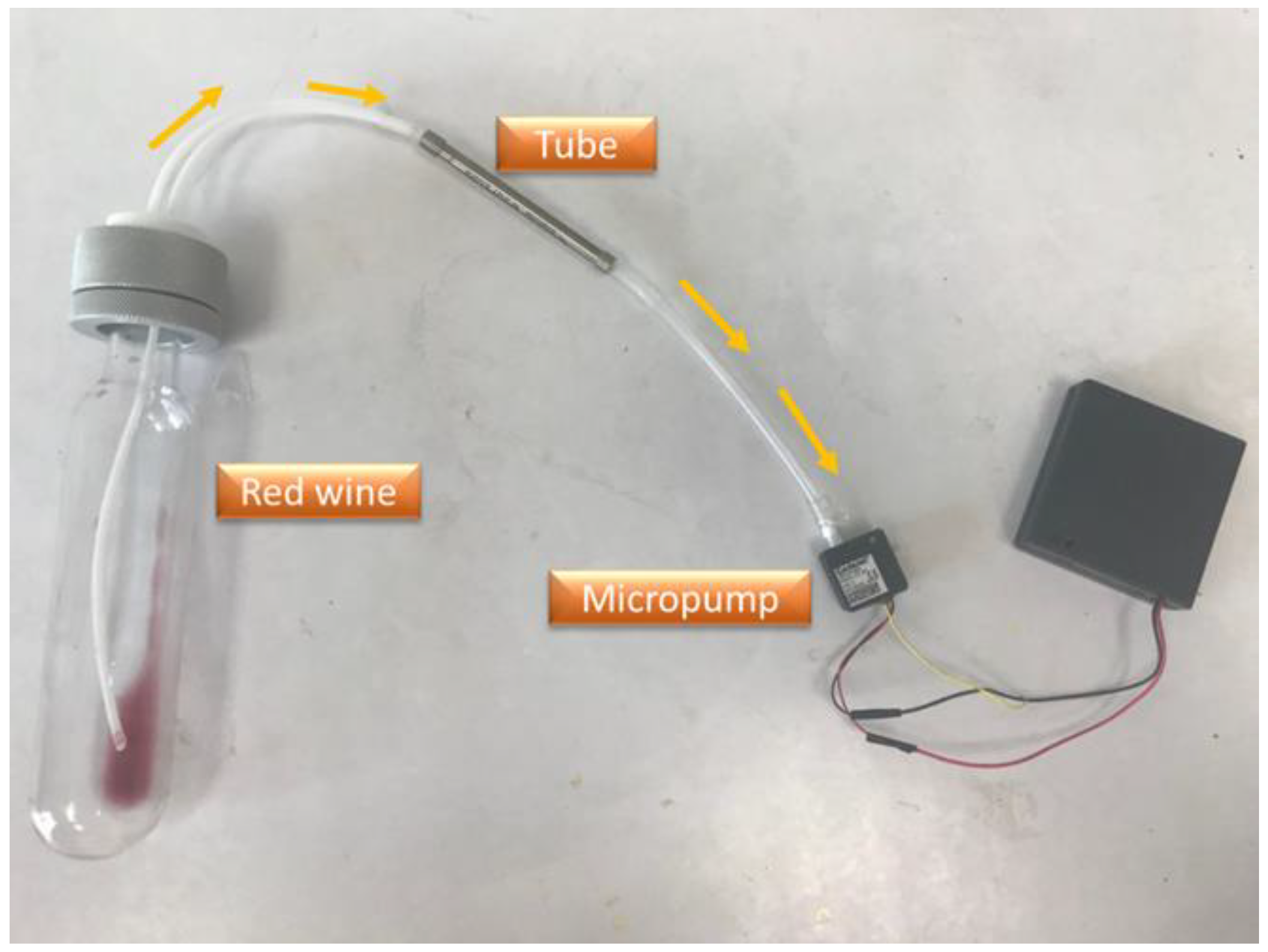
2.2. Sample Extraction
2.3. Thermal Desorption-Gas Chromatograph–Mass Spectrometer (TD-GC–MS)
2.4. Principle of a SAW Sensor
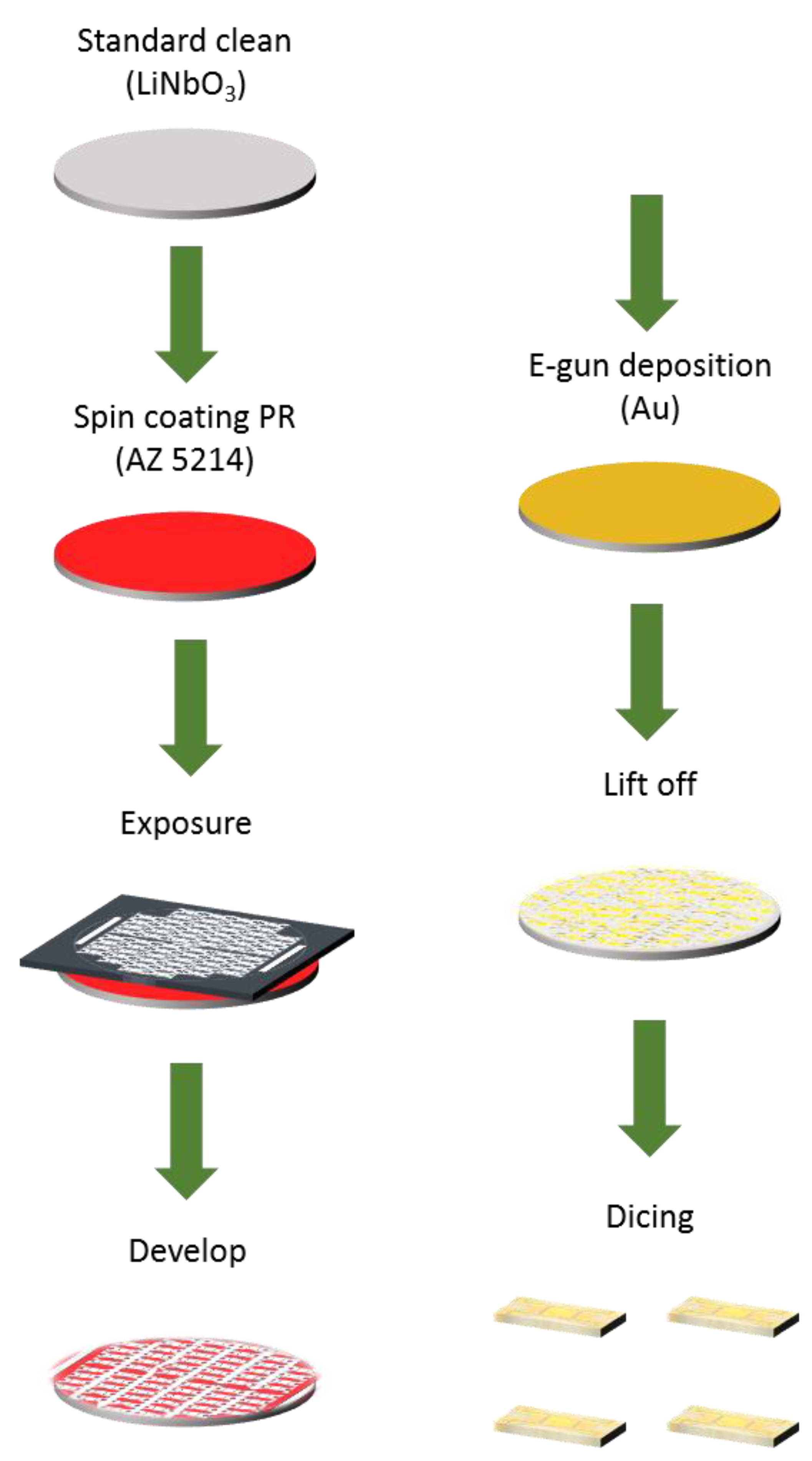
2.5. Design and Fabrication of SAW Sensors
2.6. Polymer Preparation and Coating
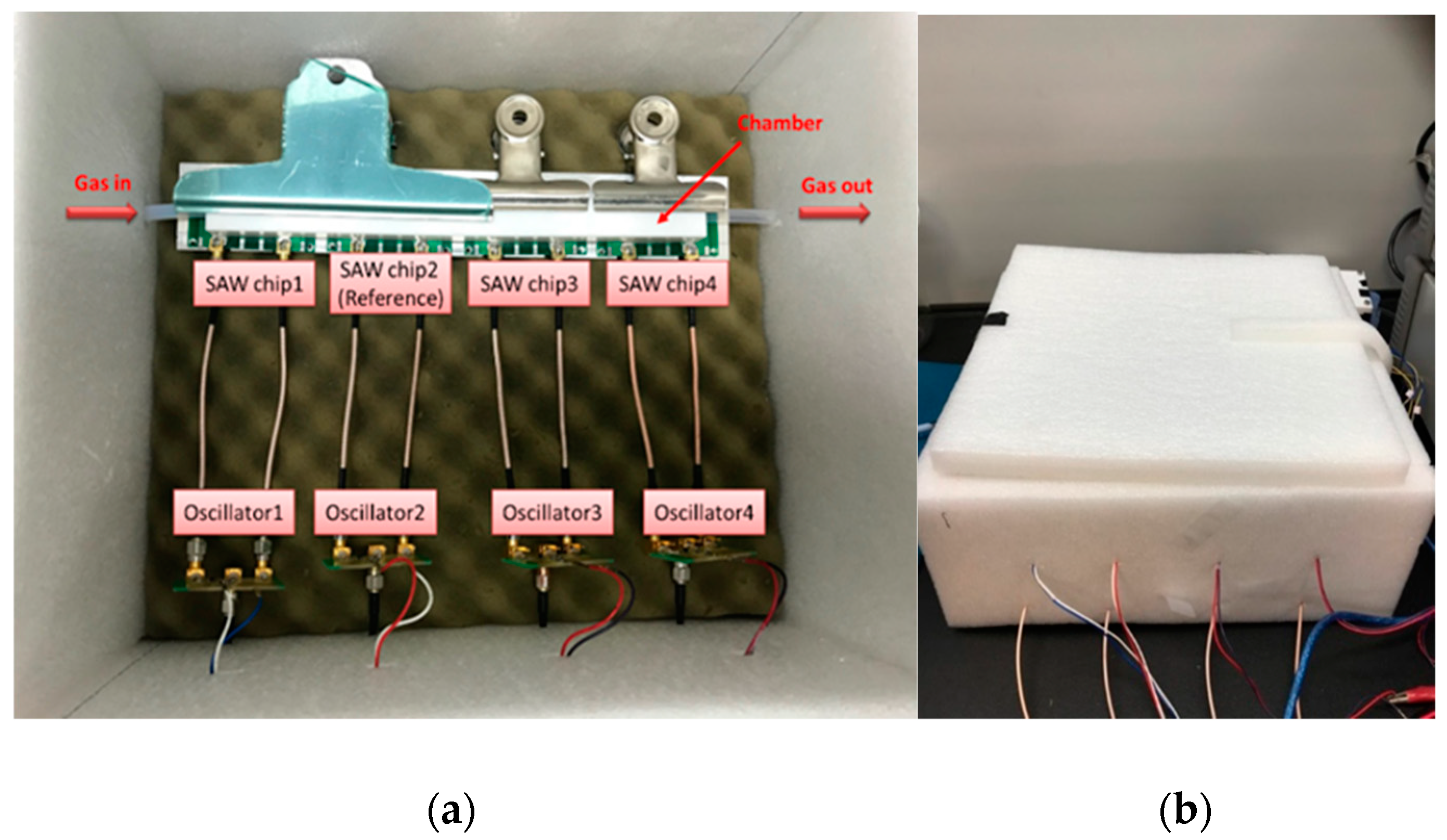
2.7. SAW Sensing System
3. Results
3.1. Polymer Preparation and Coating
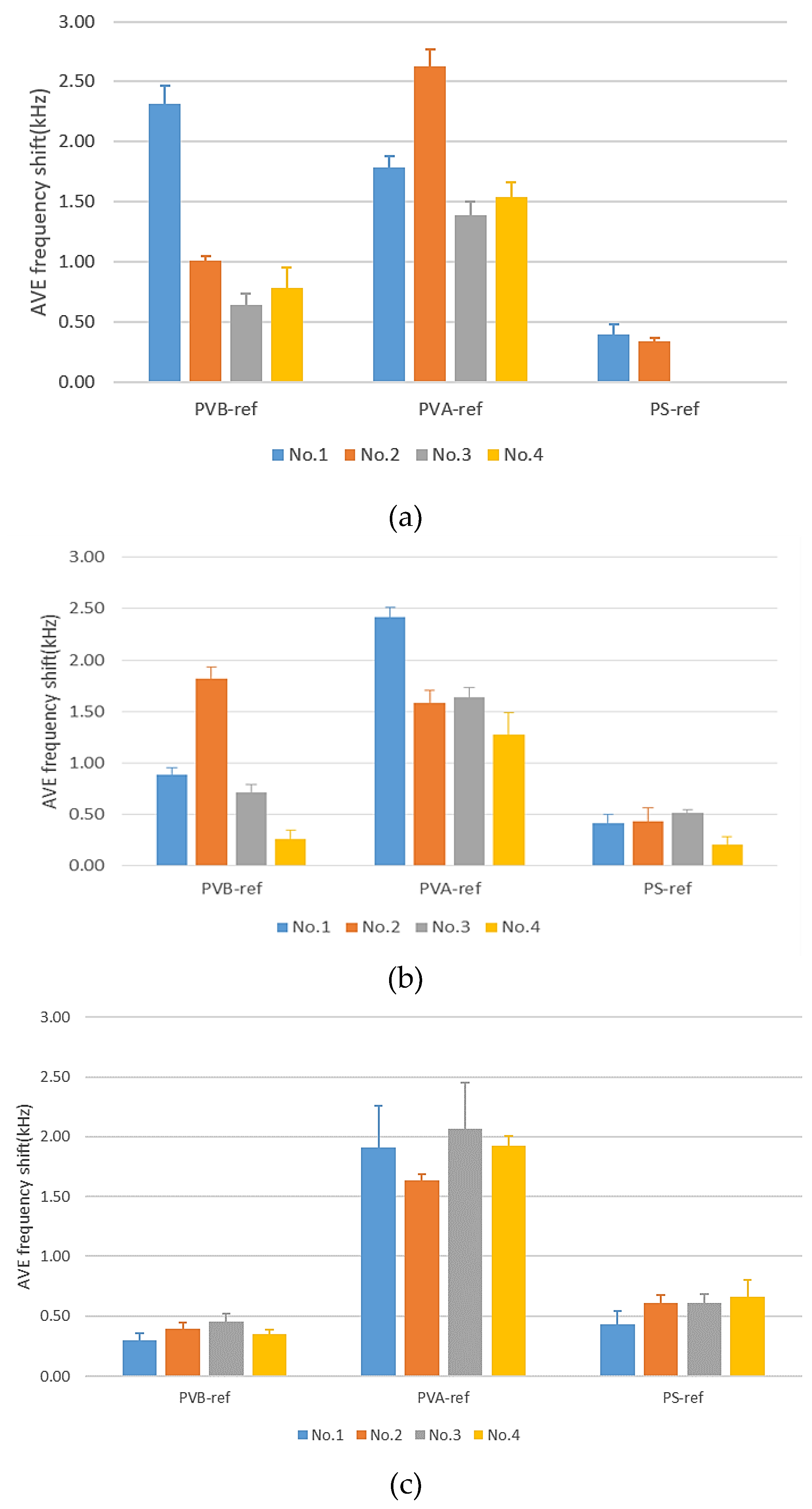
3.1.1. Chromatography Column (DB-624) Results
3.1.2. Chromatography Column (DB-WAX) Results
3.2. Polymer Preparation and Coating
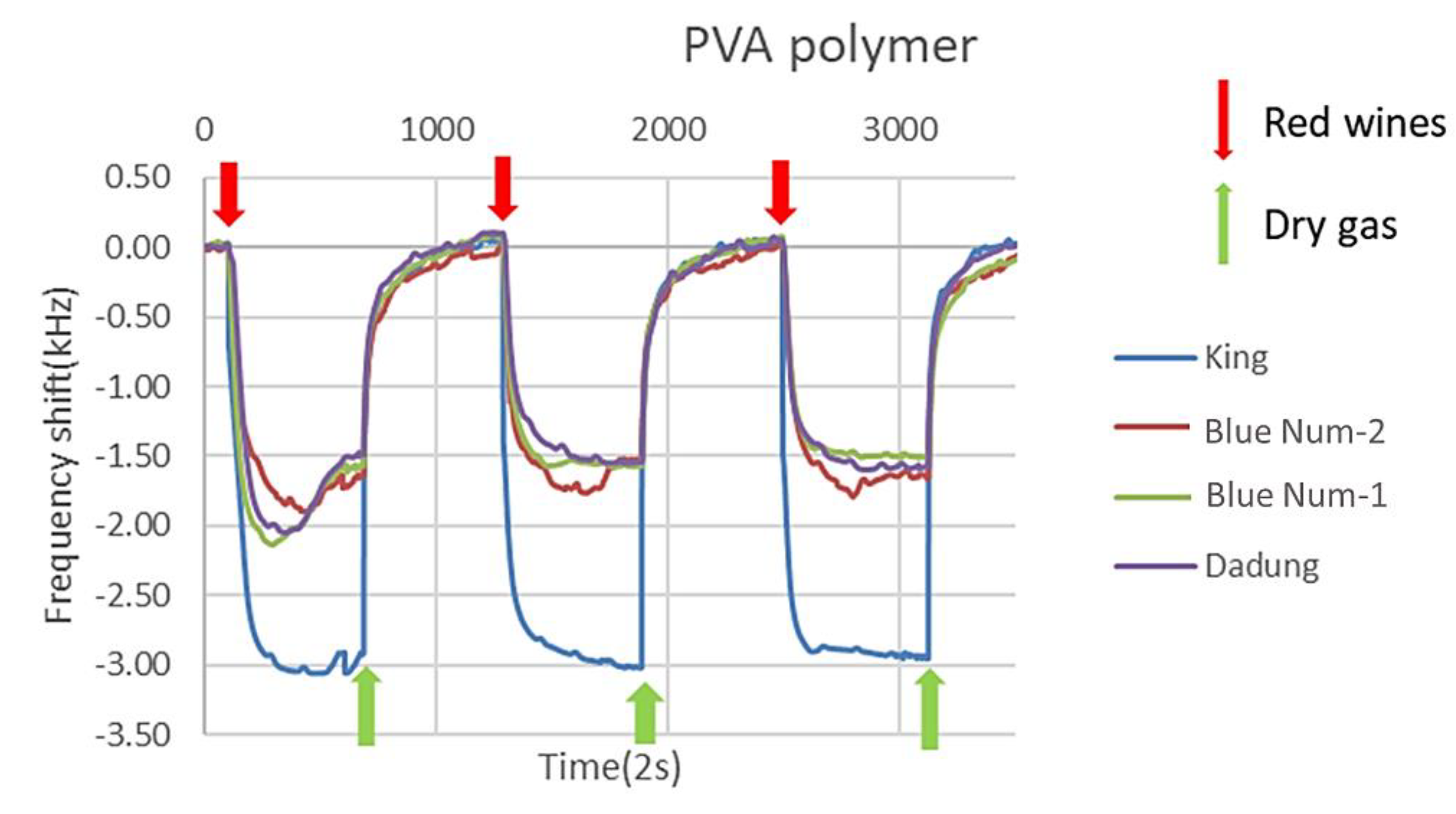
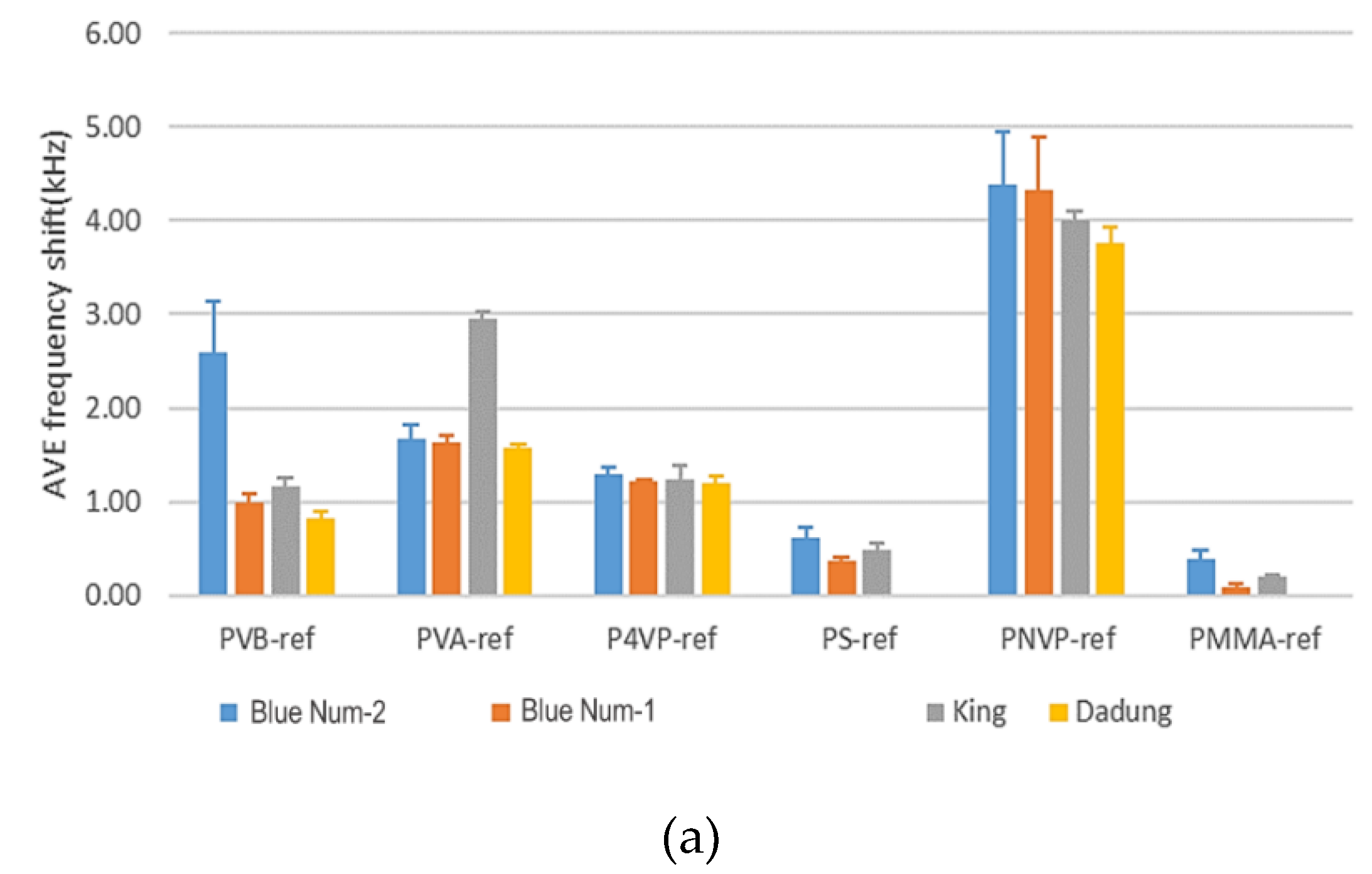
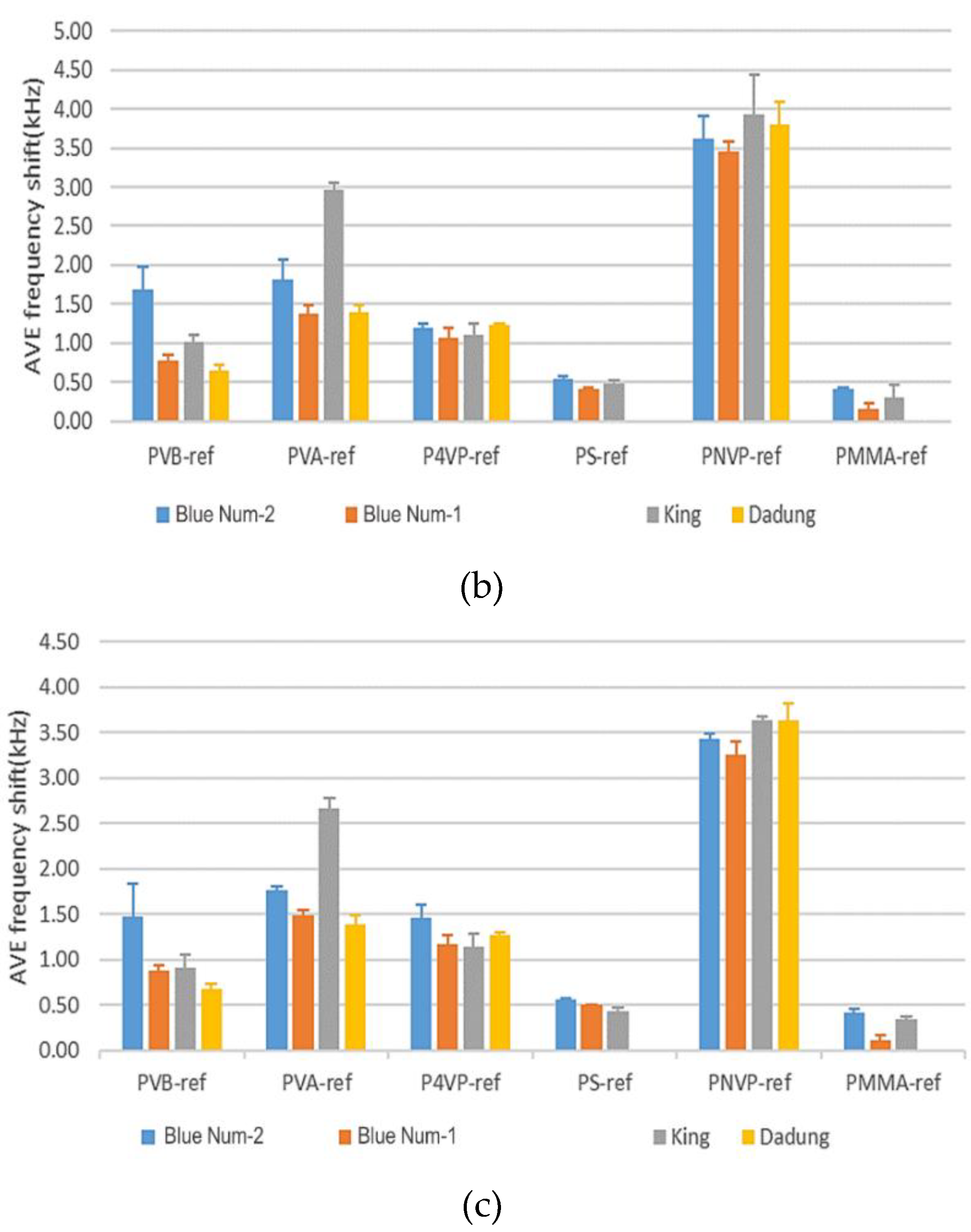
3.2.1. Results of a SAW Sensor Array Coated with Six Polymers
3.2.2. Blind Test of Red Wines and Lifetime of a Polymer Film
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aznar, M.; Guirado-López, R.A.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Guirado-López, R.A.; Cacho, J. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Álvarez, I.; Aleixandre, J.L.; García, M.J.