Neurological Disorders in a Murine Model of Chronic Renal Failure
Abstract
:1. Introduction
2. Results
2.1. Anxiety and Exploratory Behavior
2.1.1. The Openfield Test
2.1.2. The Dark/Light Box Test
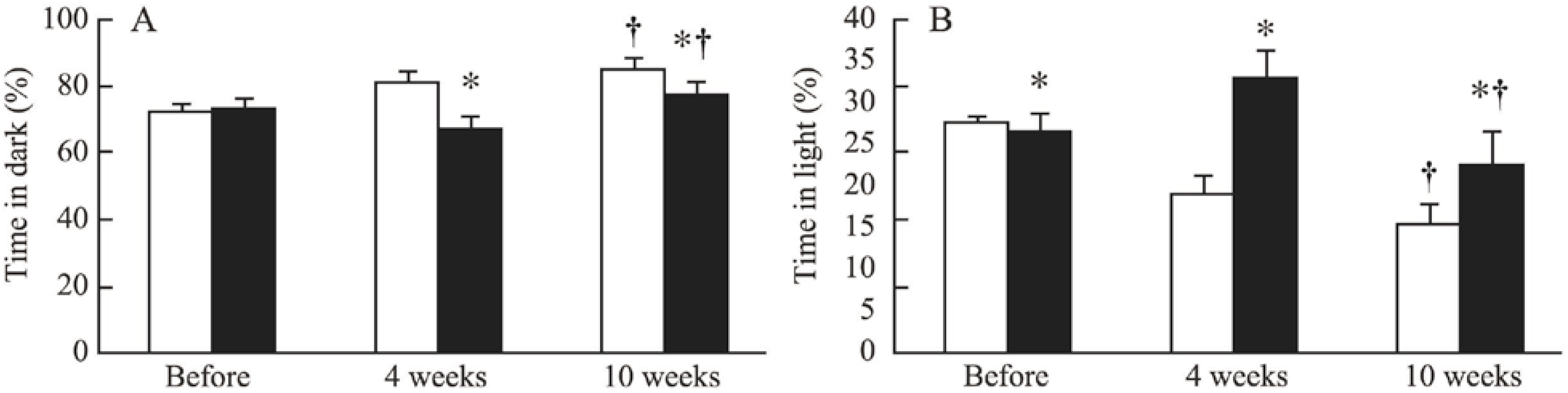
2.1.3. The Elevated Maze
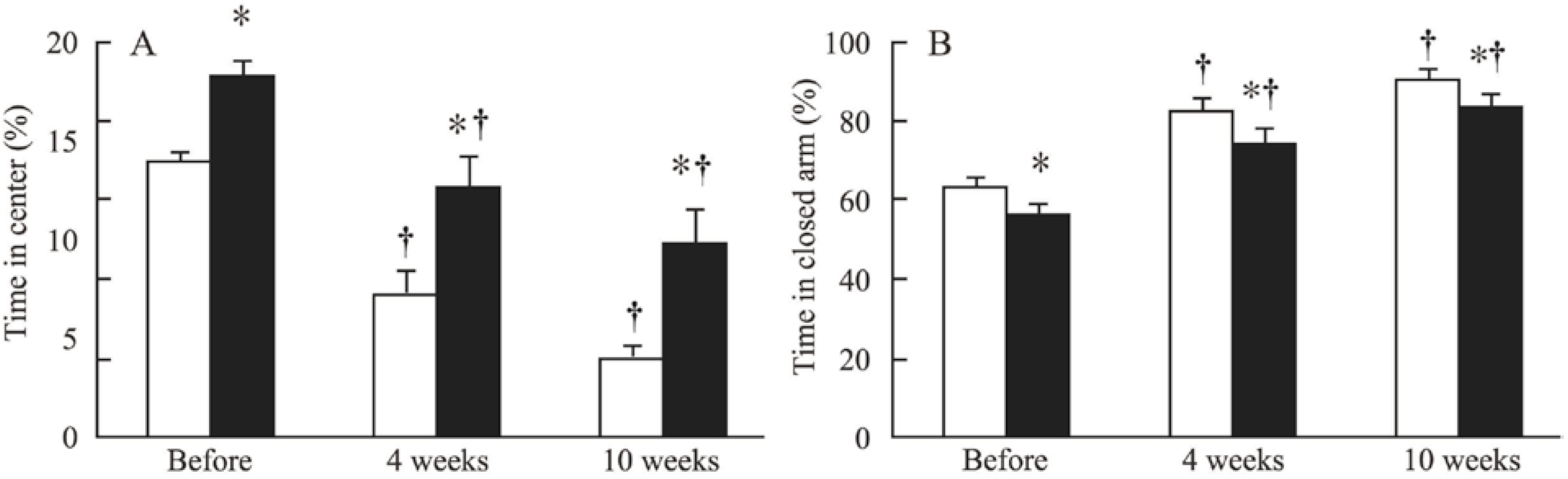
2.2. Recognition
The Y Maze
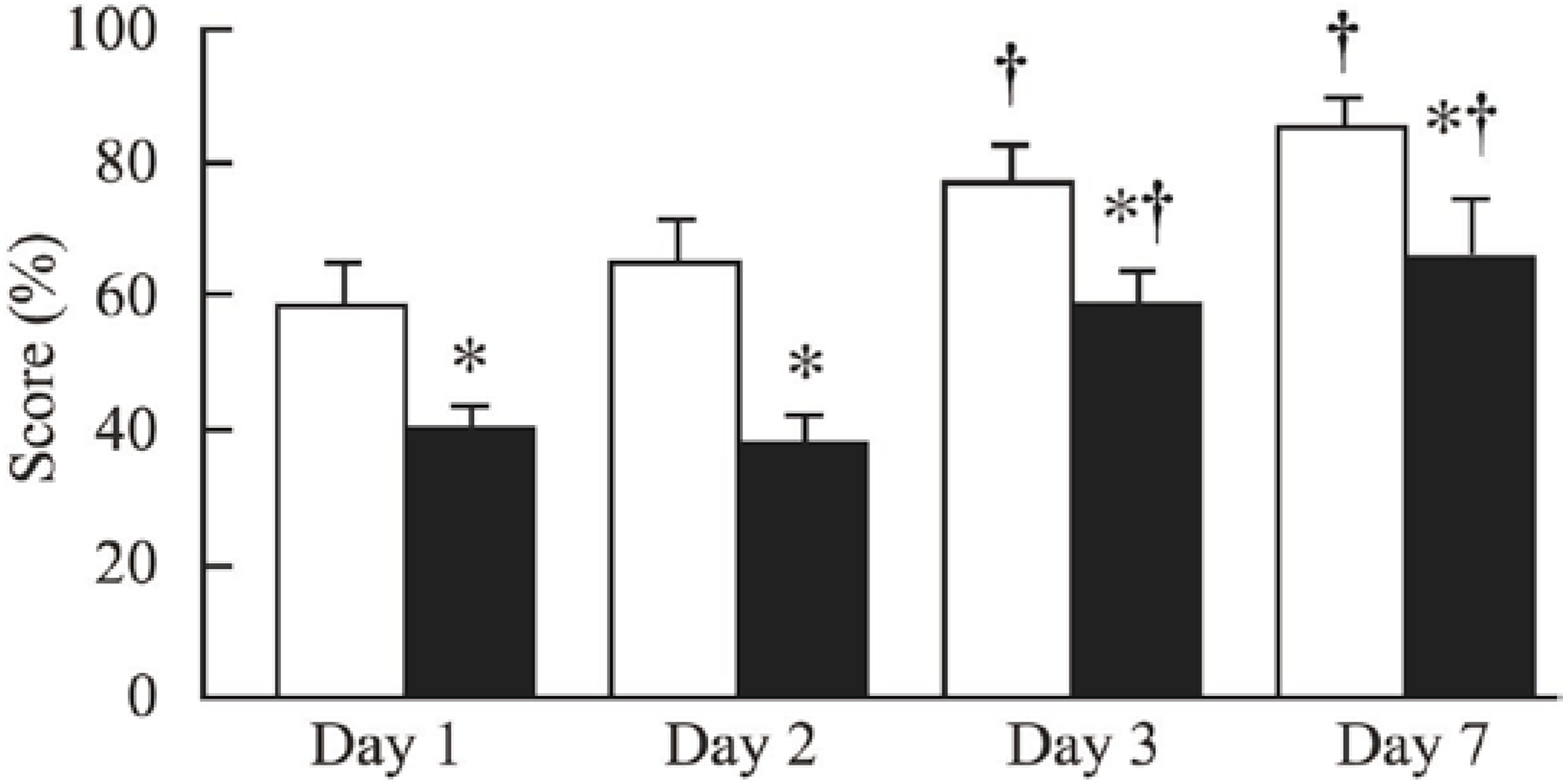
2.3. Ischemic Stroke
2.3.1. Spontaneous Mortality and Bodyweight
| Parameters | non-CRF-34w mice | CRF-34w mice | ||||||
|---|---|---|---|---|---|---|---|---|
| Days | Day 0 | Day 1 | Day 2 | Day 3 | Day 0 | Day 1 | Day 2 | Day 3 |
| n | 4 | 4 | 4 | 4 | 9 | 6 | 6 | 4 |
| Weight (g) | 24.8 ± 0.7 | 24.4 ± 0.8 † | 20.8 ± 1.1 † | 19.4 ± 0.8 † | 24.2 ± 0.4 | 22.0 ± 0.7 † | 19.9 ± 0.8 † | 18.7 ± 0.7 † |
2.3.2. Neurological Evaluation
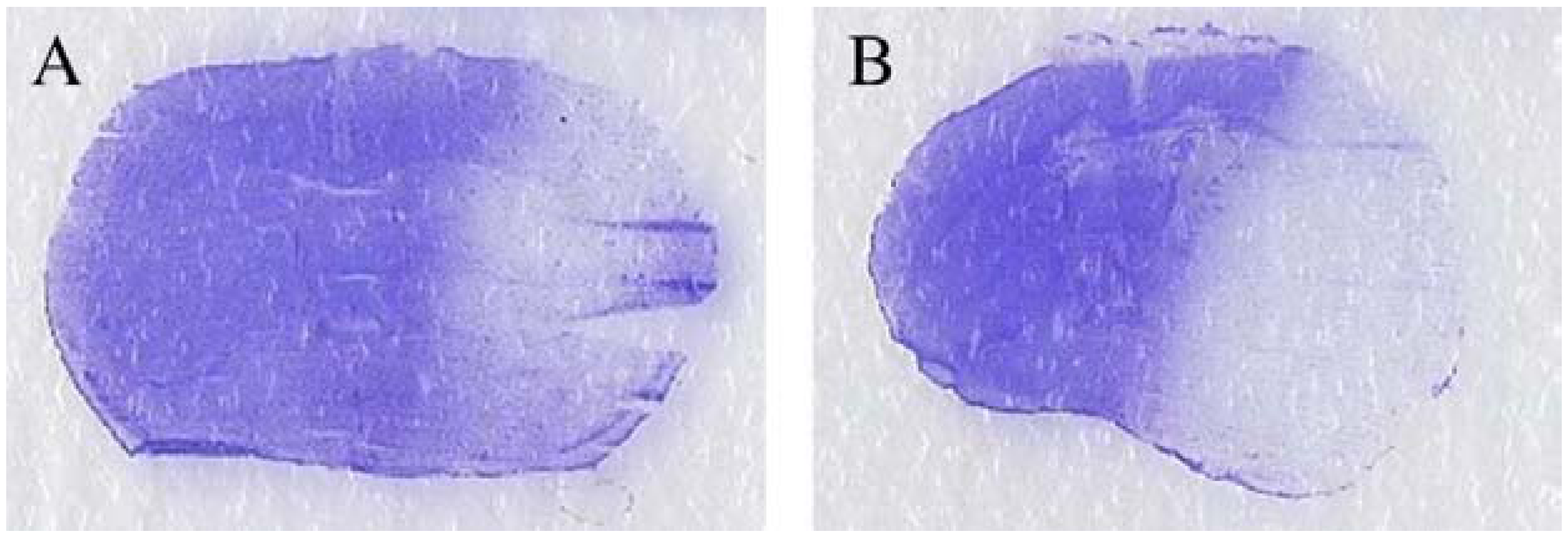
2.3.3. Histological Evaluation
3. Discussion
3.1. Characteristics of the Model
3.2. Anxiety and Exploratory Behavior
3.3. Recognition
3.4. The Severity of Ischemic Stroke
3.5. Study Limitations
4. Methods
4.1. Animals and Diet
4.2. Induction of CRF
4.3. Experimental Procedures
4.3.1. Anxiety and Exploratory Behavior in Mice
| Tests | Parameters examined |
|---|---|
| The openfield test | Anxiety, locomotor activity |
| The dark/light box test | Anxiety |
| The elevated maze test | Anxiety, time taken to make a decision |
| The Y maze | Recognition |
| The prehensile test | Post-stroke evaluation |
4.3.1.1. The Openfield Test [30]
4.3.1.2. The Dark/Light Box Test [31,32]
4.3.1.3. The Elevated Maze [33]
4.3.2. Recognition
The Y Maze [34]
4.3.3. Ischemic Stroke
4.3.3.1. The Neuroscore
4.3.3.2. The Rotarod Test [36]
4.3.3.3. The Prehensile Test [36]
4.3.3.4. Histological Evaluation
