Fabrication of 512-Channel Geometrical Passive Breakup Device for High-Throughput Microdroplet Production
Abstract
:1. Introduction
2. Materials and Methods
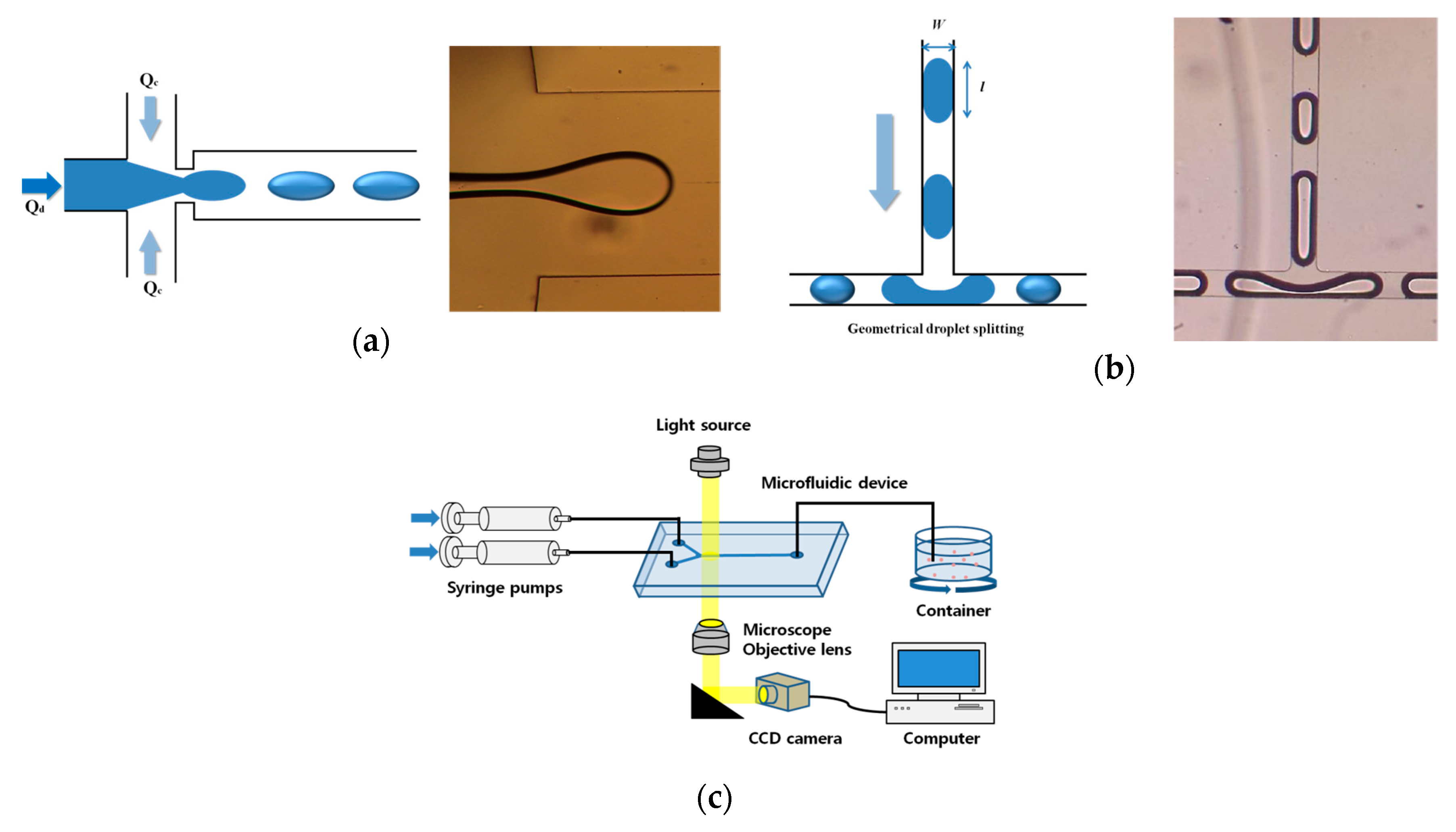
2.1. Design of Microfluidic Device
2.2. Fabrication Process for 512-Channel Geometrical Passive Breakup Device
2.3. Experimental Set-Up for Microfluidic Device
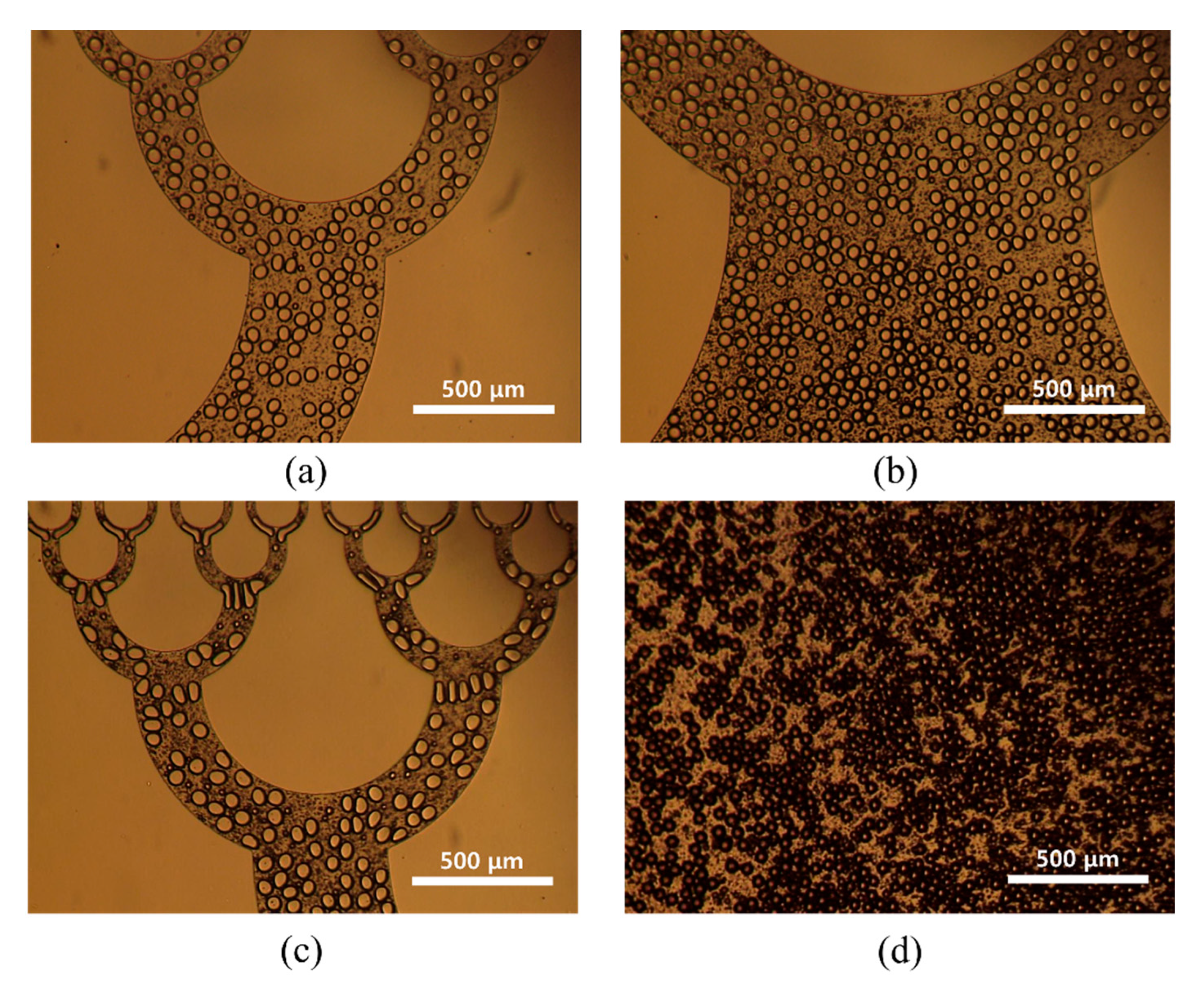
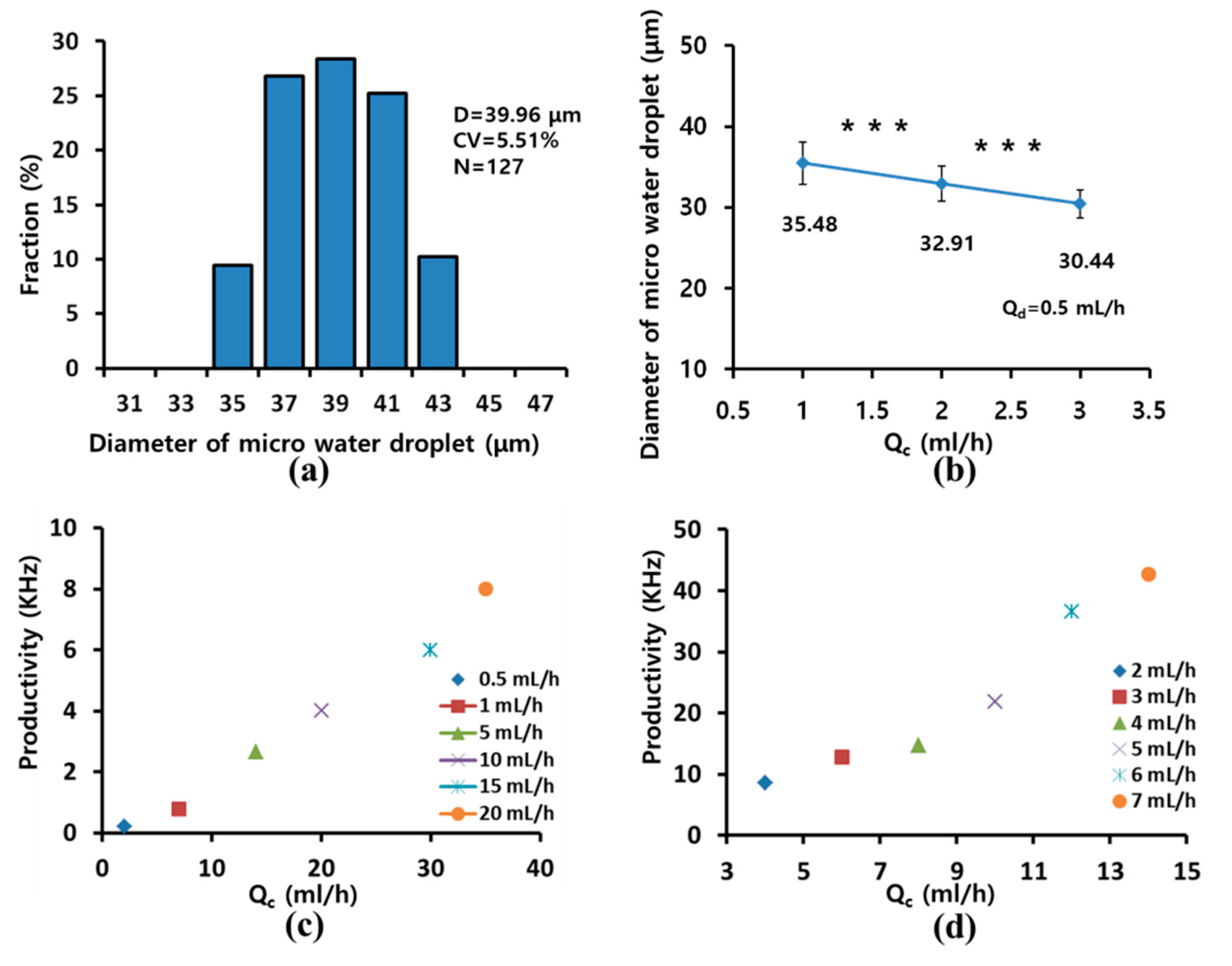
2.4. Preparation of Chitosan Microspheres with Fabricated Microfluidic Device
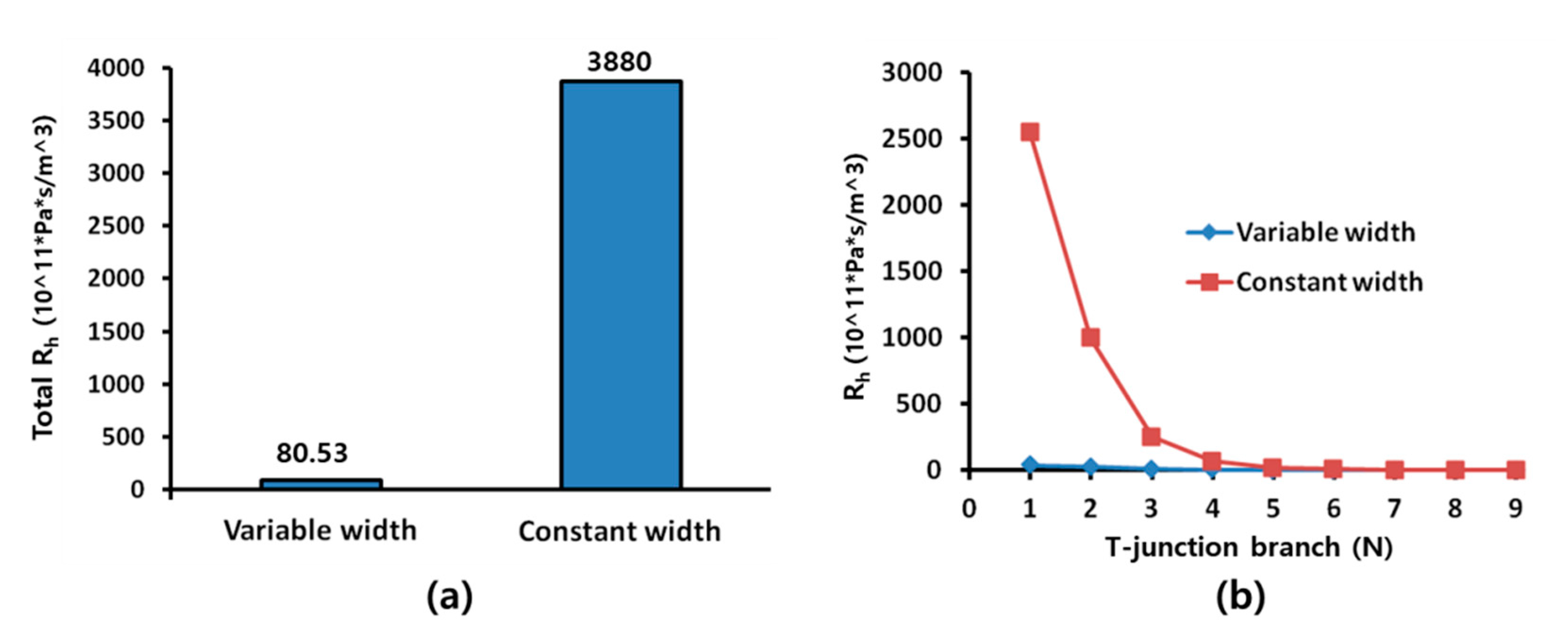
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kshirsagar, D.S.; Saudagar, R.B. Microsphere: A review. Res. J. Top. Cosmet. Sci. 2016, 7, 27–37. [Google Scholar] [CrossRef]
- Sahil, K.; Akanksha, M.; Premjeet, S.; Bilandi, A.; Kapoor, B. Microsphere: A review. Int. J. Res. Pharm. Chem. 2011, 1, 1184–1198. [Google Scholar]
- Kim, C.M.; Ullah, A.; Chang, C.H.; Kim, G.M. Preparation of lidocaine-loaded porous Poly (lactic-co-glycolic acid) microparticles using microfluidic flow focusing and phosphate buffer solution porogen. Int. J. Precis. Eng. Manuf. 2017, 18, 599–604. [Google Scholar] [CrossRef]