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Ugliano, M.; Moio, L. The influence of malolactic fermentation and Oenococcus oeni strain on glycosidic aroma precursors and related volatile compounds of red wine. J. Sci. Food Agric. 2006, 86, 2468–2476. [Google Scholar] [CrossRef]
- De Freitas, V.A.P.; Ramalho, P.S.; Azevedo, Z.; Macedo, A. Identification of some volatile descriptors of the rock-rose-like aroma of fortified red wines from Douro demarcated region. J. Agric. Food Chem. 1999, 47, 4327–4331. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic noses and tongues in wine industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, M.; Cabellos, J.M.; Arroyo, T.; Horrillo, M.C. Quantification of wine mixtures with an electronic nose and a human panel. Front. Bioeng. Biotechnol. 2018, 6, 14. [Google Scholar] [CrossRef]
- Nagle, H.T.; Gutierrez-Osuna, R.; Schiffman, S.S. The how and why of electronic noses. IEEE Spectr. 1998, 35, 22–31. [Google Scholar] [CrossRef]
- Berna, A. Metal oxide sensors for electronic noses and their application to food analysis. Sensors 2010, 10, 3882–3910. [Google Scholar] [CrossRef]
- Buratti, S.; Ballabio, D.; Giovanelli, G.; Zuluanga, D.C.M.; Moles, A.; Benedetti, S.; Sinelli, N. Monitoring of alcoholic fermentation using near infrared and mid infrared spectroscopies combined with electronic nose and electronic tongue. Anal. Chim. Acta 2011, 697, 67–74. [Google Scholar] [CrossRef]
- Gebicki, J. Application of electrochemical sensors and sensor matrixes for measurement of odorous chemical compounds. Trends Anal. Chem. 2016, 77, 1–13. [Google Scholar] [CrossRef]
- Chiang, M.-C.; Hao, H.-C.; Yang, C.-M.; Yao, D.-J. Detection of hazardous vapors including mixtures in varied conditions using a surface-acoustic-wave device. ECS J. Solid State Sci. Technol. 2018, 7, Q3120–Q3125. [Google Scholar] [CrossRef]
- Hoummady, M.; Campitelli, A.; Wlodarski, W. Acoustic wave sensors: Design, sensing mechanisms and applications. Smart Mater. Struct. 1997, 6, 647. [Google Scholar] [CrossRef]
- Rapp, M.; Reibel, J.; Stier, S.; Voigt, A.; Bahlo, J. SAGAS: Gas Analyzing Sensor Systems based on Surface Acoustic Wave Devices-an Issue of Commercialization of SAW Sensor Technology. In Proceedings of the International Frequency Control Symposium, Orlando, FL, USA, 30–30 May 1997. [Google Scholar]