4.4. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Vanholder, R.; Massy, Z.; Argiles, A.; Spasovski, G.; Verbeke, F.; Lameire, N. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol. Dial. Transplant. 2005, 20, 1048–1056. [Google Scholar] [CrossRef]
- Guérin, A.P.; London, G.M.; Marchais, S.J.; Metivier, F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol. Dial. Transplant. 2000, 15, 1014–1021. [Google Scholar] [CrossRef]
- Seliger, S.L.; Gillen, D.L.; Longstreth, W.T., Jr.; Kestenbaum, B.; Stehman-Breen, C.O. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003, 64, 603–609. [Google Scholar] [CrossRef]
- Murray, A.M. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv. Chronic Kidney Dis. 2008, 15, 123–132. [Google Scholar] [CrossRef]
- Seliger, S.L.; Siscovick, D.S.; Stehman-Breen, C.O.; Gillen, D.L.; Fitzpatrick, A.; Bleyer, A.; Kuller, L.H. Moderate renal impairment and risk of dementia among older adults: The cardiovascular health cognition study. J. Am. Soc. Nephrol. 2004, 15, 1904–1911. [Google Scholar] [CrossRef]
- Iseki, K.; Fukiyama, K. Clinical demographics and long-term prognosis after stroke in patients on chronic haemodialysis. The Okinawa dialysis study (okids) group. Nephrol. Dial. Transplant. 2000, 15, 1808–1813. [Google Scholar] [CrossRef]
- Nakatani, T.; Naganuma, T.; Uchida, J.; Masuda, C.; Wada, S.; Sugimura, T.; Sugimura, K. Silent cerebral infarction in hemodialysis patients. Am. J. Nephrol. 2003, 23, 86–90. [Google Scholar] [CrossRef]
- Lass, P.; Buscombe, J.R.; Harber, M.; Davenport, A.; Hilson, A.J. Cognitive impairment in patients with renal failure is associated with multiple-infarct dementia. Clin. Nucl. Med. 1999, 24, 561–565. [Google Scholar] [CrossRef]
- Muntner, P.; He, J.; Hamm, L.; Loria, C.; Whelton, P.K. Renal insufficiency and subsequent death resulting from cardiovascular disease in the united states. J. Am. Soc. Nephrol. 2002, 13, 745–753. [Google Scholar]
