Real-Time Single Cell Monitoring: Measurement and Counting of Motile Sperm Using LCR Impedance-Integrated Microfluidic Device
Abstract
:1. Introduction
2. Materials and Methods
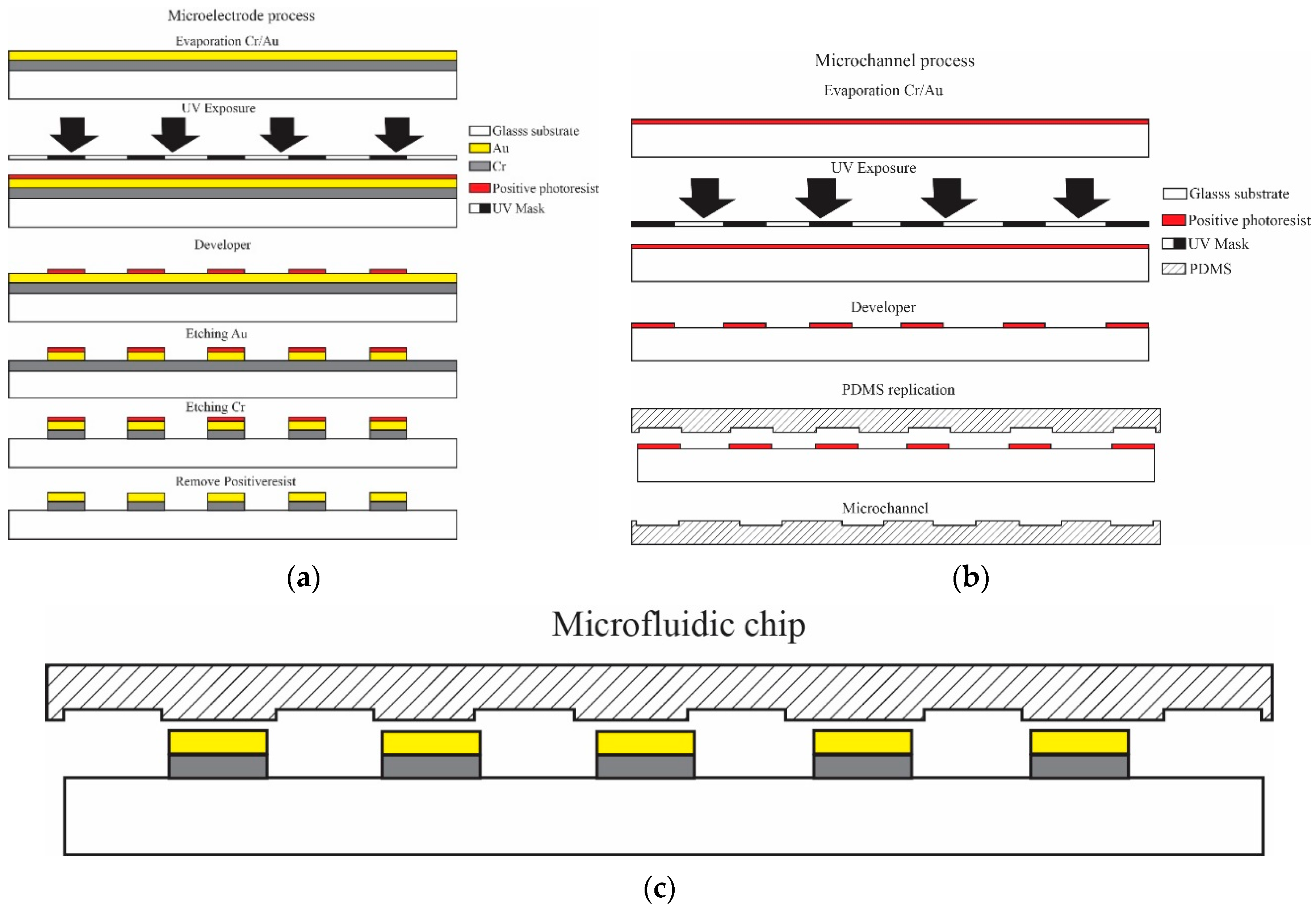
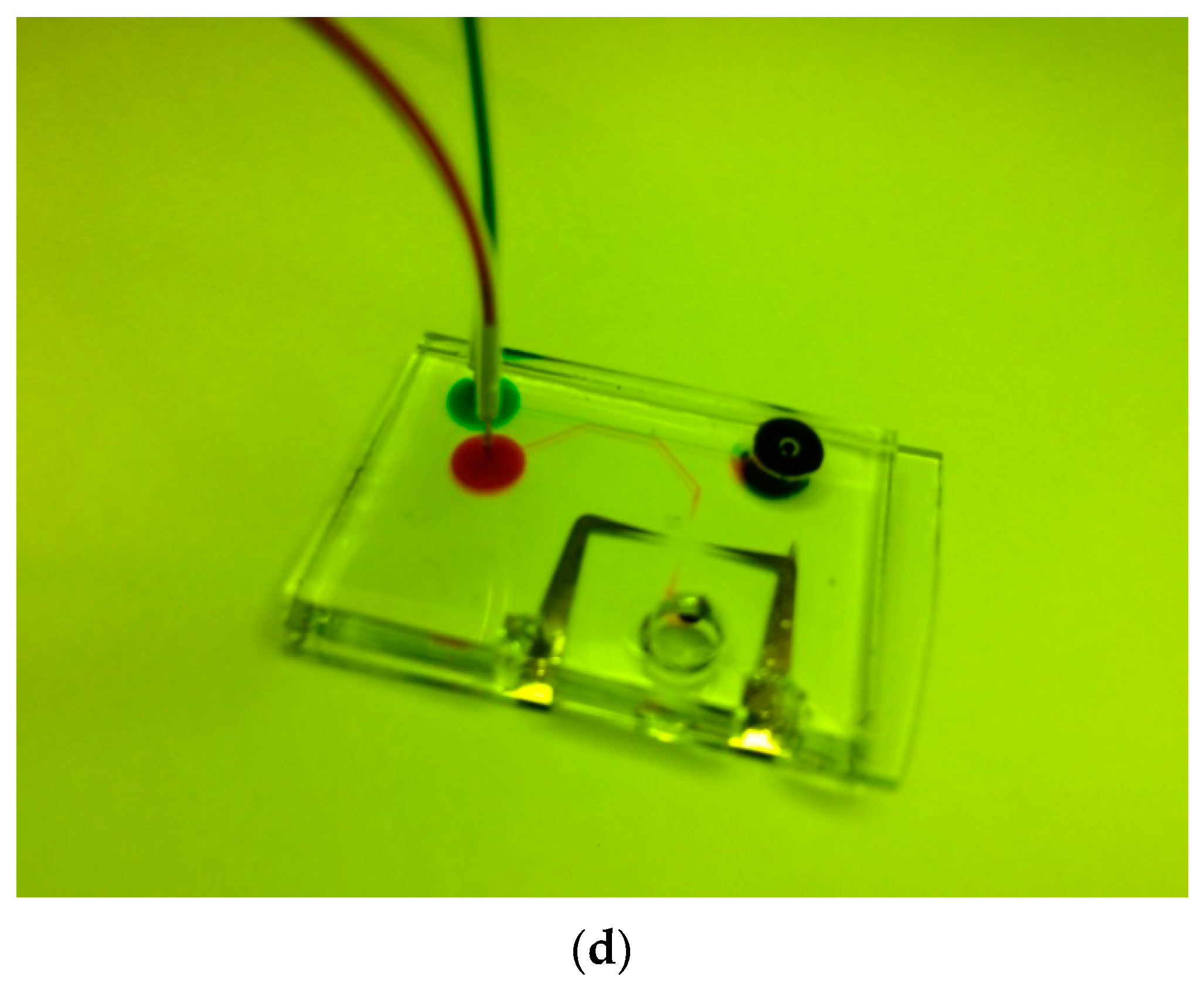
2.1. Design and Fabrication
2.2. Sample
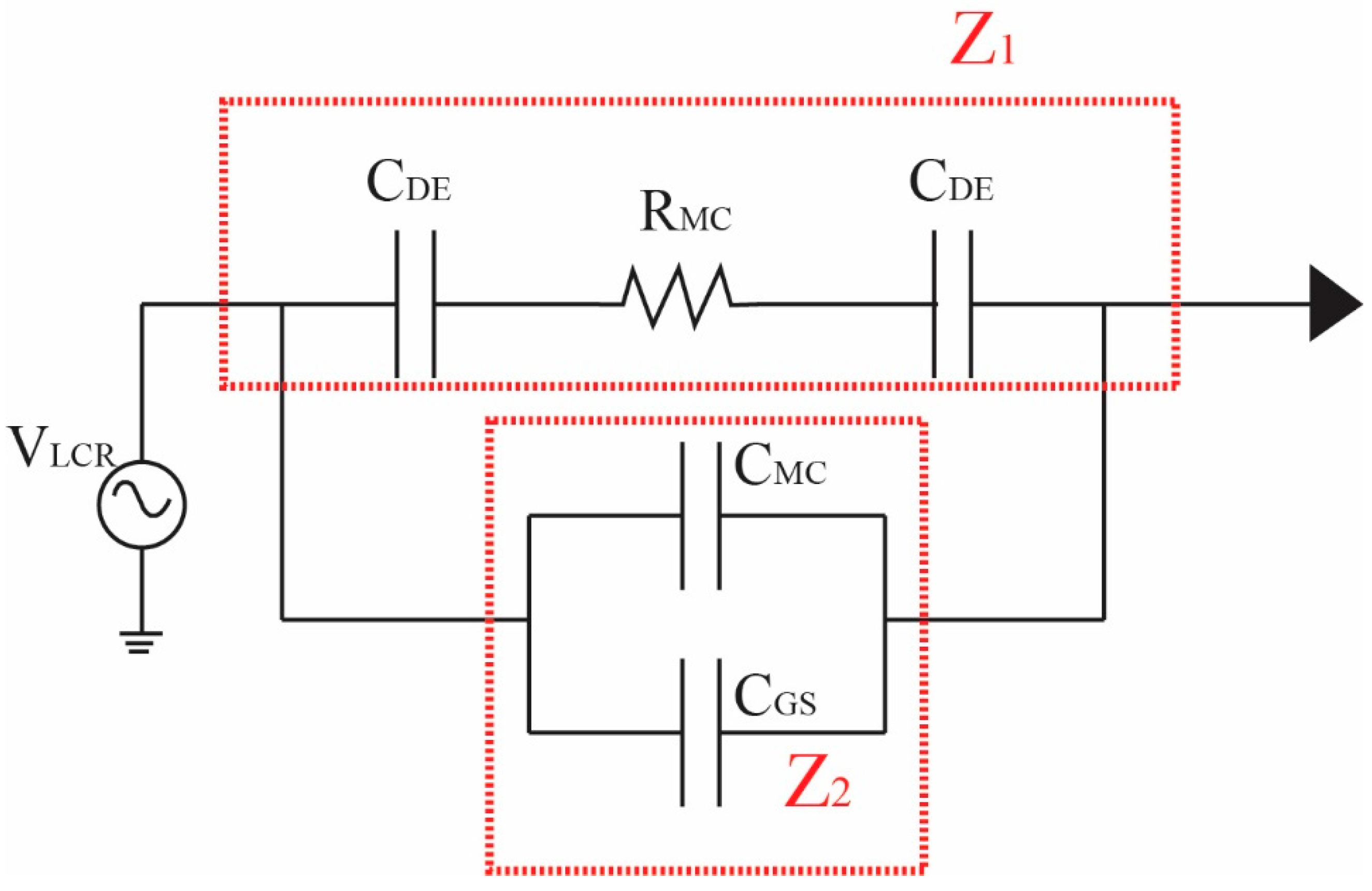
2.3. Impedance Detection and Analysis
2.3.1. Overview of LCR Impedance Program
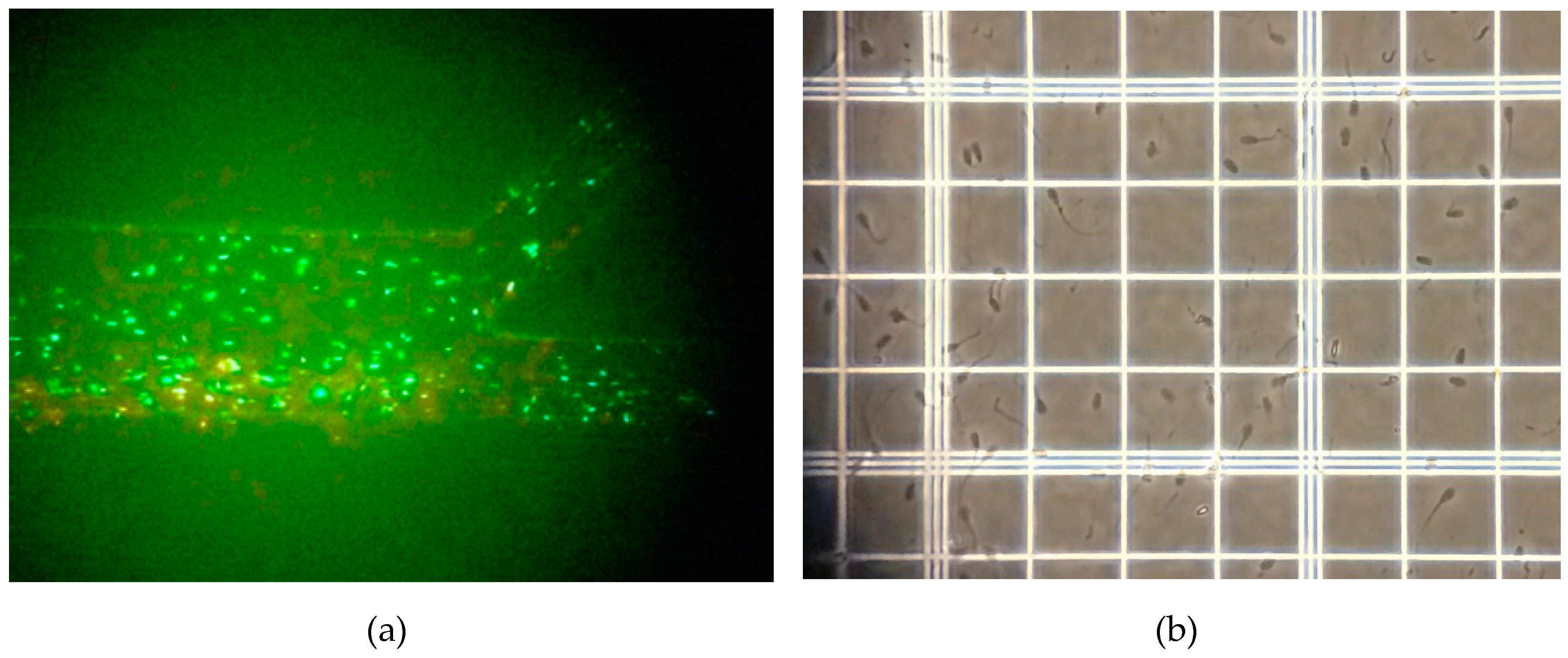
2.3.2. Detection Method for LCR Impedance and Counting Spermatozoa
2.3.3. Programming and GUI
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Frey, K.A. Male reproductive health and infertility. Prim. Care 2010, 37, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Raymond, J.G. Women as Wombs: Reproductive Technologies and the Battle over Women’s Freedom; Spinifex Press: North Geelong, Australia, 1993. [Google Scholar]