- Szulczyński, B.; Gębicki, J. Currently commercially available chemical sensors employed for detection of volatile organic compounds in outdoor and indoor air. Environments 2017, 4, 21. [Google Scholar] [CrossRef]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Kabir, K.M.; Sabri, Y.M.; Lay, B.; Ippolito, S.J.; Bhargava, S.K. A silver electrode based surface acoustic wave (SAW) mercury vapor sensor: A physio-chemical and analytical investigation. RSC Adv. 2016, 6, 36362–36372. [Google Scholar] [CrossRef]
- Grate, J.W.; McGill, R.A. Dewetting effects on polymer-coated surface acoustic wave vapor sensors. Anal. Chem. 1995, 67, 4015–4019. [Google Scholar] [CrossRef]
- D’amico, A.; Verona, E. SAW sensors. Sens. Actuators 1989, 17, 55–66. [Google Scholar] [CrossRef]
- Wohltjen, H. Mechanism of operation and design considerations for surface acoustic wave device vapour sensors. Sens. Actuators 1984, 5, 307–325. [Google Scholar] [CrossRef]
- Boeker, P. On ‘electronic nose’methodology. Sens. Actuators B Chem. 2014, 204, 2–17. [Google Scholar] [CrossRef]
- Sims, C.A.; Morris, J.R. Effects of pH, sulfur dioxide, storage time, and temperature on the color and stability of red muscadine grape wine. Am. J. Enol. Vitic. 1984, 35, 35–39. [Google Scholar]

| Sensor Type | Semiconductor | Infrared | Quartz Crystal | SAW |
|---|---|---|---|---|
| Sensor size | 0.3 × 0.3 mm2 | 6.35 × 3.18 mm2 | Diameter: 25 mm | 3 × 2 mm2 |
| Measured concentration | 200 ppm | ~ppm | 0.1–100 ppm [12] | 0–250 ppb |
| Response time | 100 s | <5 s | <1 s | ~100 s |
| Operating temperature | 200–500 °C | −10–50 °C | 0–40 °C | ~23 °C |
| Mechanism | resistance | absorption of infrared radiation | mass loading | mass loading |
| Common detection | CH4, CO, C2H4, C2H2, NO, CO2, CH2CHCl, EtOH | CH4, CO2, hydrocarbons | organic or inorganic film layers | VOC, H2, H2O, H2S, CO, CO2 |
| Disadvantages | operation at high temperature | easily influenced by temperature and humidity | interface electronics and detection range less than for SAW | interface electronics |
| Wine Brand | King | Blue Nun-1 | Blue Nun-2 | Dadung | |
|---|---|---|---|---|---|