- Kielstein, J.T.; Zoccali, C. Asymmetric dimethylarginine: A novel marker of risk and a potential target for therapy in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2008, 17, 609–615. [Google Scholar] [CrossRef]
- Maizel, J.; Six, I.; Slama, M.; Tribouilloy, C.; Sevestre, H.; Poirot, S.; Giummelly, P.; Atkinson, J.; Choukroun, G.; Andrejak, M.; et al. Mechanisms of aortic and cardiac dysfunction in uremic mice with aortic calcification. Circulation 2009, 119, 306–313. [Google Scholar] [CrossRef]
- Pannier, B.; Guerin, A.P.; Marchais, S.J.; Metivier, F.; Safar, M.E.; London, G.M. Postischemic vasodilation, endothelial activation, and cardiovascular remodeling in end-stage renal disease. Kidney Int. 2000, 57, 1091–1099. [Google Scholar] [CrossRef]
- Van Guldener, C.; Janssen, M.J.; Lambert, J.; Steyn, M.; Donker, A.J.; Stehouwer, C.D. Endothelium-dependent vasodilatation is impaired in peritoneal dialysis patients. Nephrol. Dial. Transplant. 1998, 13, 1782–1786. [Google Scholar] [CrossRef]
- Bugnicourt, J.M.; Da Silveira, C.; Bengrine, A.; Godefroy, O.; Baumbach, G.; Sevestre, H.; Bode-Boeger, S.M.; Kielstein, J.T.; Massy, Z.A.; Chillon, J.M. Chronic renal failure alters endothelial function in cerebral circulation in mice. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1143–H1152. [Google Scholar] [CrossRef]
- Massy, Z.A.; Ivanovski, O.; Nguyen-Khoa, T.; Angulo, J.; Szumilak, D.; Mothu, N.; Phan, O.; Daudon, M.; Lacour, B.; Drueke, T.B.; et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein e knockout mice. J. Am. Soc. Nephrol. 2005, 16, 109–116. [Google Scholar]
