Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gelatin Methacryloyl (GelMA) Synthesis
2.2. Three-Dimensional Bioprinting Procedure
2.3. Mechanical and Rheological Properties of Hydrogels
2.4. Cell Culture and Cell Viability Assay
2.5. Quantification of Cell Metabolic Activity
2.6. Immunofluorescent Staining
2.7. Statistical Analysis
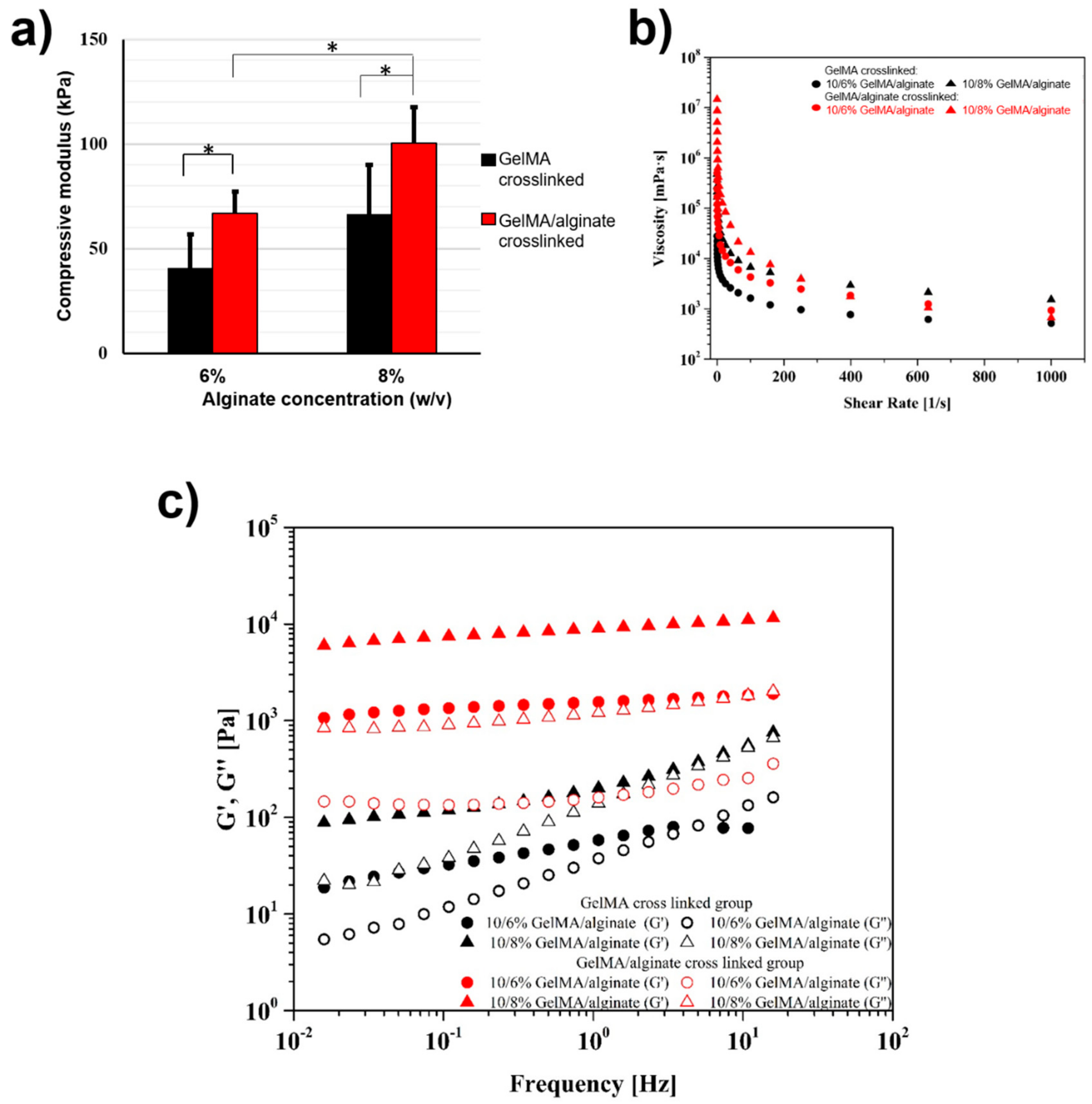
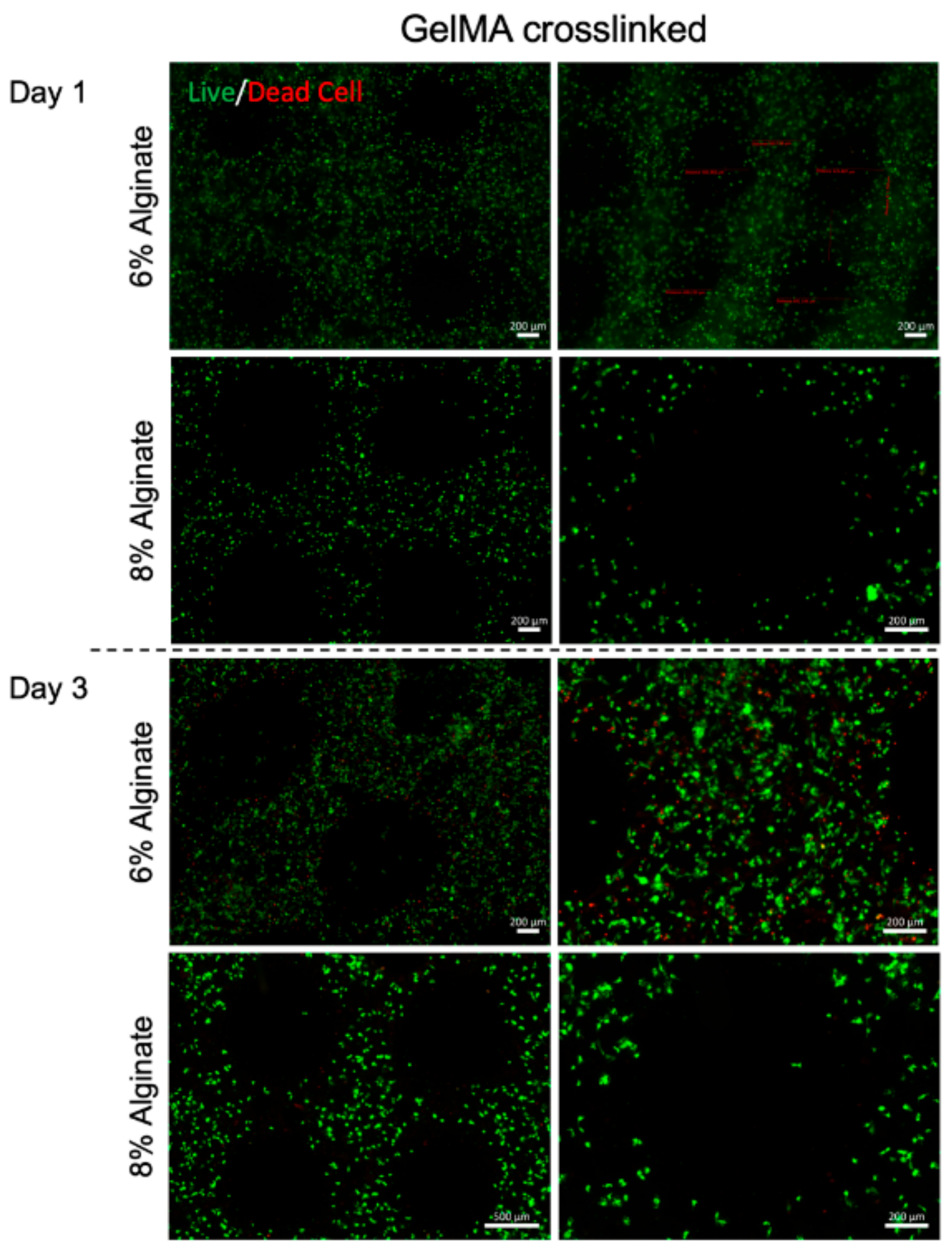
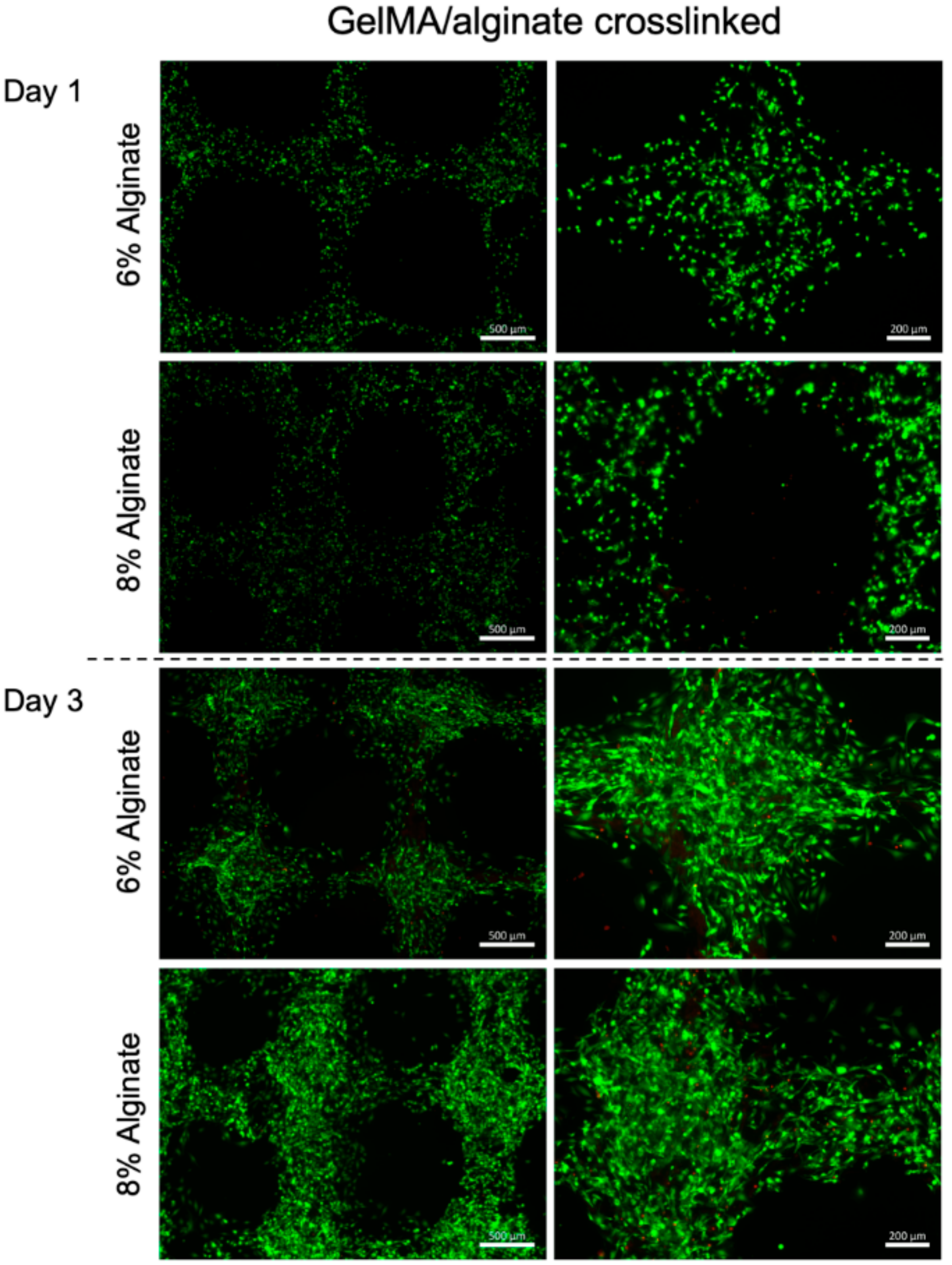
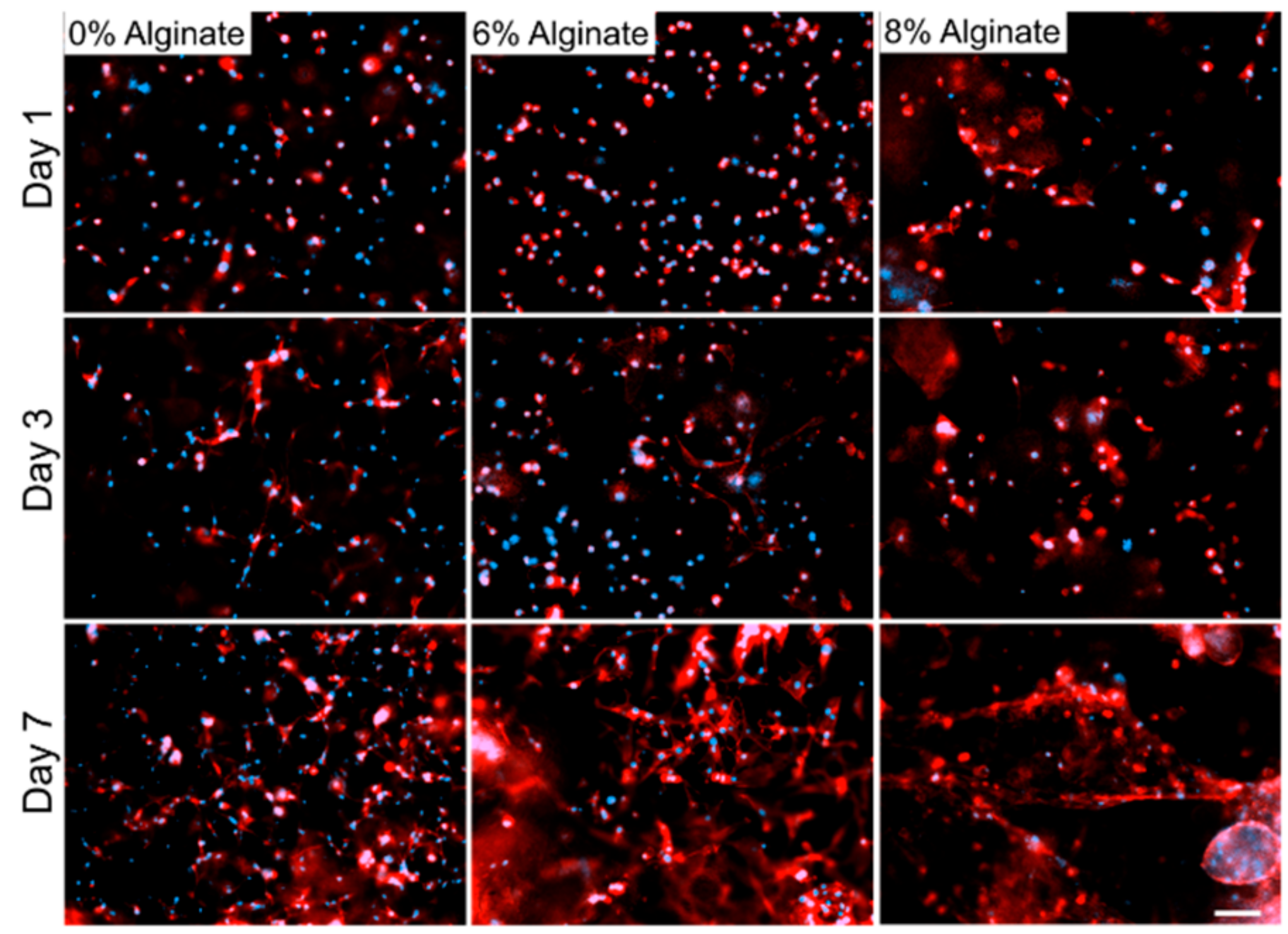
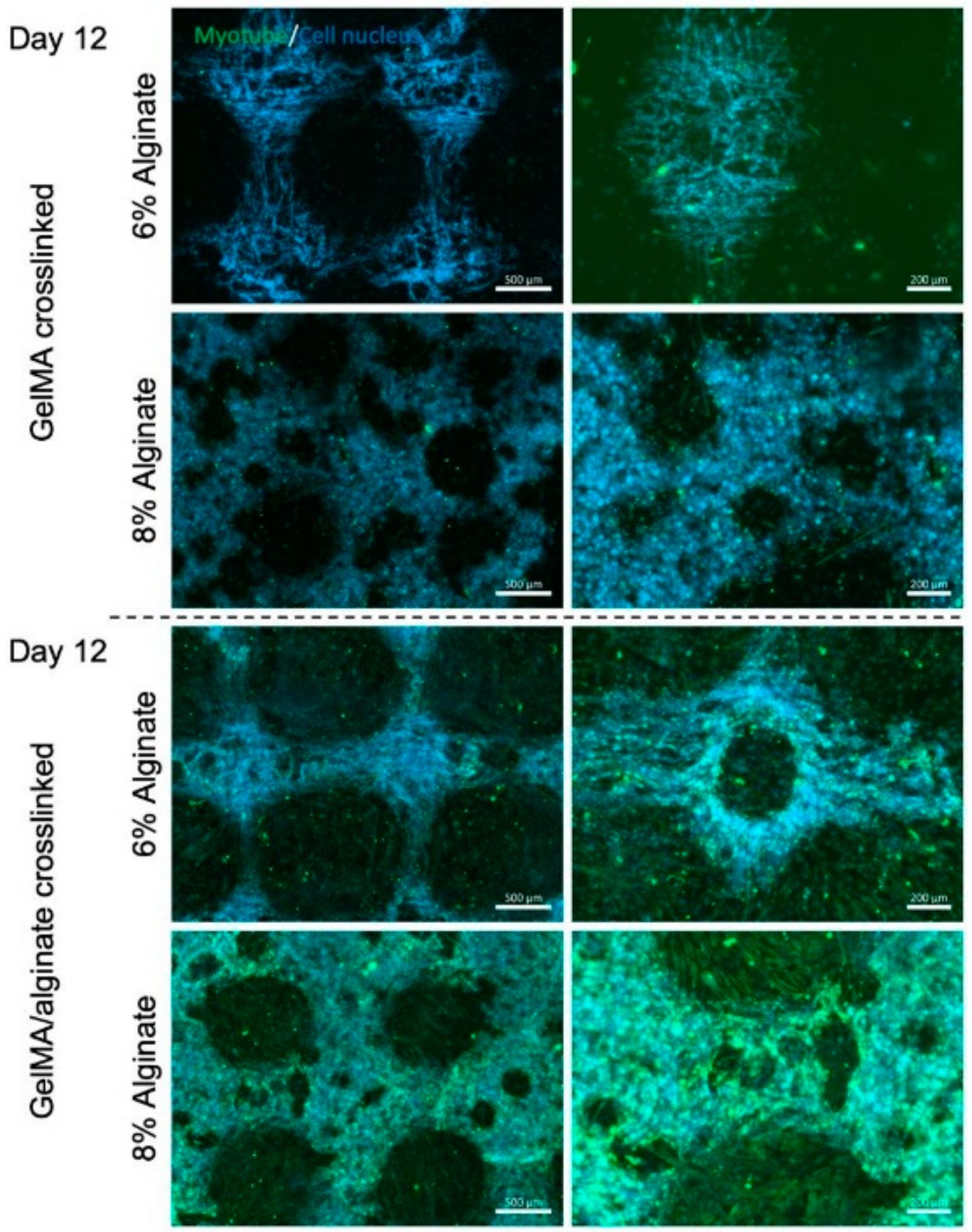
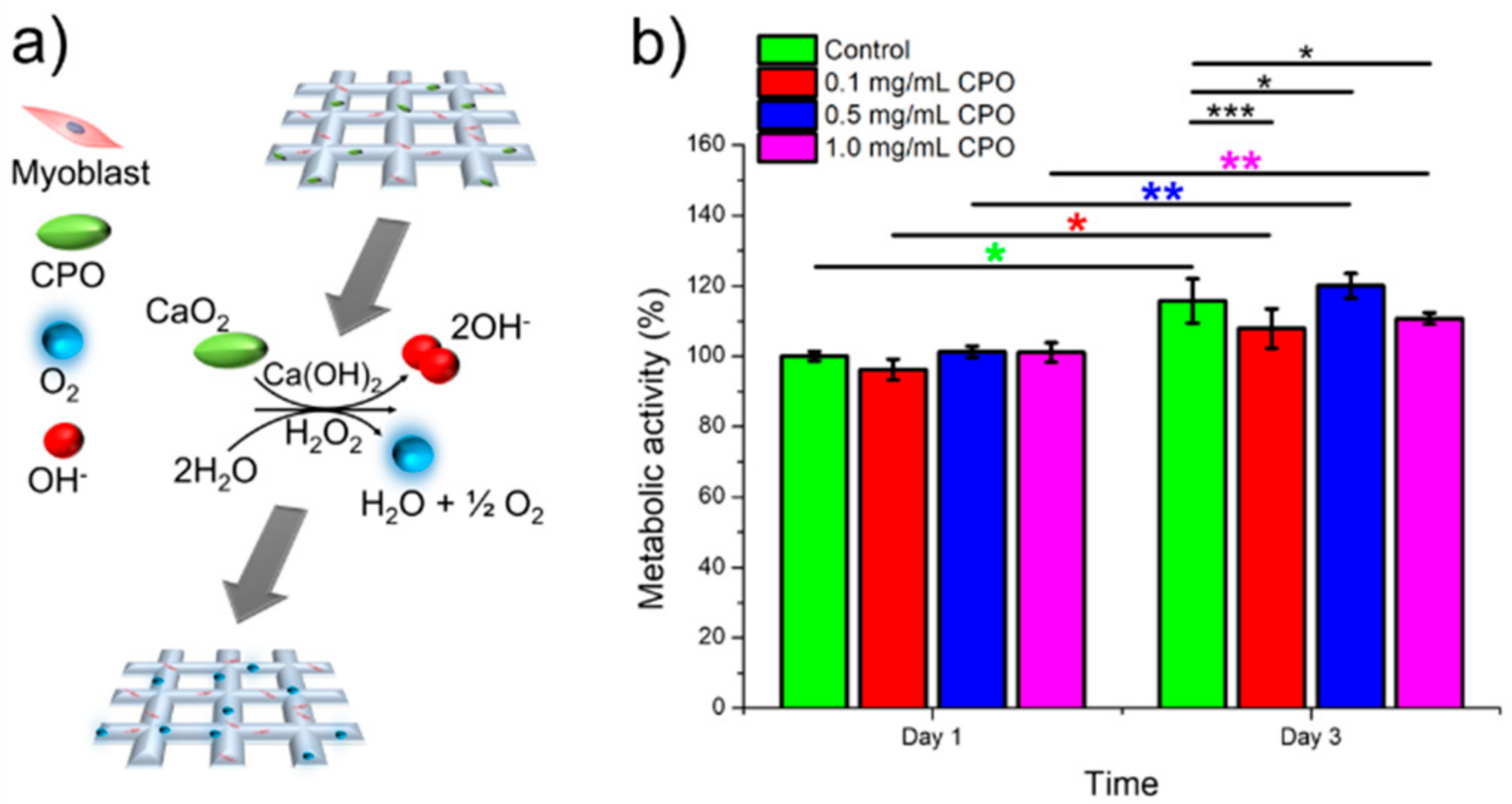
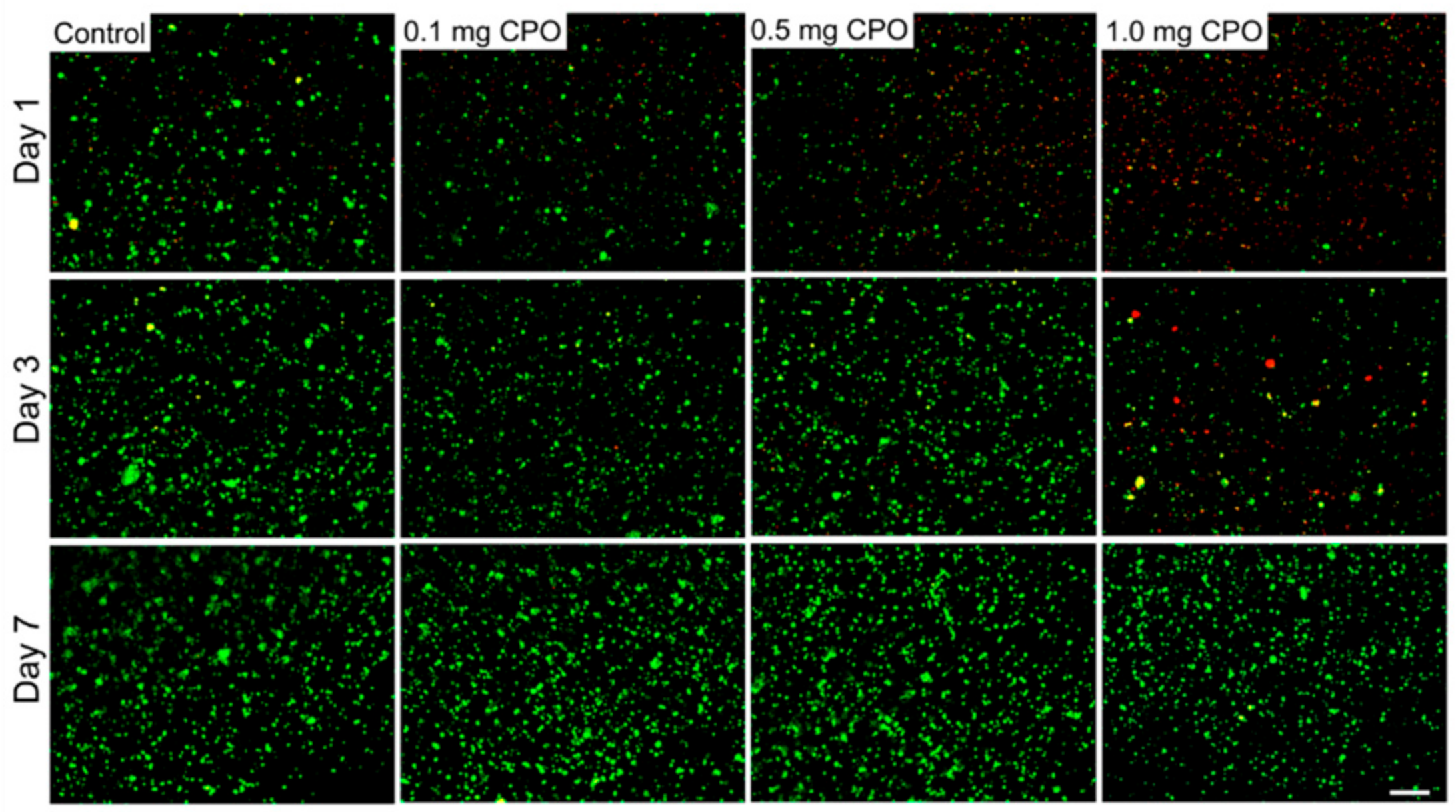
3. Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrovidov, S.; Hosseini, V.; Ahadian, S.; Fujie, T.; Parthiban, S.P.; Ramalingam, M.; Bae, H.; Kaji, H.; Khademhosseini, A. Skeletal muscle tissue engineering: Methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 2014, 20, 403–436. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Liang, X.; Ostrovidov, S.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; Bae, H.; et al. Hybrid hydrogels containing vertically aligned carbon nanotubes with anisotropic electrical conductivity for muscle myofiber fabrication. Sci. Rep. 