Dextran as a Resorbable Coating Material for Flexible Neural Probes
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Materials
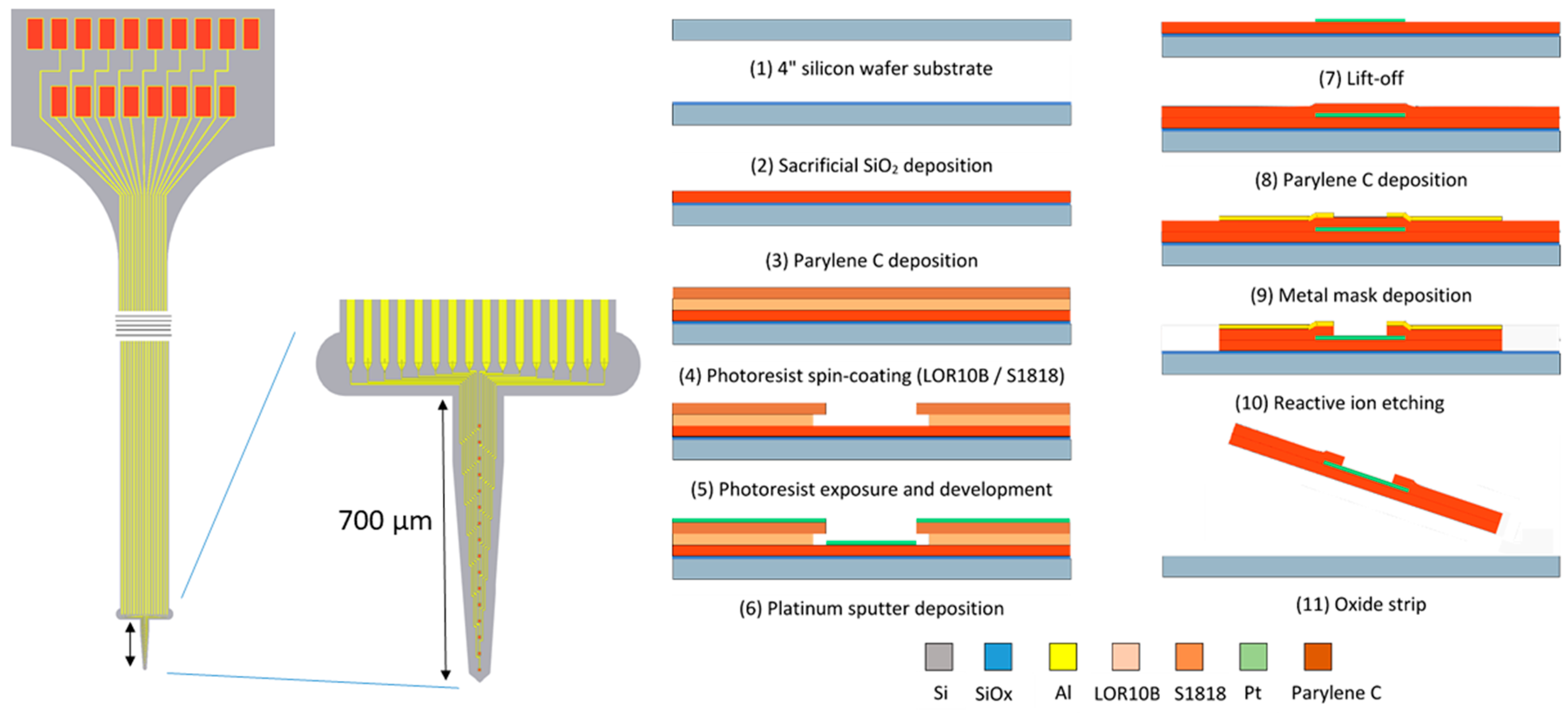
2.2. Electrode Design and Fabrication
- -
- The 4 inch silicon carrier wafers were thoroughly cleaned using piranha etchant (4 H2SO4:1 H2O2) to remove any organic contaminants. Afterwards, a HF-dip (2% HF) was performed followed by a rinsing cycle in DI water. The substrate was dried using purified nitrogen.
- -
- A 400 nm thin layer of silicon oxide was grown on the substrate using a wet thermal oxidation process. The oxide served as a sacrificial layer which aids in the final release of the devices from the carrier wafer in a later step.
- -
- A first insulation layer of Parylene-C was deposited, yielding a 7 µm thick layer.