- Phan, O.; Ivanovski, O.; Nguyen-Khoa, T.; Mothu, N.; Angulo, J.; Westenfeld, R.; Ketteler, M.; Meert, N.; Maizel, J.; Nikolov, I.G.; et al. Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein e-deficient mice. Circulation 2005, 112, 2875–2882. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, M.S.; Cho, S.; Kim, S.R. Association of depression and anxiety with reduced quality of life in patients with predialysis chronic kidney disease. Int. J. Clin. Pract. 2013, 67, 363–368. [Google Scholar] [CrossRef]
- Cukor, D.; Cohen, S.D.; Peterson, R.A.; Kimmel, P.L. Psychosocial aspects of chronic disease: Esrd as a paradigmatic illness. J. Am. Soc. Nephrol. 2007, 18, 3042–3055. [Google Scholar] [CrossRef]
- Hsu, H.J.; Yen, C.H.; Chen, C.K.; Wu, I.W.; Lee, C.C.; Sun, C.Y.; Chang, S.J.; Chou, C.C.; Hsieh, M.F.; Chen, C.Y.; et al. Association between uremic toxins and depression in patients with chronic kidney disease undergoing maintenance hemodialysis. Gen. Hosp. Psychiatry 2013, 35, 23–27. [Google Scholar] [CrossRef]
- Monge, M.; Houben, T.; De Boer, H.; Aleksinskaya, M.; Massy, Z.; Rabelink, T.; Meijer, J.H.; Van Zonneveld, A.J. Chronic renal failure does not affect the mouse locomotor activity in darkness conditions. Biol. Rhytm Res. 2013, in press. [Google Scholar]
- Bugnicourt, J.M.; Godefroy, O.; Chillon, J.M.; Choukroun, G.; Massy, Z.A. Cognitive disorders and dementia in ckd: The neglected kidney-brain axis. J. Am. Soc. Nephrol. 2013, 24, 353–363. [Google Scholar] [CrossRef]
- Kumai, Y.; Kamouchi, M.; Hata, J.; Ago, T.; Kitayama, J.; Nakane, H.; Sugimori, H.; Kitazono, T. Proteinuria and clinical outcomes after ischemic stroke. Neurology 2012, 78, 1909–1915. [Google Scholar] [CrossRef]
- Vanholder, R.; Baurmeister, U.; Brunet, P.; Cohen, G.; Glorieux, G.; Jankowski, J. A bench to bedside view of uremic toxins. J. Am. Soc. Nephrol. 2008, 19, 863–870. [Google Scholar] [CrossRef]
- Allen, C.L.; Bayraktutan, U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int. J. Stroke Off. J. Int. Stroke Soc. 2009, 4, 461–470. [Google Scholar] [CrossRef]
