Methylglyoxal (MG) and Cerebro-Renal Interaction: Does Long-Term Orally Administered MG Cause Cognitive Impairment in Normal Sprague-Dawley Rats?
Abstract
:1. Introduction
2. Results
2.1. Physical Findings and Laboratory Tests
| Phase | Age in weeks (W) | MG exposure (W) | Behavior test | Urine & blood test | Mean BP (mmHg) | p Value | Body weight (g) | p Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 9) | MG (n = 10) | Control (n = 9) | MG (n = 10) | |||||||
| Phase I | 8 | START | - | - | 101 ± 2.1 | 105 ± 2.0 | 0.221 | 281 ± 3.3 | 277 ± 3.8 | 0.527 |
| 16 | +8 | - | Urine Test (1) | 99.0 ± 4.0 | 99.0 ± 3.3 | 0.935 | 506 ± 10.5 | 487 ± 10.7 | 0.238 | |
| 16 | +8 | Object exploration Test (1) | - | - | - | - | - | - | - | |
| 20 ~ 23 | +12~+15 | Radial-arm Maze Test (1) | - | 82.0 ± 2.1 | 79.7 ± 3.4 | 0.594 | 533 ± 10.3 | 509 ± 11.9 | 0.159 | |
| Phase II | 31 | +23 | Object exploration Test (2) | - | - | - | - | 569 ± 11.3 | 539 ± 12.8 | 0.116 |
| 42 ~ 44 | +34~+36 | Radial-arm Maze Test (2) | - | - | - | - | - | - | - | |
| 45 | +37 | - | Urine Test (2) | - | - | - | - | - | - | |
| 47 | +39 | (Sacrificed) | Blood Test | 93.4 ± 3.3 | 94.2 ± 4.5 | 0.674 | 603 ± 14.2 | 566 ± 14.5 | 0.083 | |
| Parameters | Phase I | Phase II | ||||
|---|---|---|---|---|---|---|
| Control (n = 9) | MG (n = 10) | p Value | Control (n = 9) | MG (n = 10) | p Value | |
| Uric Protein (g/gCrea) | 0.7 ± 0.2 | 1.0 ± 0.5 | 0.068 | 3.0 ± 0.7 | 1.8 ± 0.6 | 0.201 |
| Urinary 8-OHdG (ng/day) | 252.2 ± 21.1 | 218.9 ± 14.5 | 0.356 | 211.9 ± 12.0 | 205.0 ± 16.3 | 0.549 |
| Urinary MDA (nmol/day) | 106.8 ± 9.2 | 101.3 ± 12.6 | 0.733 | 27.0 ± 4.9 | 27.7 ± 4.8 | 0.927 |
| BUN (mg/dL) | - | - | - | 20.7 ± 0.6 | 21.9 ± 0.8 | 0.241 |