2014, 4, 4271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beldjilali-Labro, M.; Garcia, A.; Farhat, F.; Bedoui, F.; Grosset, J.F.; Dufresne, M.; Legallais, C. Biomaterials in tendon and skeletal muscle tissue engineering: Current trends and challenges. Materials 2018, 11, 1116. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, V.; Ahadian, S.; Ostrovidov, S.; Camci-Unal, G.; Chen, S.; Kaji, H.; Ramalingam, M.; Khademhosseini, A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng. Part A 2012, 18, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Ostrovidov, S.; Ahadian, S.; Ramón-Azcón, J.; Hosseini, V.; Fujie, T.; Parthiban, S.P.; Shiku, H.; Matsue, T.; Kaji, H.; Ramalingam, M.; et al. Three-dimensional co-culture of C2C12/PC12 cells improves skeletal muscle tissue formation and function. J. Tissue Eng. Regen. Med. 2017, 11, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Ramón-Azcón, J.; Estili, M.; Obregon, R.; Shiku, H.; Matsue, T. Facile and rapid generation of 3D chemical gradients within hydrogels for high-throughput drug screening applications. Biosens. Bioelectron. 2014, 59, 166–173. [Google Scholar] [CrossRef]
- Ahadian, S.; Ramón-Azcón, J.; Chang, H.; Liang, X.; Kaji, H.; Shiku, H.; Nakajima, K.; Ramalingam, M.; Wu, H.; Matsue, T.; et al. Electrically regulated differentiation of skeletal muscle cells on ultrathin graphene-based FILMS. RSC Adv. 2014, 4, 9534–9541. [Google Scholar] [CrossRef]
- Monico, M.D.; Tahriri, M.; Fahmy, M.D.; Ghassemi, H.; Vashaee, D.; Tayebi, L. Cartilage and facial muscle tissue engineering and regeneration: A mini review. Bio Des. Manuf. 2018, 1, 115–122. [Google Scholar] [CrossRef]
- Bartolacci, J.; Dziki, J.; Badylak, S.F. Scaffolds for skeletal muscle tissue engineering. In Handbook of Tissue Engineering Scaffolds; Woodhead Publishing: Cambridge, UK, 2019; Volume 1, pp. 245–258. [Google Scholar]
- Ahadian, S.; Khademhosseini, A. A perspective on 3D bioprinting in tissue regeneration. Bio-Des. Manuf. 2018, 1, 157–160. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Zengjie, F.; Suthiwanich, K.; Lorestani, F.; Orive, G.; Ostrovidov, S.; Khademhosseini, A. Advances and future perspectives in 4D bioprinting. Biotechnol. J. 2018, 13, e1800148. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Darabi, M.A.; El Tahchi, M.; Lee, J.; Suthiwanich, K.; Sheikhi, A.; Dokmeci, M.R.; Oklu, R.; Khademhosseini, A. Minimally invasive and regenerative therapeutics. Adv. Mater. 