- Rallidis, L.S.; Vikelis, M.; Panagiotakos, D.B.; Rizos, I.; Zolindaki, M.G.; Kaliva, K.; Kremastinos, D.T. Inflammatory markers and in-hospital mortality in acute ischaemic stroke. Atherosclerosis 2006, 189, 193–197. [Google Scholar] [CrossRef]
- Niu, P.P.; Yang, G.; Zheng, B.K.; Guo, Z.N.; Jin, H.; Yang, Y. Relationship between endothelial nitric oxide synthase gene polymorphisms and ischemic stroke: A meta-analysis. Acta Neurol. Scand. 2013, 128, 202–212. [Google Scholar] [CrossRef]
- Carbonell, T.; Rama, R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Curr. Med. Chem. 2007, 14, 857–874. [Google Scholar] [CrossRef]
- Heiss, W.D. The ischemic penumbra: How does tissue injury evolve? Ann. NY Acad. Sci. 2012, 1268, 26–34. [Google Scholar] [CrossRef]
- Carmichael, S.T. Rodent models of focal stroke: Size, mechanism, and purpose. NeuroRx J. Am. Soc. Exp. Neuro Ther. 2005, 2, 396–409. [Google Scholar]
- Walsh, R.N.; Cummins, R.A. The open-field test: A critical review. Psychol. bull. 1976, 83, 482–504. [Google Scholar] [CrossRef]
- Imaizumi, M.; Miyazaki, S.; Onodera, K. Effects of a non-xanthine adenosine antagonist, cgs 15943, and a phosphodiesterase inhibitor, ro 20-1724, in a light/dark test in mice. Methods Find. Exp. Clin. Pharmacol. 1994, 16, 717–721. [Google Scholar]
- Imaizumi, M.; Suzuki, T.; Machida, H.; Onodera, K. A fully automated apparatus for a light/dark test measuring anxiolytic or anxiogenic effects of drugs in mice. Jpn. J. psychopharmacol. 1994, 14, 83–91. [Google Scholar]
- Rodgers, R.J.; Dalvi, A. Anxiety, defence and the elevated plus-maze. Neurosci. Biobehav. Rev. 1997, 21, 801–810. [Google Scholar] [CrossRef]