| Grape Variety | |||||
| Black queen | ● | ● | |||
| Cabernet sauvignon | ● | ● | |||
| Merlot | ● | ||||
| Markers | King | Blue Nun-1 | Blue Nun-2 | Dadung |
|---|---|---|---|---|
| Sulfur dioxide | – | 1.70 | 2.59 | – |
| Acetaldehyde | 7.10 | – | – | 2.53 |
| Acetic acid, methyl ester (methyl acetate) | 85.22 | – | – | – |
| Acetic acid, 2-methylpropyl ester (2-methylpropyl acetate) | 50.12 | – | – | – |
| Ethanol | 20711.84 | 16530.75 | 22560.19 | 13886.27 |
| Ethyl acetate | 4872.73 | 1777.45 | 2092.90 | 2682.81 |
| 1-Propanol | 493.72 | 173.19 | 161.85 | 31.20 |
| 1-Propanol, 2-methyl- | 1861.24 | 686.80 | 994.77 | 230.52 |
| 1-Butanol | 35.59 | 12.89 | 9.03 | – |
| 2-Pentanone | 86.84 | – | – | – |
| Propanoic acid, ethyl ester | 82.78 | 25.08 | 63.20 | 11.02 |
| Propanoic acid, 2-methyl-, ethyl ester | 107.96 | 17.38 | 24.05 | 9.47 |
| Disulfide, dimethyl | 10.85 | – | – | – |
| 1-Butanol, 2-methyl- | 70.69 | 90.09 | 77.93 | 53.63 |
| 1-Butanol, 2-methyl-, acetate | 18.40 | 31.53 | 29.84 | – |
| 1-Butanol, 3-methyl- | 2426.55 | 3475.05 | 4362.63 | 562.89 |