| Serum Creatinine (mg/dL) | - | - | - | 0.30 ± 0.01 | 0.33 ± 0.02 | 0.210 |
| Plasma Glucose (mg/dL) | - | - | - | 236.3 ± 7.1 | 221.9 ± 6.4 | 0.149 |
| Serum MG (nM) | - | - | - | 244.8 ± 28.2 | 495.8 ± 38.1 | <0.001 |
| Plasma MDA (µM) | - | - | - | 0.16 ± 0.04 | 0.14 ± 0.01 | 0.447 |
| Plasma AGT (ng/mL) | - | - | - | 3.2 ± 0.3 | 2.1 ± 0.1 | 0.014 |
| Urinary AGT (ng/day) | 29.3 ± 6.5 | 16.3 ± 6.3 | 0.034 | |||
| Kidney GPx (mU/mg) | - | - | - | 3.6 ± 0.4 | 5.1 ± 0.4 | 0.036 |
| Kidney SOD (U/mL/mg) | - | - | - | 3.7 ± 0.9 | 6.3 ± 0.9 | 0.069 |
2.2. Results of Behavioral Testing
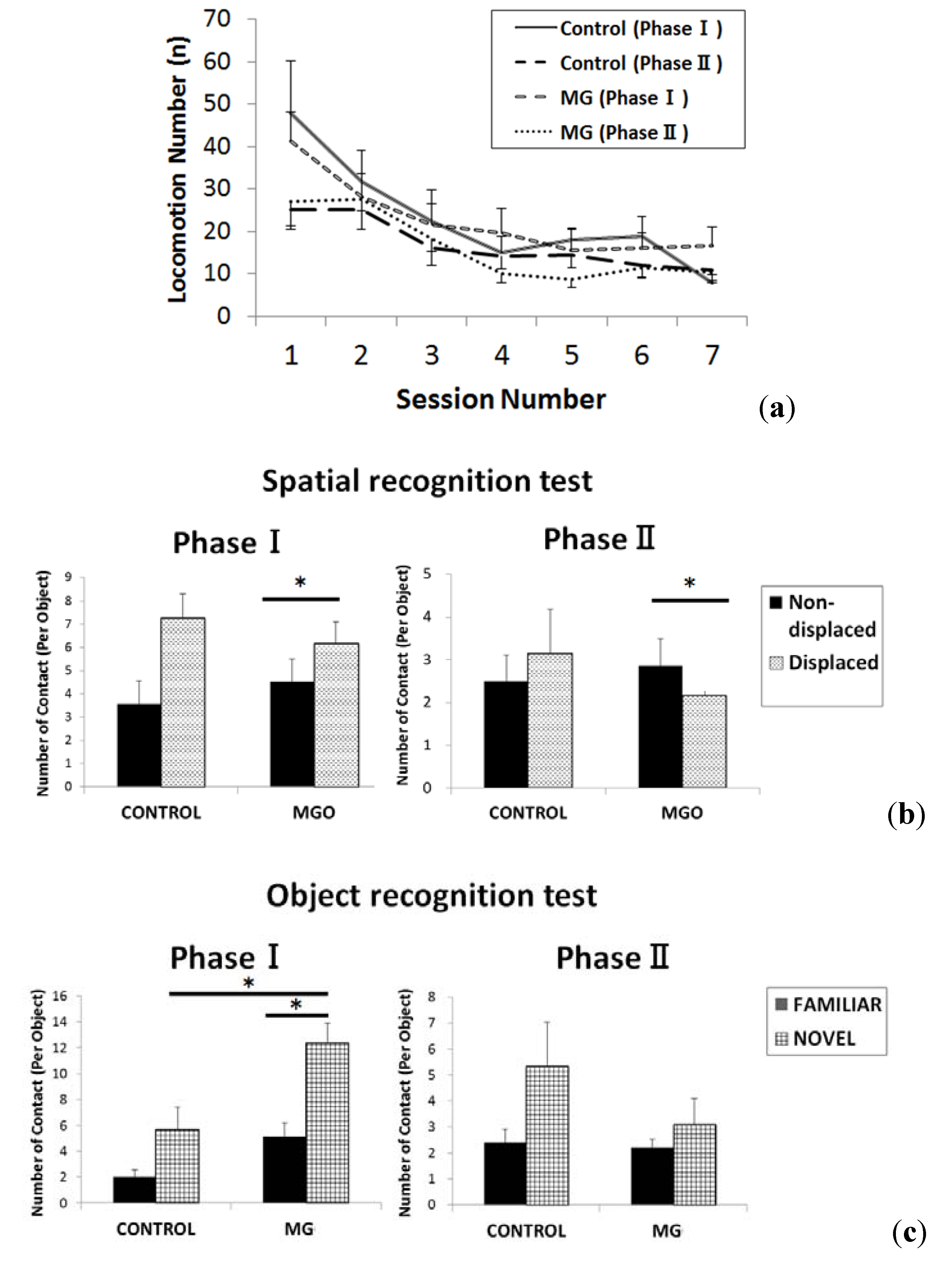
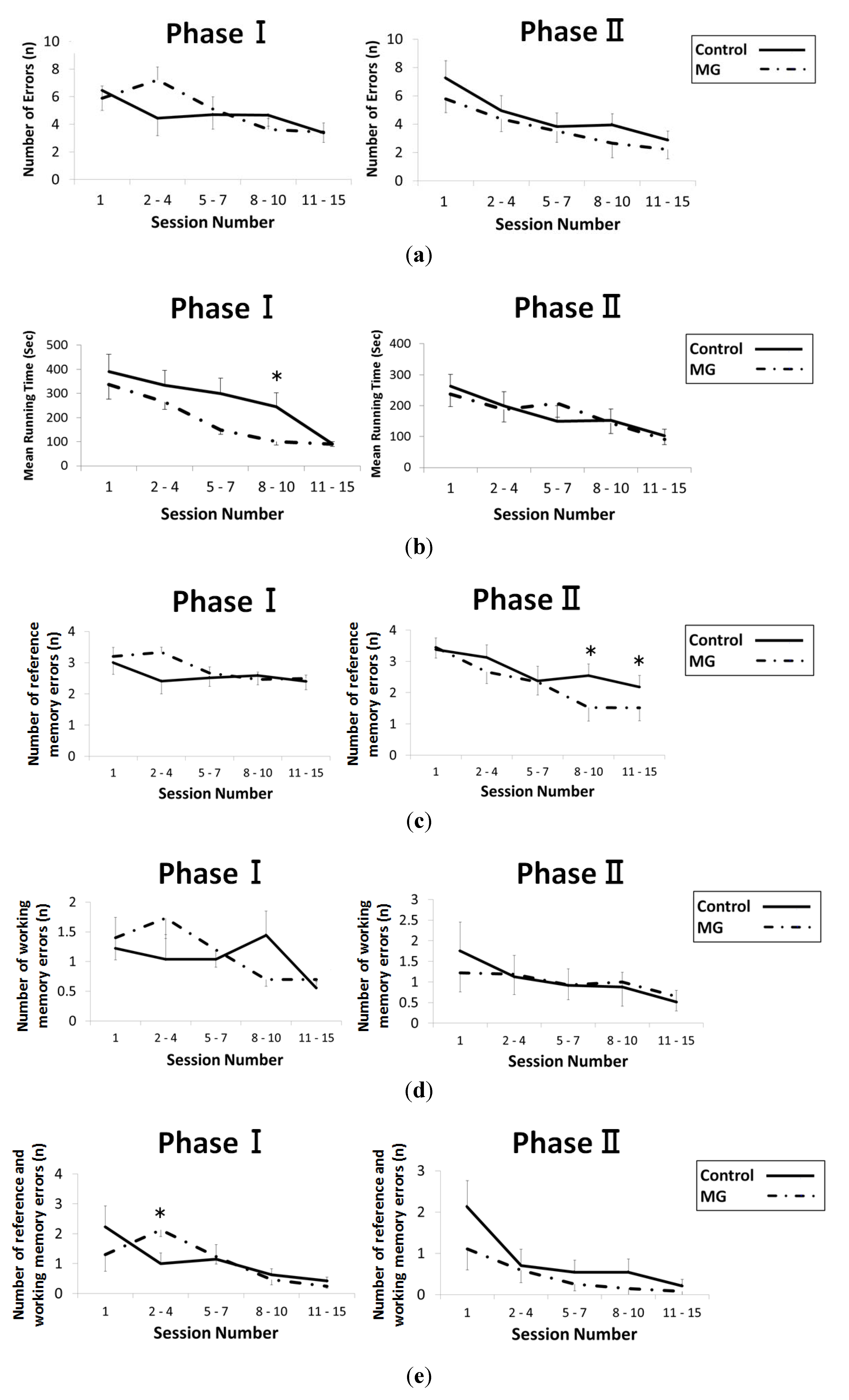
2.3. Histological Findings of Brain and Kidney
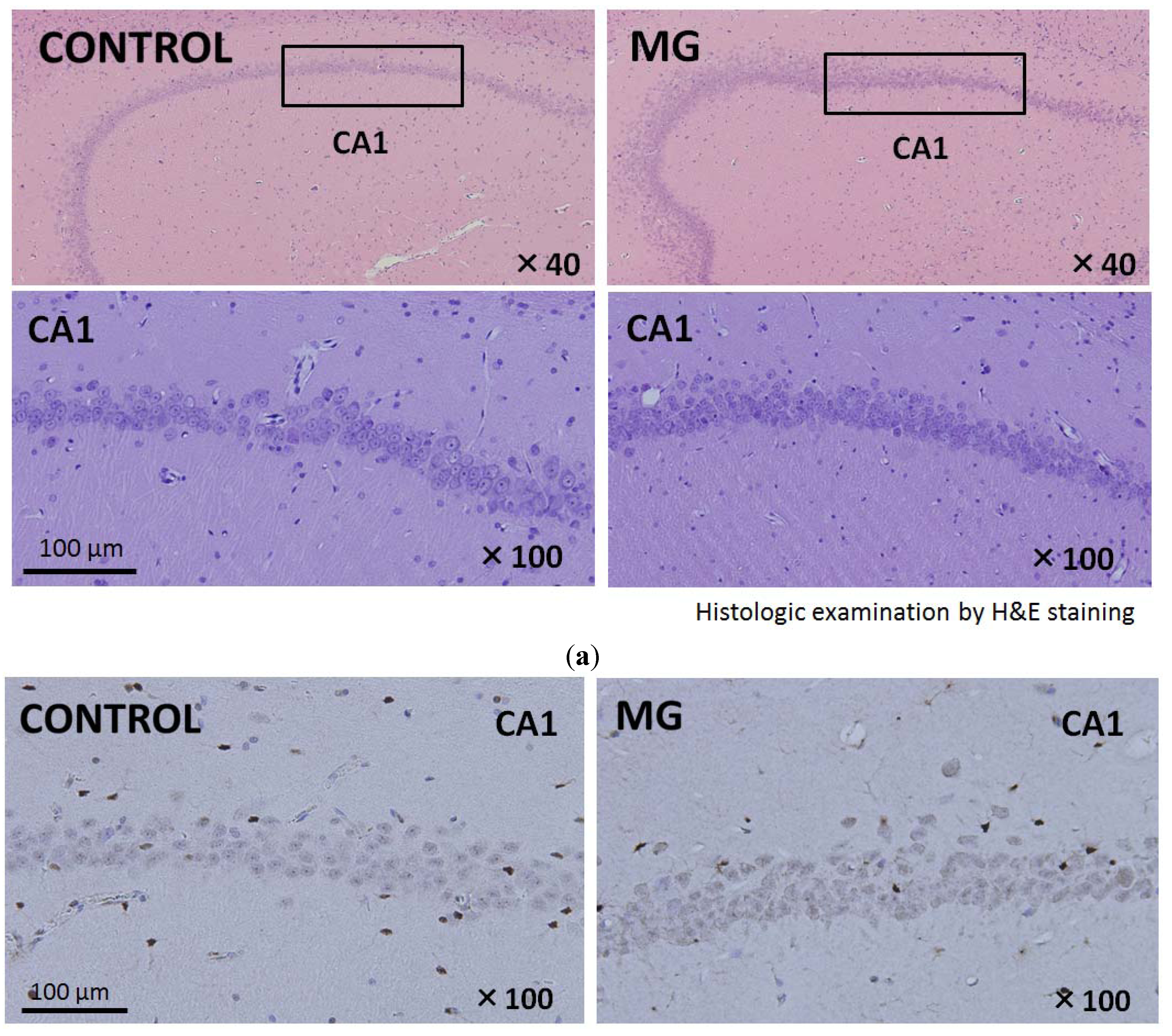
2.4. Analysis of Oxidative Stress, Antioxidants and Angiotensinogen
3. Discussion
4. Experimental Section
4.1. Animals
4.2. Treatment Protocol
4.3. Behavioral Procedure
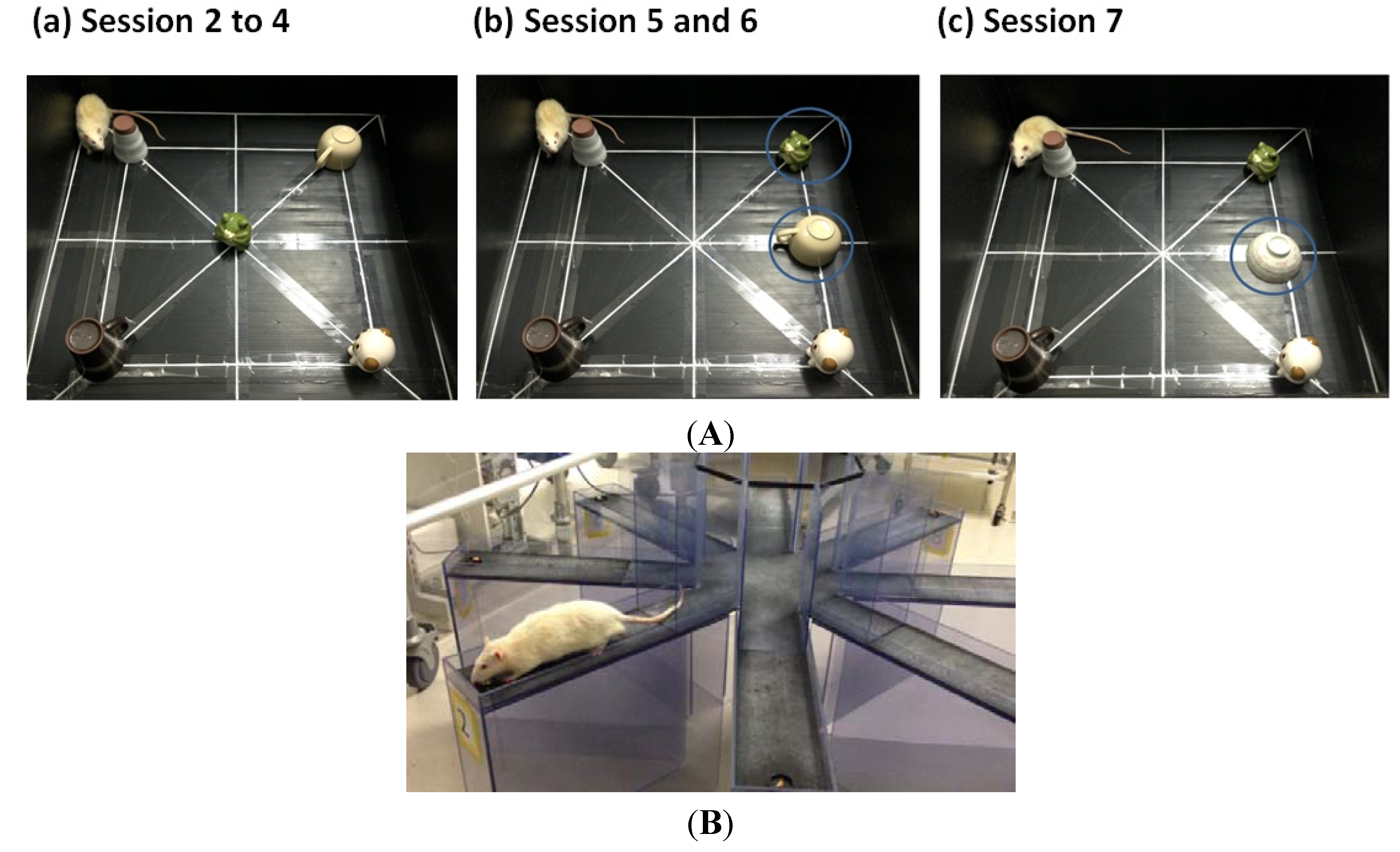
4.3.1. Object Exploration Test
4.3.2. Radial Arm Maze Test
4.4. Collection of Blood, Urine, Kidney and Brain Samples
4.5. Sample Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Odani, H.; Shinzato, T.; Matsumoto, Y.; Usami, J.; Maeda, K. Increase in three alpha, beta-dicarbonyl compound levels in human uremic plasma: Specific in vivo determination of intermediates in advanced maillard reaction. Biochem. Biophys. Res. Commun. 1999, 256, 89–93. [Google Scholar] [CrossRef]
- Matafome, P.; Sena, C.; Seica, R. Methylglyoxal, obesity, and diabetes. Endocrine 2013, 43, 472–484. [Google Scholar] [CrossRef]
- McQuillan, R.; Jassal, S.V. Neuropsychiatric complications of chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 471–479. [Google Scholar] [CrossRef]