- Kim, C.M.; Ullah, A.; Kim, K.G.; Kim, S.Y.; Kim, G.M. Preparation of carbon nanotube-wrapped porous microparticles using a microfluidic device. J. Nanosci. Nanotechnol. 2016, 16, 12003–12008. [Google Scholar] [CrossRef]
- Berkland, C.; Kipper, M.J.; Narasimhan, B.; Kim, K.K.; Pack, D.W. Microsphere size, precipitation kinetics and drug distribution control drug release from biodegradable polyanhydride microspheres. J. Control. Release 2004, 94, 129–141. [Google Scholar] [CrossRef]
- Xu, Q.; Hashimoto, M.; Dang, T.T.; Hoare, T.; Kohane, D.S.; Whitesides, G.M.; Langer, R.; Anderson, D.G. Preparation of monodisperse biodegradable polymer microparticles using a microfluidic flow-focusing device for controlled drug delivery. Small 2009, 5, 1575–1581. [Google Scholar] [CrossRef]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Teh, S.-Y.; Lin, R.; Hung, L.-H.; Lee, A.P. Droplet microfluidics. Lab Chip 2008, 8, 198–220. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977. [Google Scholar] [CrossRef]
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61, 907–914. [Google Scholar] [CrossRef]
- Kim, C.M.; Park, S.J.; Kim, G.M. Applications of PLGA microcarriers prepared using geometrically passive breakup on microfluidic chip. Int. J. Precis. Eng. Manuf. 2015, 16, 2545–2551. [Google Scholar] [CrossRef]
- Nisisako, T.; Torii, T. Microfluidic large-scale integration on a chip for mass production of monodisperse droplets and particles. Lab Chip 2008, 8, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, I.; Wada, Y.; Uemura, K.; Nakajima, M. Microchannel emulsification for mass production of uniform fine droplets: Integration of microchannel arrays on a chip. Microfluid. Nanofluid. 2010, 8, 255–262. [Google Scholar] [CrossRef]
- Fu, G.; Tor, S.B.; Loh, N.H.; Hardt, D.E. Fabrication of robust tooling for mass production of polymeric microfluidic devices. J. Micromech. Microeng. 2010, 20, 085019. [Google Scholar] [CrossRef]