| 1-Butanol, 3-methyl-, acetate | 1271.36 | 1408.16 | 1726.33 | 316.59 |
| Butanoic acid, ethyl ester | 36.87 | 3.82 | 4.17 | 84.44 |
| Butanoic acid, 2-methyl-, ethyl ester | 49.27 | 7.24 | 8.31 | – |
| Butanoic acid, 3-methyl-, ethyl ester | 54.07 | 268.77 | 225.24 | – |
| Hexanoic acid, ethyl ester | – | 21.39 | 31.99 | – |
| Quinoline, 6-ethoxy-1,2-dihydro-2,2,4-trimethyl- | – | – | 48.16 | – |
| Markers | King | Blue Nun-1 | Blue Nun-2 | Dadung |
|---|---|---|---|---|
| Ethyl acetate | 2978.03 ± 0.01 | 146.87 ± 236.22 | 1234.08 ± 373.10 | 456.69 ± 40.01 |
| Ethanol | 35789.09 ± 7854.27 | 3646.95 ± 8053.27 | 38669.95 ± 13566.44 | 15525.80 ± 1184.38 |
| 1-Propanol | 301.46 ± 97.39 | 20.11 ± 104.03 | 132.26 ±64.69 | 74.65 ± 14.28 |
| 1-Propanol, 2-methyl- | 1050.15 ± 273.24 | 85.63 ± 314.87 | 423.56 ± 229.19 | 224.30 ± 32.79 |
| 2-Pentanol | 9.40 ± 5.19 | – | – | – |
| 2-Pentanone | 300.27 ± 414.58 | – | – | – |
| Propanoic acid, ethyl ester | 185.54 ± 41.9 | 22.39 ± 87.84 | 39.23 ± 12.54 | – |
| Propanoic acid, 2-methyl-, ethyl ester | 55.94 ± 16.24 | 8.80 ± 8.14 | 72.49 ± 17.87 | – |
| 1-Butanol | 9.46 ± 6.14 | 1.46 ± 7.46 | 8.41 ± 2.52 | – |