- -
- The 400 nm thick Pt conductors were deposited by sputter coating on a lithographically patterned photoresist bilayer (LOR10B/S1818). The lift-off process was completed by soaking the wafers in n-methyl-2-pyrrolidone (NMP) overnight at room temperature.
- -
- A second layer of Parylene-C, with a thickness of 7 µm, was deposited using a similar process.
- -
- The device shape was defined by reactive ion etching (RIE) of the Parylene-C using an aluminum hard mask. The device outline was lithographically patterned using a negative photoresist on top of which a 40 nm thick layer of aluminum was thermally evaporated. After a lift-off step in acetone, the wafers were ready for RIE. As platinum is not etched by the RIE plasma, it also functions as an etch stop, which opens up the electrode contacts and bond pads.
- -
- The wafer was annealed at a temperature of 200 °C for 4 h (2 °C/min ramping rate) in a nitrogen atmosphere to relax any residual stress that was built up during the processing, as well as to prevent delamination [26].
- -
- Finally, the wafer was soaked in a 1% HF solution that removed the Al hard mask and underetched the sacrificial silicon oxide layer. After 1 h of etching, the adhesion between the Parylene-C electrode and the carrier wafer was reduced to such a level that the electrodes could be peeled off the carrier wafer using tweezers.
2.3. Micromechanical Characterization