- Murray, A.M.; Tupper, D.E.; Knopman, D.S.; Gilbertson, D.T.; Pederson, S.L.; Li, S.; Smith, G.E.; Hochhalter, A.K.; Collins, A.J.; Kane, R.L. Cognitive impairment in hemodialysis patients is common. Neurology 2006, 67, 216–223. [Google Scholar] [CrossRef]
- Kurella, M.; Yaffe, K.; Shlipak, M.G.; Wenger, N.K.; Chertow, G.M. Chronic kidney disease and cognitive impairment in menopausal women. Am. J. Kidney Dis. 2005, 45, 66–76. [Google Scholar]
- Khatri, M.; Nickolas, T.; Moon, Y.P.; Paik, M.C.; Rundek, T.; Elkind, M.S.; Sacco, R.L.; Wright, C.B. CKD Associates with cognitive decline. J. Am. Soc. Nephrol. 2009, 20, 2427–2432. [Google Scholar]
- Rakowski, D.A.; Caillard, S.; Agodoa, L.Y.; Abbott, K. Dementia as a predictor of mortality in dialysis patients. Clin. J. Am. Soc. Nephrol. 2006, 1, 1000–1005. [Google Scholar] [CrossRef]
- Beeri, M.S.; Moshier, E.; Schmeidler, J.; Godbold, J.; Uribarri, J.; Reddy, S.; Sano, M.; Grossman, H.T.; Cai, W.; Vlassara, H.; et al. Serum concentration of an inflammatory glycotoxin, methylglyoxal, is associated with increased cognitive decline in elderly individuals. Mech. Ageing Dev. 2011, 132, 583–587. [Google Scholar] [CrossRef]
- Srikanth, V.; Westcott, B.; Forbes, J.; Phan, T.G.; Beare, R.; Venn, A.; Pearson, S.; Greenaway, T.; Parameswaran, V.; Munch, G. Methylglyoxal, cognitive function and cerebral atrophy in older people. J. Gerontol. A 2013, 68, 68–73. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Nakayama, K.; Nakayama, M.; Iwabuchi, M.; Terawaki, H.; Sato, T.; Kohno, M.; Ito, S. Plasma alpha-oxoaldehyde levels in diabetic and nondiabetic chronic kidney disease patients. Am. J. Nephrol. 2008, 28, 871–878. [Google Scholar] [CrossRef]