- Valentim, A.M.; Ribeiro, P.O.; Olsson, I.A.; Antunes, L.M. The memory stages of a spatial y-maze task are not affected by a low dose of ketamine/midazolam. Eur. J. Pharmacol. 2013, 712, 39–47. [Google Scholar] [CrossRef]
- Deplanque, D.; Gele, P.; Petrault, O.; Six, I.; Furman, C.; Bouly, M.; Nion, S.; Dupuis, B.; Leys, D.; Fruchart, J.C.; et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J. Neurosci. 2003, 23, 6264–6271. [Google Scholar]
- Zausinger, S.; Hungerhuber, E.; Baethmann, A.; Reulen, H.; Schmid-Elsaesser, R. Neurological impairment in rats after transient middle cerebral artery occlusion: A comparative study under various treatment paradigms. Brain Res. 2000, 863, 94–105. [Google Scholar] [CrossRef]
- Paxinos, G.; Halliday, G.; Watson, C.; Koutcherov, Y.; Wang, H.Q. Atlas of the Developing Mouse Brain at e17.5, p0, and p6; Elsevier Academic Press Inc: San Diego, CA, USA, 2007. [Google Scholar]
- Deplanque, D.; Venna, V.R.; Bordet, R. Brain ischemia changes the long term response to antidepressant drugs in mice. Behav. Brain Res. 2011, 219, 367–372. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chillon, J.-M.; Brazier, F.; Bouquet, P.; Massy, Z.A. Neurological Disorders in a Murine Model of Chronic Renal Failure. Toxins 2014, 6, 180-193. https://doi.org/10.3390/toxins6010180
Chillon J-M, Brazier F, Bouquet P, Massy ZA. Neurological Disorders in a Murine Model of Chronic Renal Failure. Toxins. 2014; 6(1):180-193. https://doi.org/10.3390/toxins6010180
Chicago/Turabian StyleChillon, Jean-Marc, François Brazier, Philippe Bouquet, and Ziad A. Massy. 2014. "Neurological Disorders in a Murine Model of Chronic Renal Failure" Toxins 6, no. 1: 180-193. https://doi.org/10.3390/toxins6010180
APA StyleChillon, J.-M., Brazier, F., Bouquet, P., & Massy, Z. A. (2014). Neurological Disorders in a Murine Model of Chronic Renal Failure. Toxins, 6(1), 180-193. https://doi.org/10.3390/toxins6010180