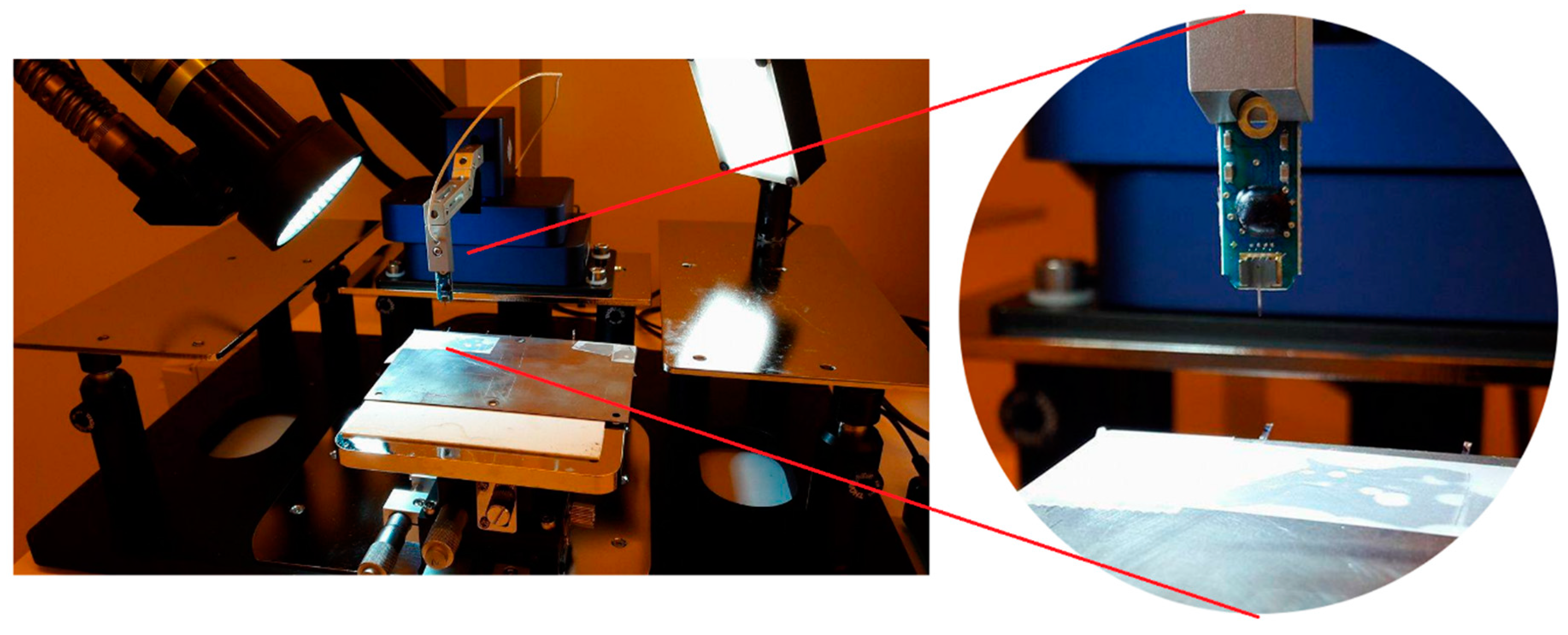
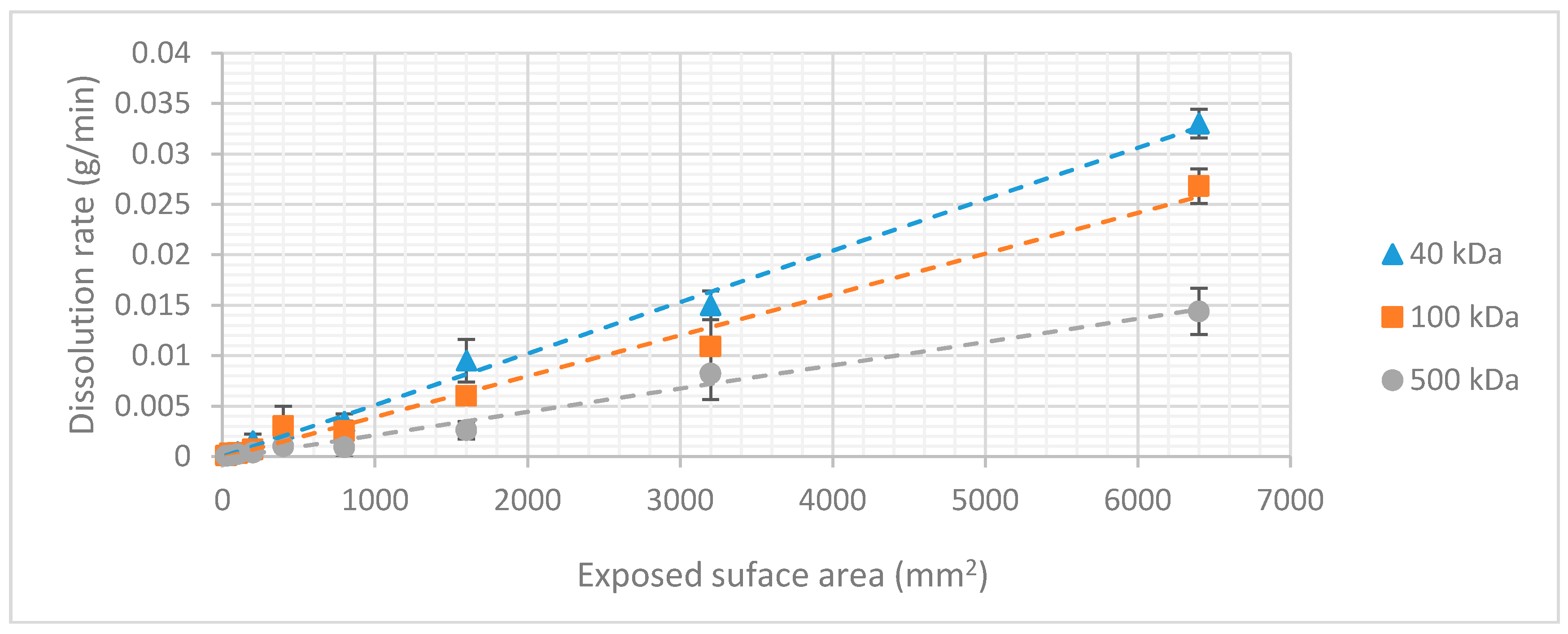
2.4. Dissolution Rate
2.5. In Vivo Experiment
2.5.1. Implantation Procedure
2.5.2. Perfusion
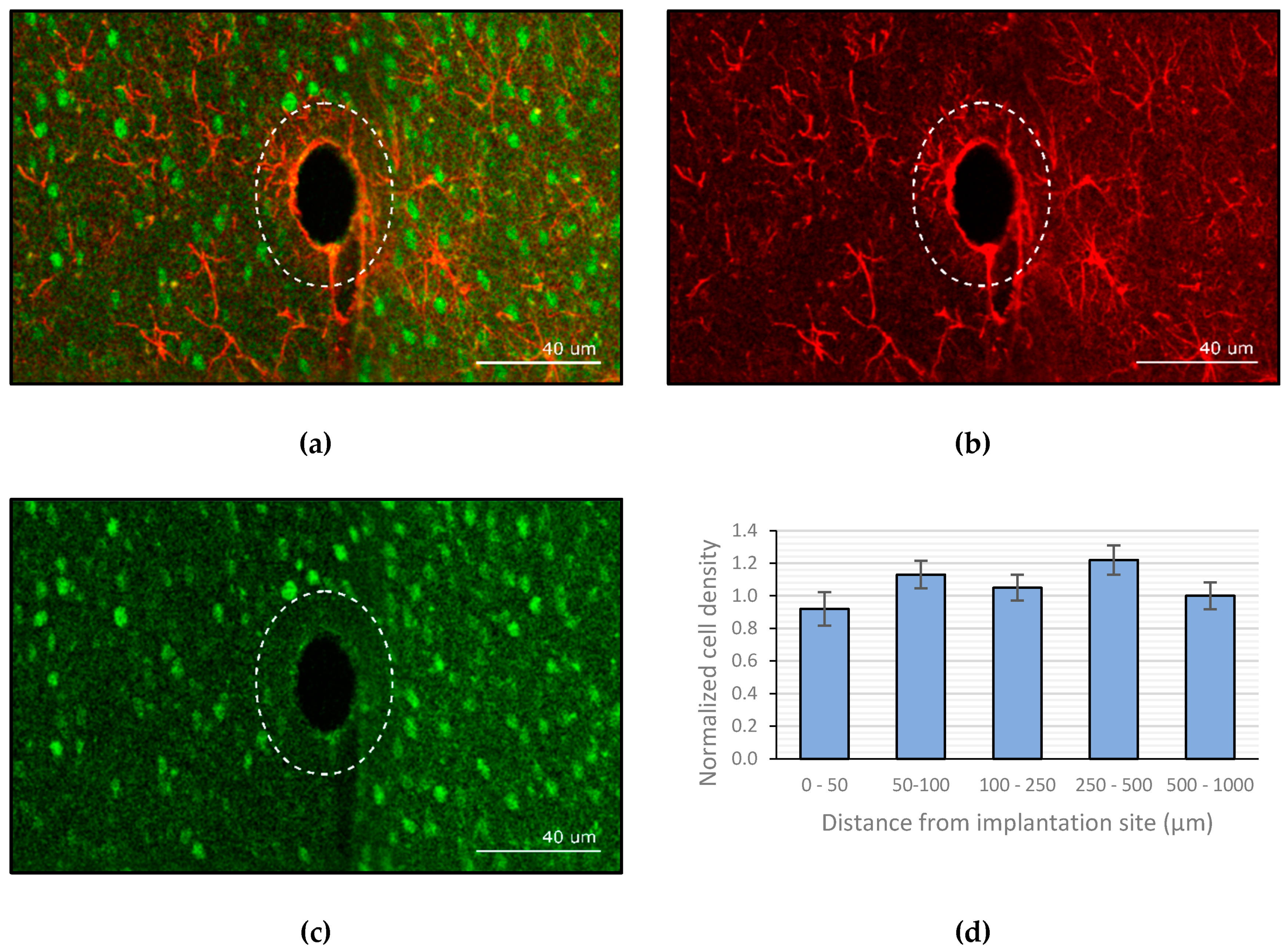
2.5.3. Histology
- -
- Overnight soak in blocking buffer (1% BSA, 0.1% Triton X100 in PBS) to reduce non-specific background staining
- -
- Application of primary antibodies (1:100 MAB377 mouse anti-NeuN; 1:500 polyclonal rabbit anti-GFAP Z0334 in blocking buffer)
- -
- Rinse in PBS (three times)
- -
- 2 h incubation in blocking buffer
- -
- Application of secondary antibodies (ALEXA fluor 488 (Abcam, Cambridge, UK) donkey anti-mouse IgG (H + L) and Cy3 Donkey Anti Rabbit IgG (H + L), 1:1000 in blocking buffer).