- Di Loreto, S.; Caracciolo, V.; Colafarina, S.; Sebastiani, P.; Gasbarri, A.; Amicarelli, F. Methylglyoxal induces oxidative stress-dependent cell injury and up-regulation of interleukin-1beta and nerve growth factor in cultured hippocampal neuronal cells. Brain Res. 2004, 1006, 157–167. [Google Scholar]
- Kikuchi, S.; Shinpo, K.; Moriwaka, F.; Makita, Z.; Miyata, T.; Tashiro, K. Neurotoxicity of methylglyoxal and 3-deoxyglucosone on cultured cortical neurons: Synergism between glycation and oxidative stress, possibly involved in neurodegenerative diseases. J. Neurosci. Res. 1999, 57, 280–289. [Google Scholar] [CrossRef]
- Rutten, B.P.F.; Schmitz, C.; Gerlach, O.H.H.; Oyen, H.M.; De Mesquita, E.B.; Steinbusch, H.W.M.; Kon, H. The aging brain: Accumulation of DNA damage or neuron loss? Neurobiol. Aging 2007, 28, 91–98. [Google Scholar] [CrossRef]
- Huang, X.; Wang, F.; Chen, W.; Chen, Y.; Wang, N.; Von Maltzan, K. Possible link between the cognitive dysfunction associated with diabetes mellitus and the neurotoxicity of methylglyoxal. Brain Res. 2012, 1469, 82–91. [Google Scholar]
- Chen, X.; Mori, T.; Guo, Q.; Hu, C.; Ohsaki, Y.; Yoneki, Y.; Zhu, W.; Jiang, Y.; Endo, S.; Nakayama, K.; et al. Carbonyl stress induces hypertension and cardio-renal vascular injury in dahl salt-sensitive rats. Hypertens. Res. 2013, 36, 361–367. [Google Scholar]
- Ogawa, S.; Nakayama, K.; Nakayama, M.; Mori, T.; Matsushima, M.; Okamura, M.; Senda, M.; Nako, K.; Miyata, T.; Ito, S. Methylglyoxal is a Predictor in Type 2 Diabetic Patients of Intima-media Thickening and Elevation of Blood Pressure. Hypertension 2010, 56, 471–476. [Google Scholar] [CrossRef]