2019, 31, e1804041. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Civitarese, R.; Bannerman, D.; Mohammadi, M.H.; Lu, R.; Wang, E.; Davenport-Huyer, L.; Lai, B.; Zhang, B.; Zhao, Y.; et al. Organ-on-a-chip platforms: A convergence of advanced materials, cells, and microscale technologies. Adv. Healthc. Mater. 2018, 7, 1700506. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Moses, J.C.; Bandyopadhyay, A.; Mandal, B.B. 3D printing/bioprinting based tailoring of in vitro tissue models: Recent advances and challenges. ACS Appl. Bio Mater. 2019, 2, 1385–1405. [Google Scholar] [CrossRef]
- Kim, J.H.; Seol, Y.J.; Ko, I.K.; Kang, H.W.; Lee, Y.K.; Yoo, J.J.; Atala, A.; Lee, S.J. 3D bioprinted human skeletal muscle constructs for muscle function restoration. Sci. Rep. 2018, 8, 12307. [Google Scholar] [CrossRef] [PubMed]
- Mozetic, P.; Giannitelli, S.M.; Gori, M.; Trombetta, M.; Rainer, A. Engineering muscle cell alignment through 3D bioprinting. J. Biomed. Mater. Res. A 2017, 105, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Costantini, M.; Testa, S.; Mozetic, P.; Barbetta, A.; Fuoco, C.; Fornetti, E.; Tamiro, F.; Bernardini, S.; Jaroszewicz, J.; Swieszkowski, W.; et