- -
- Rinse in PBS (three times)
3. Results
3.1. Micromechanical Characterization
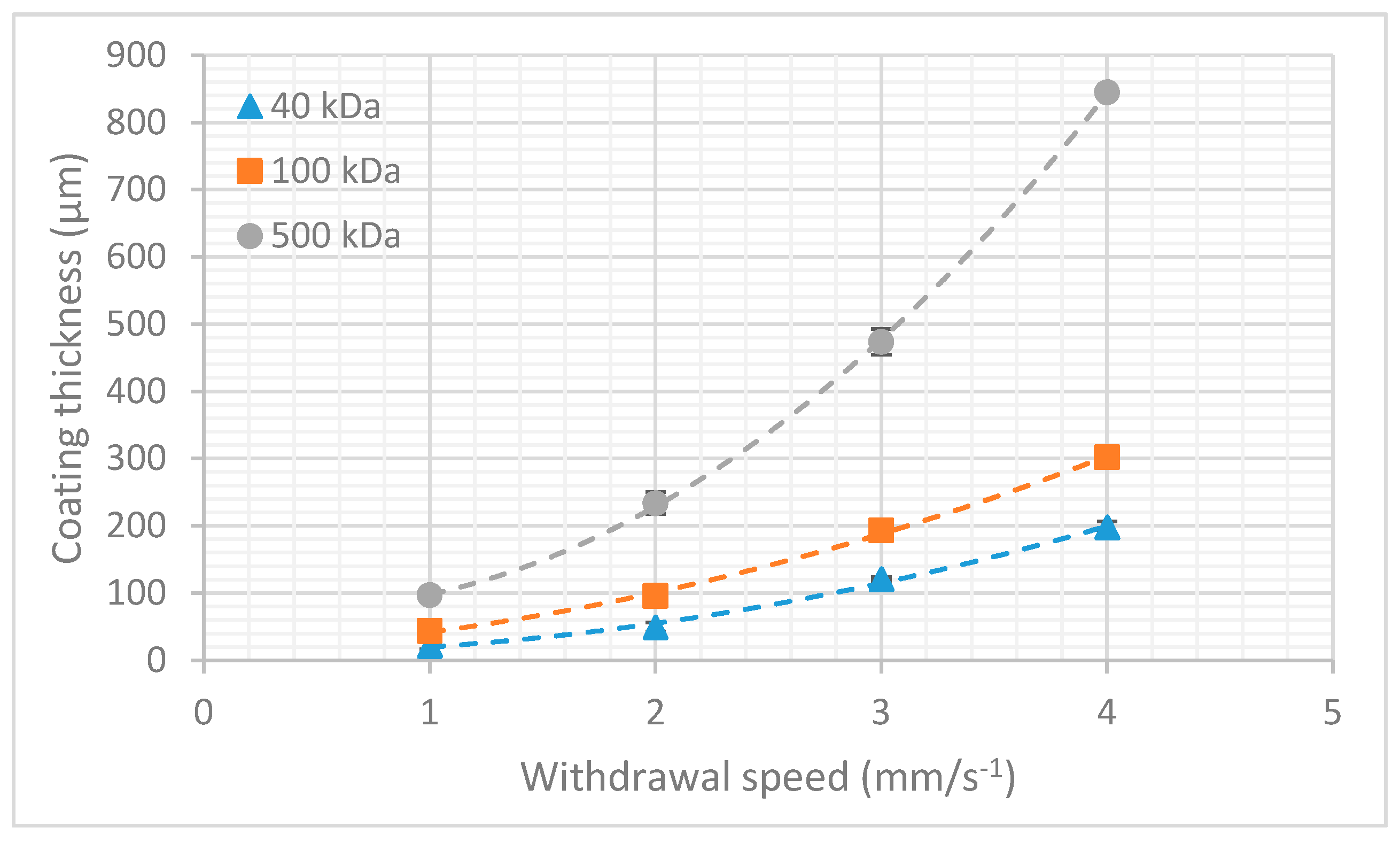
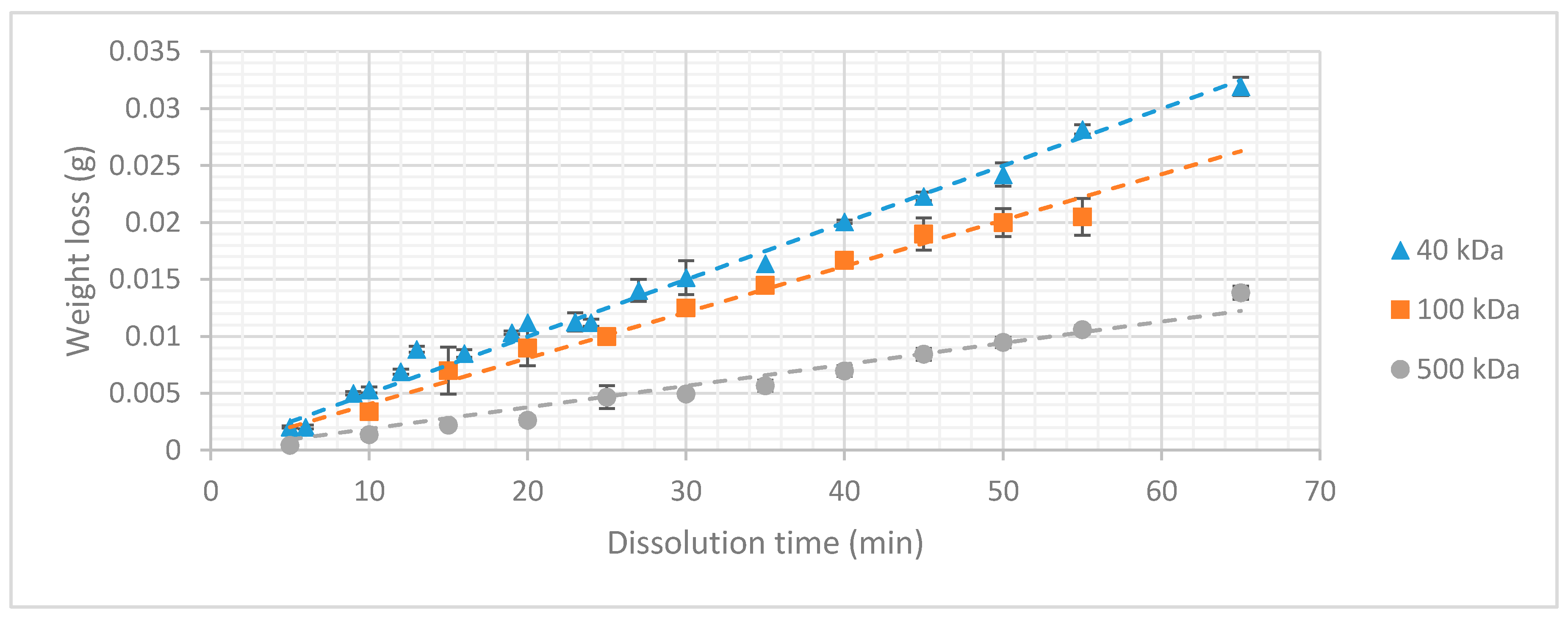
3.2. Dextran Dissolution Rate
3.3. Image Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galvani, L. De viribus eletricitatis in motu musculari commentarius. Bon. Sci. Art. Inst. Acad. Comm. 1791, 7, 363–418. [Google Scholar]
- Krucoff, M.O.; Rahimpour, S.; Slutzky, M.W.; Edgerton, R.V.; Turner, D.A. Enhancing Nervous System Recovery through Neurobiologics, Neural Interface Training, and Neurorehabilitation. Front. Neurosci. 2016, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.M. The multi-channel cochlear implant: Multi-disciplinary development of electrical stimulation of the cochlea and the resulting clinical benefit. Hear. Res. 2015, 322, 4–13. [Google Scholar] [CrossRef]
- Schwartz, A.B.; Cui, X.T.; Weber, D.J.; Moran, W. Brain-controlled interfaces: Movement restoration with neural prosthetics. Neuron 2006, 52, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Szarowski, D.H.; Anderse, M.D.; Retterer, S.; Spence, A.J.; Isaacson, M.; Craighead, H.G.; Shain, W. Brain responses to micro-machined silicon devices. Brain Res. 2003, 983, 23–35. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. J. Neurosci. Methods 2005, 148, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Biran, R.; Martin, D.C.; Tresco, P.A. Neuronal cell loss accompanies the brain tissue response to chronicaly implanted silicon microelectrode arrays. Exp. Neurol. 2005, 195, 115–126. [Google Scholar] [CrossRef]
- Rolfe, B.; Mooney, J.; Zhang, B.; Jahnke, S.; Le, S.J.; Chau, Y.Q.; Campbell, J. The fibrotic response to implanted biomaterials: Implications for tissue engineering. In Regenerative Medicine and Tissue Engineering-Cells and Biomaterials; InTech: London, UK, 2011. [Google Scholar]
- Roitbak, T.; Sykova, E. Diffusion barriers evoked in the rat cortex by reactive astrogliosis. Glia 1999, 28, 40–48. [Google Scholar] [CrossRef]
- McConnell, G.C.; Rees, H.D.; Levey, A.I.; Gutekunst, C.A.; Gross, R.E.; Bellamkonda, R.V. Implanted neural electrodes cause chronic, local inflammation that is correlated with local neurodegeneration. J. Neural Eng. 2009, 6, 056003. [Google Scholar] [CrossRef]