- Guo, Q.; Mori, T.; Jiang, Y.; Hu, C.; Osaki, Y.; Yoneki, Y.; Sun, Y.; Hosoya, T.; Kawamata, A.; Ogawa, S. Methylglyoxal contributes to the development of insulin resistance and salt sensitivity in sprague-dawley rats. J. Hypertens. 2009, 27, 1664–1671. [Google Scholar] [CrossRef]
- Wong-Goodrich, S.J.; Glenn, M.J.; Mellott, T.J.; Blusztajn, J.K.; Meck, W.H.; Williams, C.L. Spatial memory and hippocampal plasticity are differentially sensitive to the availability of choline in adulthood as a function of choline supply in utero. Brain Res. 2008, 1237, 153–166. [Google Scholar]
- Silva, A.F.; Aguiar, M.S.; Carvalho, O.S.; de Nazare Santana, L.; Franco, E.C.; Lima, R.R.; de Siqueira, N.V.; Feio, R.A.; Faro, L.R.; Gomes-Leal, W. Hippocampal neuronal loss, decreased GFAP immunoreactivity and cognitive impairment following experimental intoxication of rats with aluminum citrate. Brain Res. 2013, 1491, 23–33. [Google Scholar] [CrossRef]
- Kocahan, S.; Akillioglu, K.; Binokay, S.; Sencar, L.; Polat, S. The effects of N-Methyl-D-Aspartate receptor blockade during the early neurodevelopmental period on emotional behaviors and cognitive functions of adolescent Wistar rats. Neurochem. Res. 2013, 38, 989–996. [Google Scholar] [CrossRef]
- Rushaidhi, M.; Zhang, H.; Liu, P. Effects of prolonged agmatine treatment in aged male Sprague-Dawley rats. Neuroscience 2013, 234, 116–124. [Google Scholar] [CrossRef]
- Wang, W.; Tian, L.; Li, Y.; Wang, X.; Xia, F.; Li, L.; Li, J.; Zhang, Z. Effects of hydrogen-rich saline on rats with acute carbon monoxide poisoning. J. Emerg. Med. 2013, 44, 107–115. [Google Scholar] [CrossRef]
- Fedotova, J.; Soultanov, V.; Nikitina, T.; Roschin, V.; Ordayn, N. Ropren((R)) is a polyprenol preparation from coniferous plants that ameliorates cognitive deficiency in a rat model of beta-amyloid peptide-(25–35)-induced amnesia. Phytomedicine 2012, 19, 451–456. [Google Scholar] [CrossRef]