- Link, D.R.; Anna, S.L.; Weitz, D.A.; Stone, H.A. Geometrically mediated breakup of drops in microfluidic devices. Phys. Rev. Lett. 2004, 92, 054503. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Datta, S.S.; Weitz, D.A. The microfluidic post-array device: High throughput production of single emulsion drops. Lab Chip 2014, 14, 705–709. [Google Scholar] [CrossRef]
- Tan, Y.C.; Cristini, V.; Lee, A.P. Monodispersed microfluidic droplet generation by shear focusing microfluidic device. Sens. Actuators B Chem. 2006, 114, 350–356. [Google Scholar] [CrossRef]
- Derzsi, L.; Kasprzyk, M.; Plog, J.P.; Garstecki, P. Flow focusing with viscoelastic liquids. Phys. Fluids 2013, 25, 092001. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.H.; Yelleswarapu, V.R.; Yadavali, S.; Issadore, D.; Lee, D. Kilo-scale droplet generation in three-dimensional monolithic elastomer device (3D MED). Lab Chip 2015, 15, 4387–4392. [Google Scholar] [CrossRef]
- Conchouso, D.; Castro, D.; Khan, S.A.; Foulds, I.G. Three-dimensional parallelization of microfluidic droplet generators for a litre per hour volume production of single emulsions. Lab Chip 2014, 14, 3011–3020. [Google Scholar] [CrossRef]
- Kobayashi, I.; Neves, M.A.; Wada, Y.; Uemura, K.; Nakajima, M. Large microchannel emulsification device for mass producing uniformly sized droplets on a liter per hour scale. Green Process. Synth. 2012, 1, 353–362. [Google Scholar] [CrossRef]
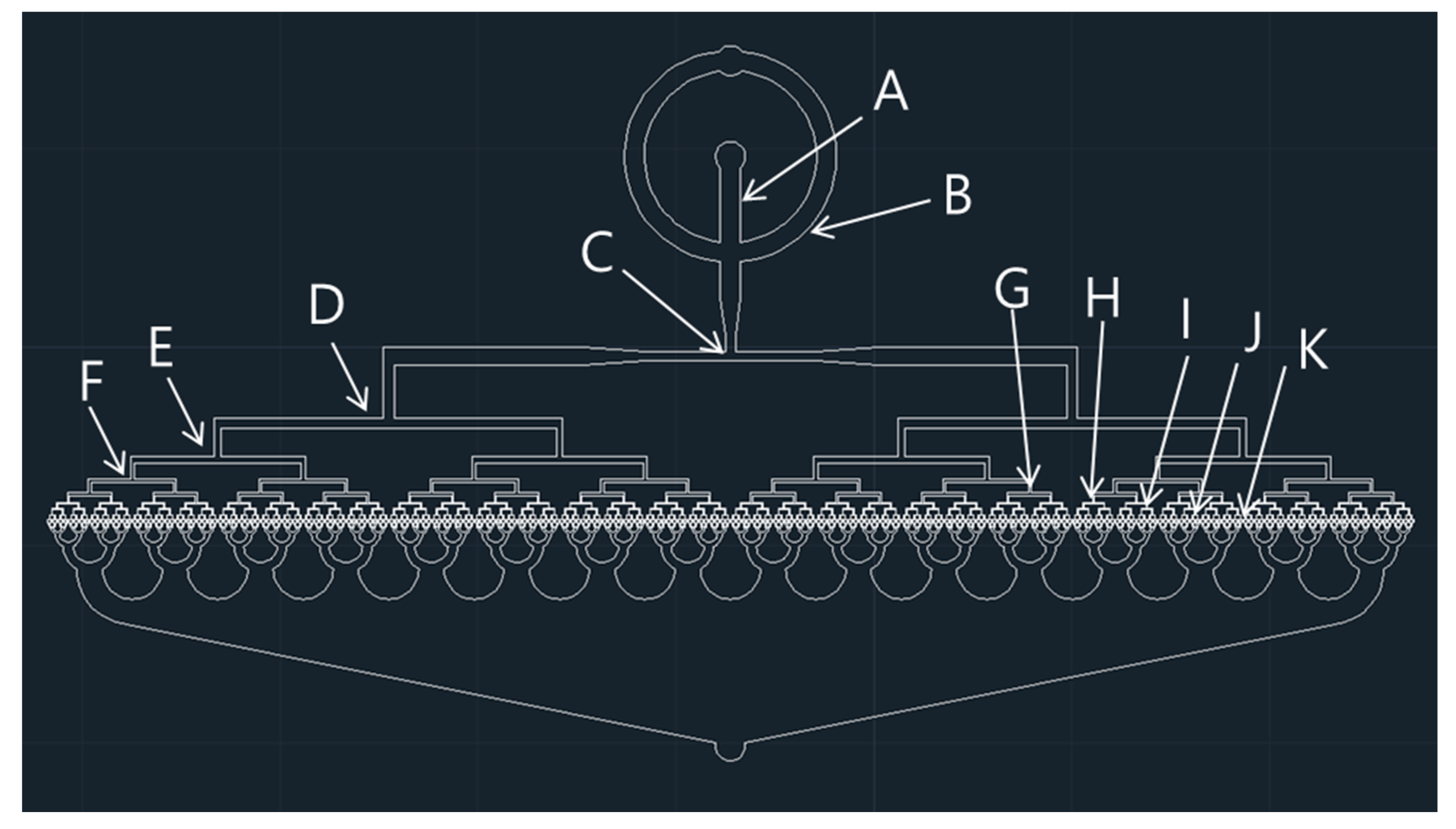



| Specifications | Width (μm) |
|---|---|
| A: Width of input A | 1000 |
| B: Width of input B | 1000 |
| C: Width of first T-junction | 500 |
| D: Width of second T-junction | 540 |
| E: Width of third T-junction | 330 |
| F: Width of fourth T-junction | 200 |
| G: Width of fifth T-junction | 130 |
| H: Width of sixth T-junction | 80 |
| I: Width of seventh T-junction | 50 |
| J: Width of eighth T-junction | 30 |
| K: Width of ninth T-junction | 30 |
| T: Thickness of the device | 40 |
| Geometry and Material | Continuous Phase | Droplet Size (μm) | Frequency (Hz) | |
|---|---|---|---|---|
| Water in oil | Channel array, silicon | Kerosene with monolaurate | 21 | −5300 (est.) |
| T-junction, acrylated urethane | Decane, tetradecane, and hexadecane with Span 80 | 10–350 | 20–80 | |
| T-junction, PMMA | High-oleic sunflower oil | 100–350 | 10–2500 | |
| T-junction, PDMS | C14F12 with C6F13(CH2)2OH | 7.5 nl | 2 | |
| Shear-focusing, PDMS | Oleic acid | 13–35 | 15–100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.M.; Kim, G.M. Fabrication of 512-Channel Geometrical Passive Breakup Device for High-Throughput Microdroplet Production. Micromachines 2019, 10, 709. https://doi.org/10.3390/mi10100709
Kim CM, Kim GM. Fabrication of 512-Channel Geometrical Passive Breakup Device for High-Throughput Microdroplet Production. Micromachines. 2019; 10(10):709. https://doi.org/10.3390/mi10100709
Chicago/Turabian StyleKim, Chul Min, and Gyu Man Kim. 2019. "Fabrication of 512-Channel Geometrical Passive Breakup Device for High-Throughput Microdroplet Production" Micromachines 10, no. 10: 709. https://doi.org/10.3390/mi10100709