- Turner, J.N.; Shain, W.; Szarowski, D.H.; Andersen, M.; Martins, S.; Isaacson, M.; Craighead, H. Cerebral astrocyte response to micromachined silicon implants. Exp. Neurol. 1999, 156, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Lago, N.; Ceballos, D.; Rodriguez, F.J.; Stieglitz, T.; Navarro, X. Long term assessment of axonal regeneration through polyimide regenerative electrodes to interface the peripheral nerve. Biomaterials 2005, 26, 2012–2031. [Google Scholar] [CrossRef] [PubMed]
- Weltman, A.; Yoo, J.; Meng, E. Flexible, Penetrating Brain Probes Enabled by Advances in Polymer Microfabrication. Micromachines 2016, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Sohal, H.S.; Clowry, G.J.; Jackson, A.; O’Neill, A.; Baker, S.N. Mechanical flexibility reduces the foreign body response to long-term implanted microelectrodes in rabbit cortex. PLoS ONE 2016, 11, e0165606. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; He, J.; Singh, A.; Massia, S.; Ehteshami, G.; Kim, B.; Raupp, G. Polyimide-based intracortical neural implant with improved structural stiffness. J. Micromech. Microeng. 2004, 14, 32–37. [Google Scholar] [CrossRef]
- Kozai, T.D.Y.; Kipke, D.R. Insertion shuttle with carboxyl terminated self-assembled monolayer coatings for implanting flexible polymer neural probes in the brain. J. Neurosci. Methods 2009, 184, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Rodger, D.C.; Pinto, A.; Meng, E.; Weiland, J.D.; Humayun, M.S.; Tai, Y.C. Parylene based integrated wireless single channel neurostimulator. Sens. Actuators A 2011, 166, 193–200. [Google Scholar] [CrossRef]
- Tien, L.; Wu, F.; Tang-Schomer, M.D.; Yoon, E.; Omenetto, F.G.; Kaplan, D.L. Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes. Adv. Funct. Mater. 2013, 23, 3185–3193. [Google Scholar] [CrossRef]
- Jeon, M.; Cho, J.; Kim, Y.K.; Jung, D.; Yoon, E.S.; Shin, S.; Cho, I.J. Partially flexible MEMS neural probe composed of polyimide and sucrose gel for reducing brain damage during and after implantation. J. Micromech. Microeng. 2014, 24, 025010. [Google Scholar] [CrossRef]
- Xiang, Z.; Yen, S.C.; Xue, N.; Sun, T.; Tsang, W.M.; Zhang, S.; Liao, L.D.; Thakor, N.V.; Lee, C. Ultra-thin flexible polyimide neural probe embedded in a dissolvable maltose-coated microneedle. J. Micromech. Microeng. 2014, 24, 065015. [Google Scholar] [CrossRef]