- Levin, E.D.; Caldwell, D.P. Low-dose mecamylamine improves learning of rats in the radial-arm maze repeated acquisition procedure. Neurobiol. Learn. Mem. 2006, 86, 117–122. [Google Scholar] [CrossRef]
- Di Loreto, S.; Zimmitti, V.; Sebastiani, P.; Cervelli, C.; Falone, S.; Amicarelli, F. Methylglyoxal causes strong weakening of detoxifying capacity and apoptotic cell death in rat hippocampal neurons. Int. J. Biochem. Cell Biol. 2008, 40, 245–257. [Google Scholar] [CrossRef]
- Heimfarth, L.; Loureiro, S.O.; Pierozan, P.; de Lima, B.O.; Reis, K.P.; Torres, E.B.; Pessoa-Pureur, R. Methylglyoxal-induced cytotoxicity in neonatal rat brain: A role for oxidative stress and MAP kinases. Metab. Brain Dis. 2013, 28, 429–438. [Google Scholar] [CrossRef]
- Amicarelli, F.; Colafarina, S.; Cattani, F.; Cimini, A.; Di Ilio, C.; Ceru, M.P.; Miranda, M. Scavenging system efficiency is crucial for cell resistance to ros-mediated methylglyoxal injury. Free Radic. Biol. Med. 2003, 35, 856–871. [Google Scholar] [CrossRef]
- Paget, C.; Lecomte, M.; Ruggiero, D.; Wiernsperger, N.; Lagarde, M. Modification of enzymatic antioxidants in retinal microvascular cells by glucose or advanced glycation end products. Free Radic. Biol. Med. 1998, 25, 121–129. [Google Scholar] [CrossRef]
- Nakayama, M.; Saito, K.; Sato, E.; Nakayama, K.; Terawaki, H.; Ito, S.; Kohno, M. Radical generation by the non-enzymatic reaction of methylglyoxal and hydrogen peroxide. Redox. Rep. 2007, 12, 125–133. [Google Scholar] [CrossRef]
- Hoffmann, G.R. A perspective on the scientific, philosophical, and policy dimensions of hormesis. Dose Response 2009, 7, 1–51. [Google Scholar] [CrossRef]