al. Microfluidic-enhanced 3D bioprinting of aligned myoblast-laden hydrogels leads to functionally organized myofibers in vitro and in vivo. Biomaterials 2017, 131, 98–110. [Google Scholar] [CrossRef]
- Testa, S.; Fuoco, C.; Costantini, M.; Belli, R.; Fascetti Leon, F.; Vitiello, L.; Rainer, A.; Cannata, S.; Gargioli, C. Designing a 3D printed human derived artificial myo-Structure for anal sphincter defects in anorectal malformations and adult secondary damage. Mater. Today Commun. 2018, 15, 120–123. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Ahadian, S.; Xu, C.; Montazerian, H.; Ko, H.; Nasiri, R.; Barros, N.; Khademhosseini, A. Bioinks and bioprinting technologies to make heterogeneous and biomimetic tissue constructs. Mater. Today Bio. 2019, 1, 100008. [Google Scholar] [CrossRef]
- Ramón-Azcón, J.; Ahadian, S.; Estili, M.; Liang, X.; Ostrovidov, S.; Kaji, H.; Shiku, H.; Ramalingam, M.; Nakajima, K.; Sakka, Y.; et al. Dielectrophoretically aligned carbon nanotubes to control electrical and mechanical properties of hydrogels to fabricate contractile muscle myofibers. Adv. Mater. 2013, 25, 4028–4034. [Google Scholar] [CrossRef]