- Lecomte, A.; Descamps, E.; Bergaud, C. A review on mechanical considerations for chronically-implanted neural probes. J. Neural Eng. 2018, 15, 31001. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Payne, D.; Hayes, P.; Naylor, R.; Bell, P.; Thompson, M.; Goodall, A. The antithrobotic effect for dextran-40 is due to enhanced fibrinolysis in vivo. J. Vasc. Surg. 2008, 48, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Massia, S.P.; Stark, J.; Letbetter, D.S. Surface-immobilized dextran limits cell adhesion and spreading. Biomaterials 2000, 21, 2253–2261. [Google Scholar] [CrossRef]
- Kil, D.; De Vloo, P.; Fierens, G.; Ceyssens, F.; Hunyadi, B.; Bertrand, A.; Nuttin, B.; Puers, R. A foldable electrode array for 3D recording of deep-seated abnormal brain cavities. J. Neural Eng. 2018, 15, 036029. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-T.; Kim, C.-D.; Lee, S.-Y.; Sohn, Y.-S. Effects of Annealing Temperature on Parylene-C Films Formed by Chemical Vapor Condensation Method. Mol. Cryst. Liquid Cryst. 2015, 618, 139–145. [Google Scholar] [CrossRef]
- Rio, E.; Boulogne, F. Withdrawing a solid from a bath: How much liquid is coated? Adv. Colloid Interface Sci. 2017, 247, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Elkin, B.S.; Ilankovan, A.; Morrison, B. Age-dependent regional mechanical properties of the rat hippocampus and cortex. J. Biomech. Eng. 2010, 132, 011010. [Google Scholar] [CrossRef]
- Jensen, W.; Yoshida, K.; Hofmann, U.G. In vivo implant mechanics of flexible, silicon-based ACREO microelectrode arrays in rat cerebral cortex. IEEE Trans. Biomed. Eng. 2006, 53, 934–940. [Google Scholar] [CrossRef]
- Sharp, A.; Ortage, A.M.; Restrepo, D.; Curran-Everett, D.; Gall, K. In vivo penetration mechanics and mechanical properties of mouse brain tissue at micrometer scales. IEEE Trans. Biomed. Eng. 2009, 56, 45–53. [Google Scholar] [CrossRef]
- Kil, D.; Brancato, L.; Puers, R. Dextran as a fast resorbable and mechanically stiff coating for flexible neural probes. J. Phys. Conf. Ser. 2017, 922, 012016. [Google Scholar] [CrossRef]
- Wehrl, H.F.; Bezrukov, I.; Wiehr, S.; Lehnhoff, M.; Fuchs, K.; Mannheim, J.G.; Quintanilla-Martinez, L.; Kohlhofer, U.; Kneilling, M.; Pichler, B.J.; et al. Assessment of murine brain tissue shrinkage caused by different histological fixatives using magnetic resonance and computed tomography imaging. Histol. Histopathol. 2015, 30, 601–613. [Google Scholar] [PubMed]