- Steptoe, P.C.; Edwards, R.G. Birth after the reimplantation of a human embryo. Lancet 1978, 312, 366. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Zini, A.; Finelli, A.; Phang, D.; Jarvi, K.A. Influence of semen processing technique on human sperm DNA integrity. Urology 2000, 56, 1081–1084. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; McClure, N.; Lewis, S.E.M. The effects of cryopreservation on sperm morphology, motility and mitochondrial function. Hum. Reprod. Update 2002, 17, 704–709. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.; Pappas, D. Recent advances in microfluidic cell separations. Analyst 2013, 138, 4714. [Google Scholar] [CrossRef]
- Zheng, Y.; Nguyen, J.; Wei, Y.; Sun, Y. Recent advances in microfluidic techniques for single-cell biophysical characterization. Lab Chip 2013, 13, 2464. [Google Scholar] [CrossRef]
- Haandbæk, N.; Bürgel, S.C.; Heer, F.; Hierlemann, A. Characterization of subcellular morphology of single yeast cells using high frequency microfluidic impedance cytometer. Lab Chip 2014, 14, 369. [Google Scholar] [CrossRef]
- Seger, U.; Gawad, S.; Johann, R.; Bertsch, A.; Renaud, P. Cell immersion and cell dipping in microfluidic devices. Lab Chip 2004, 4, 148. [Google Scholar] [CrossRef]
- Fitzpatrick, E.; McBride, S.; Yavelow, J.; Najmi, S.; Zanzucchi, P.; Wieder, R. Microfluidic techniques for single-cell protein expression analysis. Clin. Chem. 2006, 52, 1080. [Google Scholar] [CrossRef] [PubMed]
- Shields IV, C.W.; Reyesab, C.D.; López, G.P. Microfluidic cell sorting: A review of the advances in the separation of cells from debulking to rare cell isolation. Lab Chip 2015, 15, 1230–1249. [Google Scholar] [CrossRef] [PubMed]
- Giannitsis, A.T. Microfabrication of biomedical lab-on-chip devices. A review. Est. J. Eng. 2011, 17, 109–139. [Google Scholar] [CrossRef]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nature 2006, 442, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Yoon, D.S.; Kim, S.K.; Kim, T.S.; Kim, S.; Pak, E.Y.; No, K. AC frequency characteristics of coplanar impedance sensors as design parameters. Lab Chip 2005, 5, 270. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance biosensors applications to sustainability and remaining technical challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- K’Owino, I.O.; Sadik, A.O. Impedance spectroscopy a powerful tool for rapid biomolecular screening and cell culture monitoring. Electroanalysis 2005, 17, 2101. [Google Scholar] [CrossRef]
- Wagner, T.; Lazar, J.; Schnakenberg, U.; Böker, A. In situ electrochemical impedance spectroscopy of electrostatically driven selective gold nanoparticle adsorption on block copolymer lamellae. ACS Appl. Mater. Interfaces 2016, 8, 27282–27290. [Google Scholar] [CrossRef]
- Lazar, J.; Rosencrantz, R.R.; Elling, L.; Schnakenberg, U. Simultaneous electrochemical impedance spectroscopy and localized surface plasmon resonance in a microfluidic chip: New insights into the spatial origin of the signal. Anal. Chem. 2016, 88, 9590–9596. [Google Scholar] [CrossRef]
- Lazar, J.; Park, H.; Rosencrantz, R.R.; Böker, A.; Elling, L.; Schnakenberg, U. Evaluating the thickness of multivalent glycopolymer brushes for lectin binding. Macromol. Rapid Commun. 2015, 36, 1472. [Google Scholar] [CrossRef]
- Sun, T.; Morgan, H. Single-cell microfluidic impedance cytometry: A review. Microfluid. Nanofluid. 2010, 8, 423–443. [Google Scholar] [CrossRef]
- Hong, J.-H.; Lan, K.-C.; Jang, L.-S. Electrical characteristics analysis of various cancer cells using a microfluidic device based on single-cell impedance measurement. Sens. Actuators B 2012, 173, 927–934. [Google Scholar] [CrossRef]
- Jung, W.; Han, J.; Choi, J.-W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2015, 132, 46–57. [Google Scholar] [CrossRef]
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectron. Eng. 2002, 61–62, 907–914. [Google Scholar] [CrossRef]
- Gawad, S.; Schild, L.; Renaud, P. Micromachined impedance spectroscopy flow cytometer for cell analysis and particle sizing. Lab Chip 2001, 1, 76–82. [Google Scholar] [CrossRef]
- de Wagenaar, B.; Geijs, D.J.; de Boer, H.; Bomer, J.G.; Olthuis, W.; van den Berg, A.; Segerink, L.I. Spermometer: Electrical characterization of single boar sperm motility. Fertil. Steril. 2016, 106, 773–780. [Google Scholar] [CrossRef] [PubMed]