- Ahadian, S.; Ostrovidov, S.; Hosseini, V.; Kaji, H.; Ramalingam, M.; Bae, H.; Khademhosseini, A. Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis 2013, 9, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold nanocomposite bioink for printing 3D cardiac constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef] [PubMed]
- Ramón-Azcón, J.; Ahadian, S.; Obregon, R.; Camci-Unal, G.; Ostrovidov, S.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; et al. Gelatin methacrylate as a promising hydrogel for 3D microscale organization and proliferation of dielectrophoretically patterned cells. Lab Chip 2012, 12, 2959–2969. [Google Scholar] [CrossRef] [PubMed]
- Aviss, K.J.; Gough, J.E.; Downes, S. Aligned electrospun polymer fibres for skeletal muscle regeneration. Eur. Cells Mater. 2010, 19, 193–204. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, B. Electroactive 3D scaffolds based on silk fibroin and water-borne polyaniline for skeletal muscle tissue engineering. Macromol. Biosci. 2017, 17, 1700147. [Google Scholar] [CrossRef] [PubMed]
- Ahadian, S.; Ramón-Azcón, J.; Ostrovidov, S.; Camci-Unal, G.; Hosseini, V.; Kaji, H.; Ino, K.; Shiku, H.; Khademhosseini, A.; Matsue, T. Interdigitated array of Pt electrodes for electrical stimulation and engineering of aligned muscle tissue. Lab Chip 2012, 12, 3491–3503. [Google Scholar] [CrossRef]