| 1-Butanol, 3-methyl- | 1841.12 ± 1269.01 | 553.18 ± 4012.7 | 2300.56 ± 876.22 | 3596.22 ± 512.76 |
| 1-Butanol, 3-methyl-, acetate | – | 0.65 ± 2.09 | 5.77 ± 4.45 | – |
| 2,3-Butanedione | – | 8.10 ± 33.74 | – | – |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.-H.; Ke, L.-Y.; Yao, D.-J. Discrimination of Red Wines with a Gas-Sensor Array Based on a Surface-Acoustic-Wave Technique. Micromachines 2019, 10, 725. https://doi.org/10.3390/mi10110725
Lin M-H, Ke L-Y, Yao D-J. Discrimination of Red Wines with a Gas-Sensor Array Based on a Surface-Acoustic-Wave Technique. Micromachines. 2019; 10(11):725. https://doi.org/10.3390/mi10110725
Chicago/Turabian StyleLin, Min-Han, Ling-Yi Ke, and Da-Jeng Yao. 2019. "Discrimination of Red Wines with a Gas-Sensor Array Based on a Surface-Acoustic-Wave Technique" Micromachines 10, no. 11: 725. https://doi.org/10.3390/mi10110725
APA StyleLin, M.-H., Ke, L.-Y., & Yao, D.-J. (2019). Discrimination of Red Wines with a Gas-Sensor Array Based on a Surface-Acoustic-Wave Technique. Micromachines, 10(11), 725. https://doi.org/10.3390/mi10110725