- de Wagenaar, B.; Dekker, S.; de Boer, H.; Bomer, J.G.; Olthuisa, W.; van den Berg, A.; Segerink, L.I. Towards microfluidic sperm refinement: Impedance-based analysis and sorting of sperm cells. Lab Chip 2016, 16, 1514–1522. [Google Scholar] [CrossRef]
- Chung, Y.; Zhu, X.; Gu, W.; Smith, G.D.; Takayama, S. Microscale Integrated Sperm Sorter. Microfluid. Tech. 2006, 321, 227–244. [Google Scholar]
- Shields IV, C.W.; Ohiri, K.A.; Szott, L.M.; Lopez, G.P. Translating microfluidics: Cell separation technologies and their barriers to commercialization. Cytometry B 2017, 92B, 115–125. [Google Scholar] [CrossRef]
- Salisbury, G.W.; Lodge, J.R.; Vandemark, N.L. Physiology of Reproduction and Artificial Insemination of Cattle, 2nd ed.; W.H. Freeman and Company: San Francisco, CA, USA, 1978. [Google Scholar]
- Danie, J.C., Jr. Methods in Mammalian Reproduction; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]

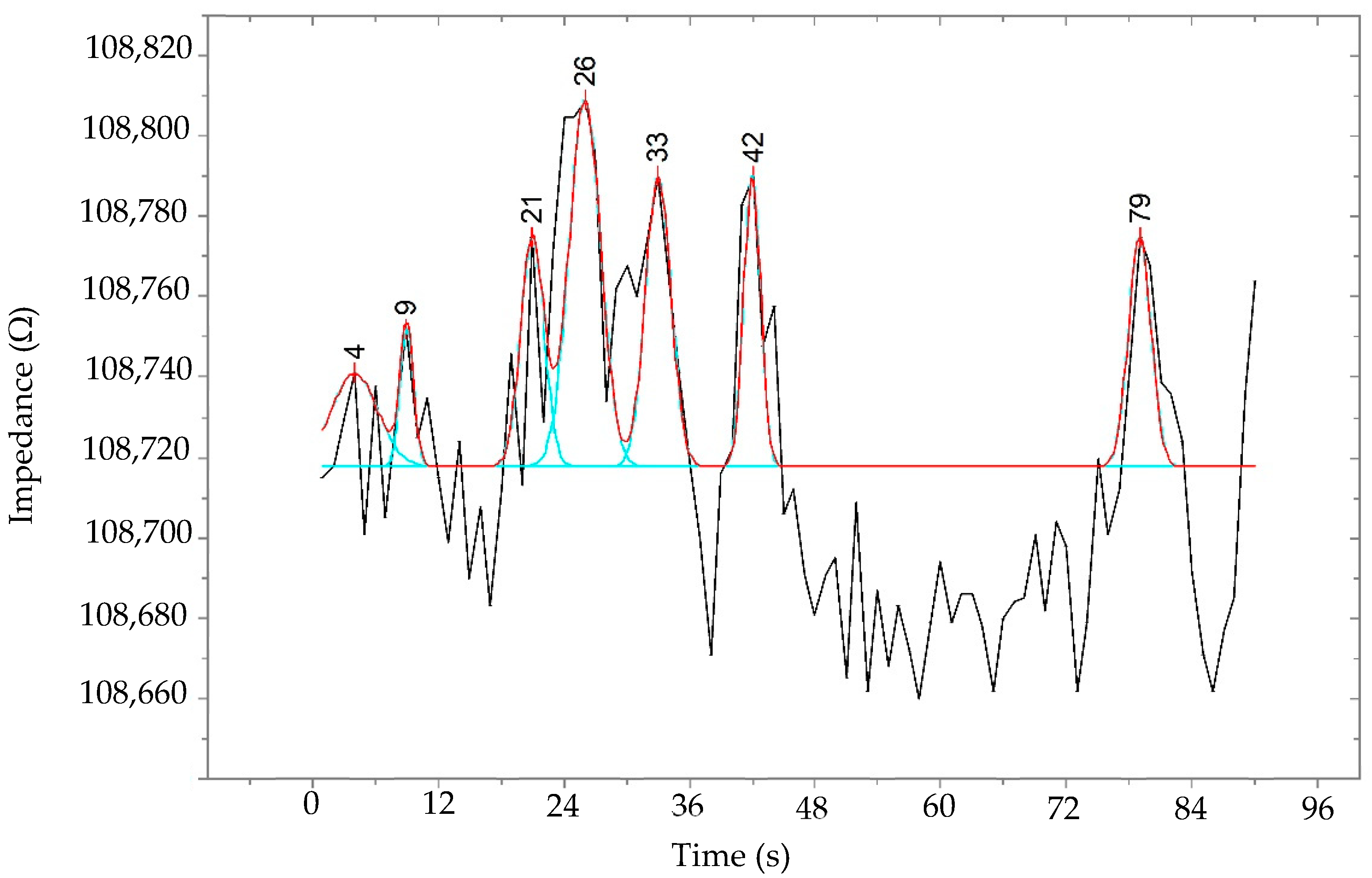
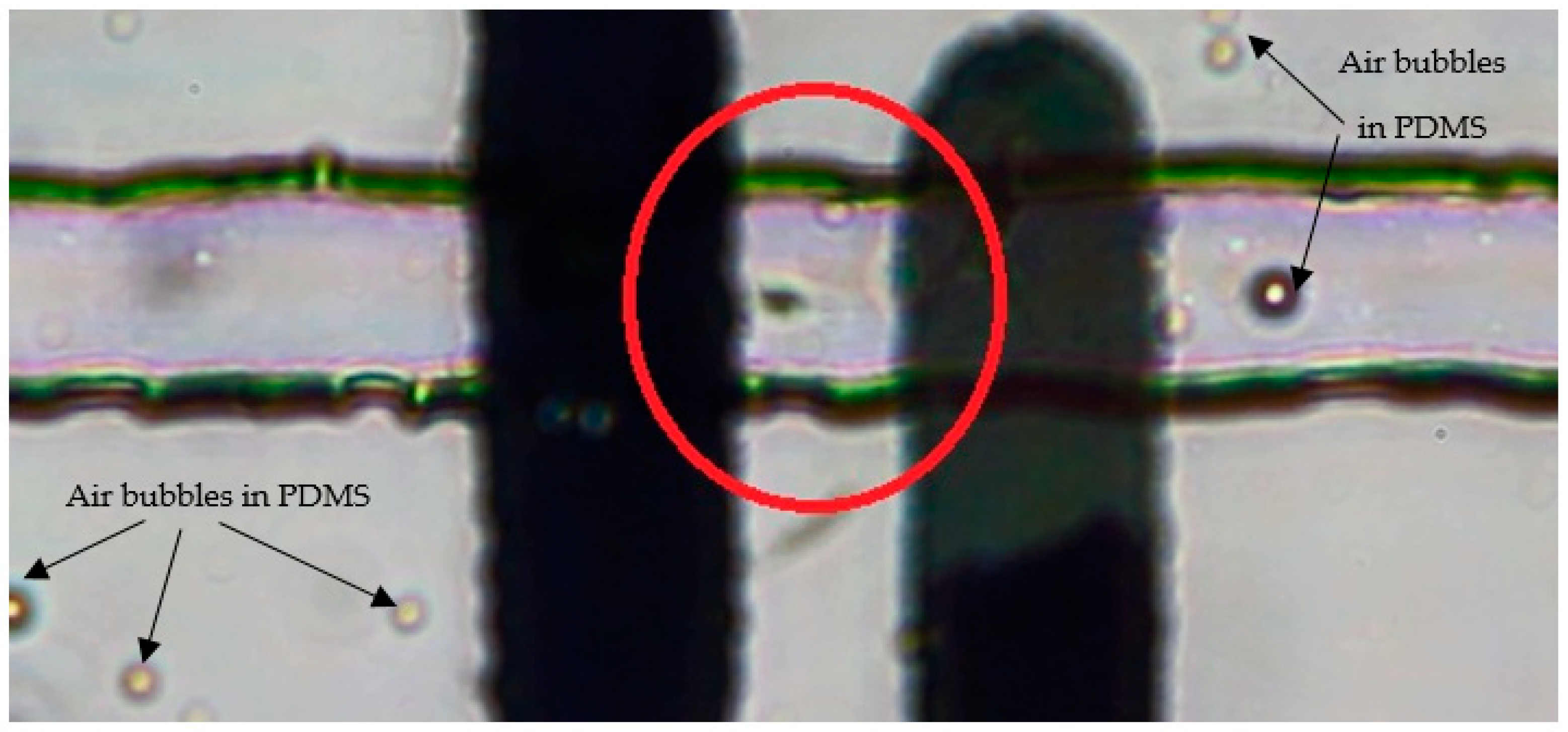
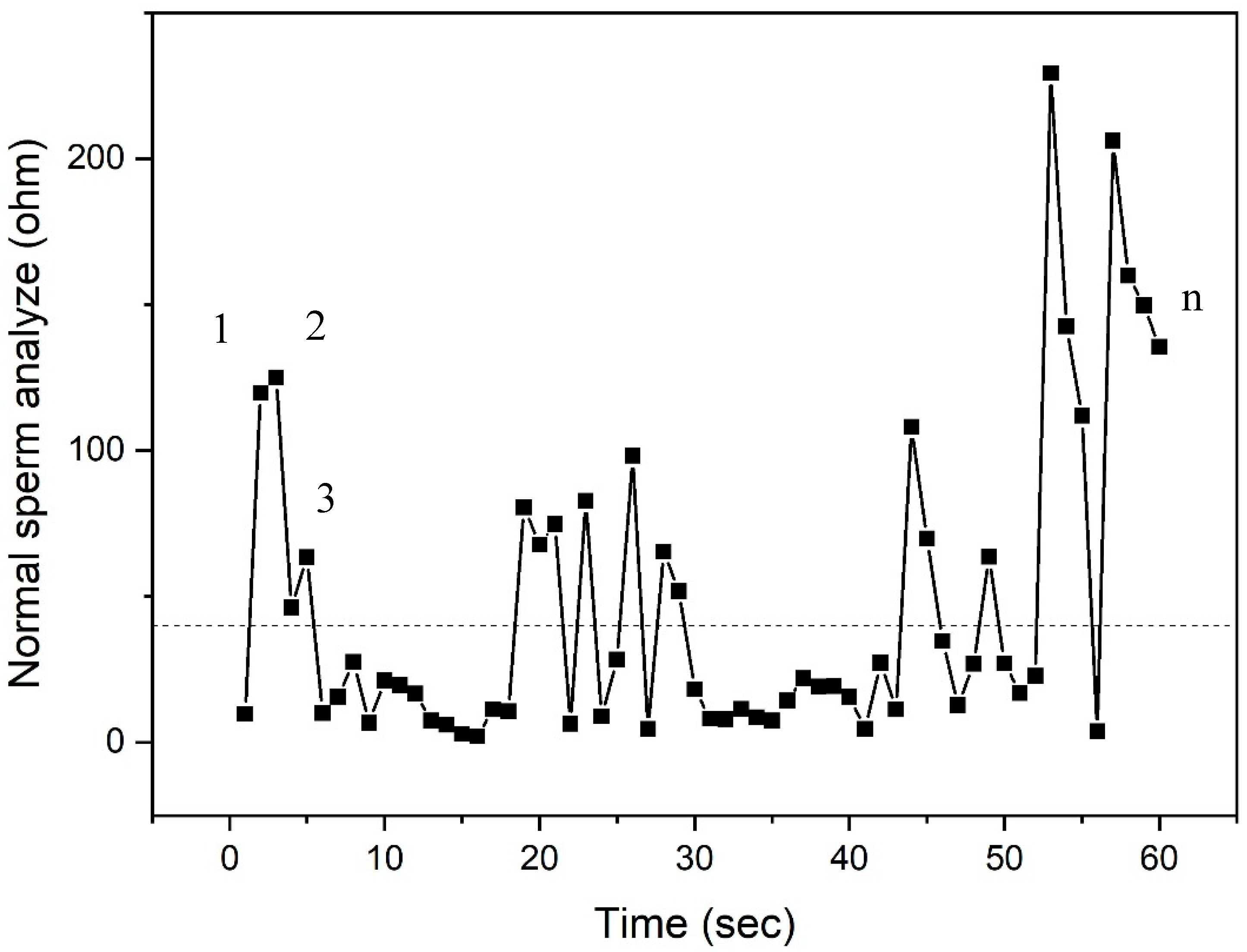

| Source | DF | SS | MS | F | p |
|---|---|---|---|---|---|
| Factor | 1 | 4,435,877,635 | 24,435,877,635 | 20,697.51 | 0.000 |
| Error | 6 | 7,083,715 | 1,180,619 | - | - |
| Total | 7 | 24,442,961,351 | - | - | - |
| S = 1087R2 = 99.97% R2 (adj) = 99.97% | |||||
| Sample | Baseline Impedance (kΩ) | Standard Error | Count/90 s |
|---|---|---|---|
| 1 | 112.48 | 1.76% | 37 |
| 2 | 110.49 | 0.05% | 42 |
| 3 | 110.47 | 0.06% | 32 |
| 4 | 108.72 | 1.65% | 40 |
| Average | 110.54 | 0.88% | 38 |
| Analysis | Volume (µL) | Time (min) | Total No. of Sperm Analyzed | Method | Validation (100%) |
|---|---|---|---|---|---|
| CASA | 10 | 15–20 | 517 | Image Programming | Reference Standard 100% |
| LCR Impedance | 10 | 5–10 | 488 | Difference in Impedance | 94.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phiphattanaphiphop, C.; Leksakul, K.; Phatthanakun, R.; Suthummapiwat, A. Real-Time Single Cell Monitoring: Measurement and Counting of Motile Sperm Using LCR Impedance-Integrated Microfluidic Device. Micromachines 2019, 10, 647. https://doi.org/10.3390/mi10100647
Phiphattanaphiphop C, Leksakul K, Phatthanakun R, Suthummapiwat A. Real-Time Single Cell Monitoring: Measurement and Counting of Motile Sperm Using LCR Impedance-Integrated Microfluidic Device. Micromachines. 2019; 10(10):647. https://doi.org/10.3390/mi10100647
Chicago/Turabian StylePhiphattanaphiphop, Chalinee, Komgrit Leksakul, Rungrueang Phatthanakun, and Apirak Suthummapiwat. 2019. "Real-Time Single Cell Monitoring: Measurement and Counting of Motile Sperm Using LCR Impedance-Integrated Microfluidic Device" Micromachines 10, no. 10: 647. https://doi.org/10.3390/mi10100647
APA StylePhiphattanaphiphop, C., Leksakul, K., Phatthanakun, R., & Suthummapiwat, A. (2019). Real-Time Single Cell Monitoring: Measurement and Counting of Motile Sperm Using LCR Impedance-Integrated Microfluidic Device. Micromachines, 10(10), 647. https://doi.org/10.3390/mi10100647