- Falone, S.; D’Alessandro, A.; Mirabilio, A.; Petruccelli, G.; Cacchio, M.; Di Ilio, C.; Di Loreto, S.; Amicarelli, F. Long term running biphasically improves methylglyoxal-related metabolism, redox homeostasis and neurotrophic support within adult mouse brain cortex. PLoS ONE 2012, 7, e31401. [Google Scholar]
- Benzie, I.F. Evolution of antioxidant defence mechanisms. Eur. J. Nutr. 2000, 39, 53–61. [Google Scholar] [CrossRef]
- Miura, Y. Oxidative stress, radiation-adaptive responses, and aging. J. Radiat. Res. 2004, 45, 357–372. [Google Scholar] [CrossRef]
- Arumugam, T.V.; Gleichmann, M.; Tang, S.C.; Mattson, M.P. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res. Rev. 2006, 5, 165–178. [Google Scholar] [CrossRef]
- Falone, S.; D’Alessandro, A.; Mirabilio, A.; Cacchio, M.; Di Ilio, C.; Di Loreto, S.; Amicarelli, F. Late-onset running biphasically improves redox balance, energy- and methylglyoxal-related status, as well as SIRT1 expression in mouse hippocampus. PLoS ONE 2013, 7, e48334. [Google Scholar]
- Save, E.; Poucet, B.; Foreman, N.; Buhot, M.C. Object exploration and reactions to spatial and nonspatial changes in hooded rats following damage to parietal cortex or hippocampal formation. Behav. Neurosci. 1992, 106, 447–456. [Google Scholar] [CrossRef]
- Okaichi, H.; Oshima, Y.; Jarrard, L.E. Scopolamine impairs both working and reference memory in rats: A replication and extension. Pharmacol. Biochem. Behav. 1989, 34, 599–602. [Google Scholar] [CrossRef]
- Cutler, R.G. Antioxidants and aging. Am. J. Clin. Nutr. 1991, 53, 373–379. [Google Scholar]