- Zhou, T.; Hong, G.; Fu, T.M.; Yang, X.; Schuhmann, T.G.; Viveros, R.D.; Lieber, C.M. Syringe-injectable mesh electronics integrate seamlessly with minimal chronic immune response in the brain. Proc. Natl. Acad. Sci. USA 2017, 114, 5894–5899. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Liu, J.; Fu, T.M.; Dai, X.; Zhou, W.; Lieber, C.M. Three-dimensional macroporous nanoelectronic networks as minimally invasive brain probes. Nat. Mater. 2015, 14, 1286. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.D.Y.; Gugel, Z.; Li, X.; Gilgunn, P.J.; Khilwani, R.; Ozdoganlar, O.B.; Fedder, G.K.; Weber, D.J.; Cui, X.T. Chronic tissue response to carboxymethyl cellulose based dissolvable insertion needle for ultra-small neural probes. Biomaterials 2014, 35, 9255–9268. [Google Scholar] [CrossRef] [PubMed]
- Lind, G.; Linsmeier, C.E.; Thelin, J.; Schouenborg, J. Gelatine-embedded electrodes—A novel biocompatible vehicle allowing implantation of highly flexible microelectrodes. J. Neural Eng. 2010, 7, 046005. [Google Scholar] [CrossRef] [PubMed]
- Lecomte, A.; Castagnola, V.; Descamps, E.; Dahan, L.; Blatché, M.C.; Dinis, T.M.; Leclerc, E.; Egles, C.; Bergaud, C. Silk and PEG as means to stiffen a parylene probe for insertion in the brain: Toward a double time-scale tool for local drug delivery. J. Micromech. Microeng. 2015, 25, 125003. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kil, D.; Bovet Carmona, M.; Ceyssens, F.; Deprez, M.; Brancato, L.; Nuttin, B.; Balschun, D.; Puers, R. Dextran as a Resorbable Coating Material for Flexible Neural Probes. Micromachines 2019, 10, 61. https://doi.org/10.3390/mi10010061
Kil D, Bovet Carmona M, Ceyssens F, Deprez M, Brancato L, Nuttin B, Balschun D, Puers R. Dextran as a Resorbable Coating Material for Flexible Neural Probes. Micromachines. 2019; 10(1):61. https://doi.org/10.3390/mi10010061
Chicago/Turabian StyleKil, Dries, Marta Bovet Carmona, Frederik Ceyssens, Marjolijn Deprez, Luigi Brancato, Bart Nuttin, Detlef Balschun, and Robert Puers. 2019. "Dextran as a Resorbable Coating Material for Flexible Neural Probes" Micromachines 10, no. 1: 61. https://doi.org/10.3390/mi10010061
APA StyleKil, D., Bovet Carmona, M., Ceyssens, F., Deprez, M., Brancato, L., Nuttin, B., Balschun, D., & Puers, R. (2019). Dextran as a Resorbable Coating Material for Flexible Neural Probes. Micromachines, 10(1), 61. https://doi.org/10.3390/mi10010061