- Gholipourmalekabadi, M.; Zhao, S.; Harrison, B.S.; Mozafari, M.; Seifalian, A.M. Oxygen-generating biomaterials: A new, viable paradigm for tissue engineering? Trends Biotechnol. 2016, 34, 1010–1021. [Google Scholar] [CrossRef]
- Menon, N.V.; Chuah, Y.J.; Cao, B.; Lim, M.; Kang, Y. A microfluidic co-culture system to monitor Tumor-Stromal interactions on a chip. Biomicrofluidics 2014, 8, 064118. [Google Scholar] [CrossRef]
- Abdi, S.I.H.; Ng, S.M.; Lim, J.O. An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. Int. J. Pharm. 2011, 409, 203–205. [Google Scholar] [CrossRef]
- Tang, Y.L.; Zhu, W.; Cheng, M.; Chen, L.; Zhang, J.; Sun, T.; Kishore, R.; Phillips, M.I.; Losordo, D.W.; Qin, G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ. Res. 2009, 104, 1209–1216. [Google Scholar] [CrossRef]


| Fixed Parameters | Value | Note |
|---|---|---|
| GelMA percentage (w/v) | 10 | - |
| Printing speed (mm/s) | 60 | - |
| Printing temperature (°C) | 21 | - |
| Variable Parameters | - | - |
| Alginate percentage (w/v) | 6–8 | - |