- De Zwart, L.L.; Meerman, J.H.; Commandeur, J.N.; Vermeulen, N.P. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999, 26, 202–226. [Google Scholar] [CrossRef]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar]
- Martin, J.P., Jr.; Dailey, M.; Sugarman, E. Negative and positive assays of superoxide dismutase based on hematoxylin autoxidation. Arch. Biochem. Biophys. 1987, 255, 329–336. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Watanabe, K.; Okada, K.; Fukabori, R.; Hayashi, Y.; Asahi, K.; Terawaki, H.; Kobayashi, K.; Watanabe, T.; Nakayama, M. Methylglyoxal (MG) and Cerebro-Renal Interaction: Does Long-Term Orally Administered MG Cause Cognitive Impairment in Normal Sprague-Dawley Rats? Toxins 2014, 6, 254-269. https://doi.org/10.3390/toxins6010254
Watanabe K, Okada K, Fukabori R, Hayashi Y, Asahi K, Terawaki H, Kobayashi K, Watanabe T, Nakayama M. Methylglyoxal (MG) and Cerebro-Renal Interaction: Does Long-Term Orally Administered MG Cause Cognitive Impairment in Normal Sprague-Dawley Rats? Toxins. 2014; 6(1):254-269. https://doi.org/10.3390/toxins6010254
Chicago/Turabian StyleWatanabe, Kimio, Kana Okada, Ryoji Fukabori, Yoshimitsu Hayashi, Koichi Asahi, Hiroyuki Terawaki, Kazuto Kobayashi, Tsuyoshi Watanabe, and Masaaki Nakayama. 2014. "Methylglyoxal (MG) and Cerebro-Renal Interaction: Does Long-Term Orally Administered MG Cause Cognitive Impairment in Normal Sprague-Dawley Rats?" Toxins 6, no. 1: 254-269. https://doi.org/10.3390/toxins6010254
APA StyleWatanabe, K., Okada, K., Fukabori, R., Hayashi, Y., Asahi, K., Terawaki, H., Kobayashi, K., Watanabe, T., & Nakayama, M. (2014). Methylglyoxal (MG) and Cerebro-Renal Interaction: Does Long-Term Orally Administered MG Cause Cognitive Impairment in Normal Sprague-Dawley Rats? Toxins, 6(1), 254-269. https://doi.org/10.3390/toxins6010254