| Post crosslinking mechanism a | UV light 30 s | 0.1 M CaCl2 bath (2 min) followed by exposing to UV light 30 s |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seyedmahmoud, R.; Çelebi-Saltik, B.; Barros, N.; Nasiri, R.; Banton, E.; Shamloo, A.; Ashammakhi, N.; Dokmeci, M.R.; Ahadian, S. Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines 2019, 10, 679. https://doi.org/10.3390/mi10100679
Seyedmahmoud R, Çelebi-Saltik B, Barros N, Nasiri R, Banton E, Shamloo A, Ashammakhi N, Dokmeci MR, Ahadian S. Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines. 2019; 10(10):679. https://doi.org/10.3390/mi10100679
Chicago/Turabian StyleSeyedmahmoud, Rasoul, Betül Çelebi-Saltik, Natan Barros, Rohollah Nasiri, Ethan Banton, Amir Shamloo, Nureddin Ashammakhi, Mehmet Remzi Dokmeci, and Samad Ahadian. 2019. "Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks" Micromachines 10, no. 10: 679. https://doi.org/10.3390/mi10100679
APA StyleSeyedmahmoud, R., Çelebi-Saltik, B., Barros, N., Nasiri, R., Banton, E., Shamloo, A., Ashammakhi, N., Dokmeci, M. R., & Ahadian, S. (2019). Three-Dimensional Bioprinting of Functional Skeletal Muscle Tissue Using Gelatin Methacryloyl-Alginate Bioinks. Micromachines, 10(10), 679. https://doi.org/10.3390/mi10100679









