Evaluation of the Formation of Six Beta-Carboline Alkaloids, a Class of Natural Toxins, in Meat Products Using Liquid Chromatography Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results
2.1. Effects of Cooking Time and Temperature on Six βC-Alkaloids in Pan-Fried Meats and Seafood
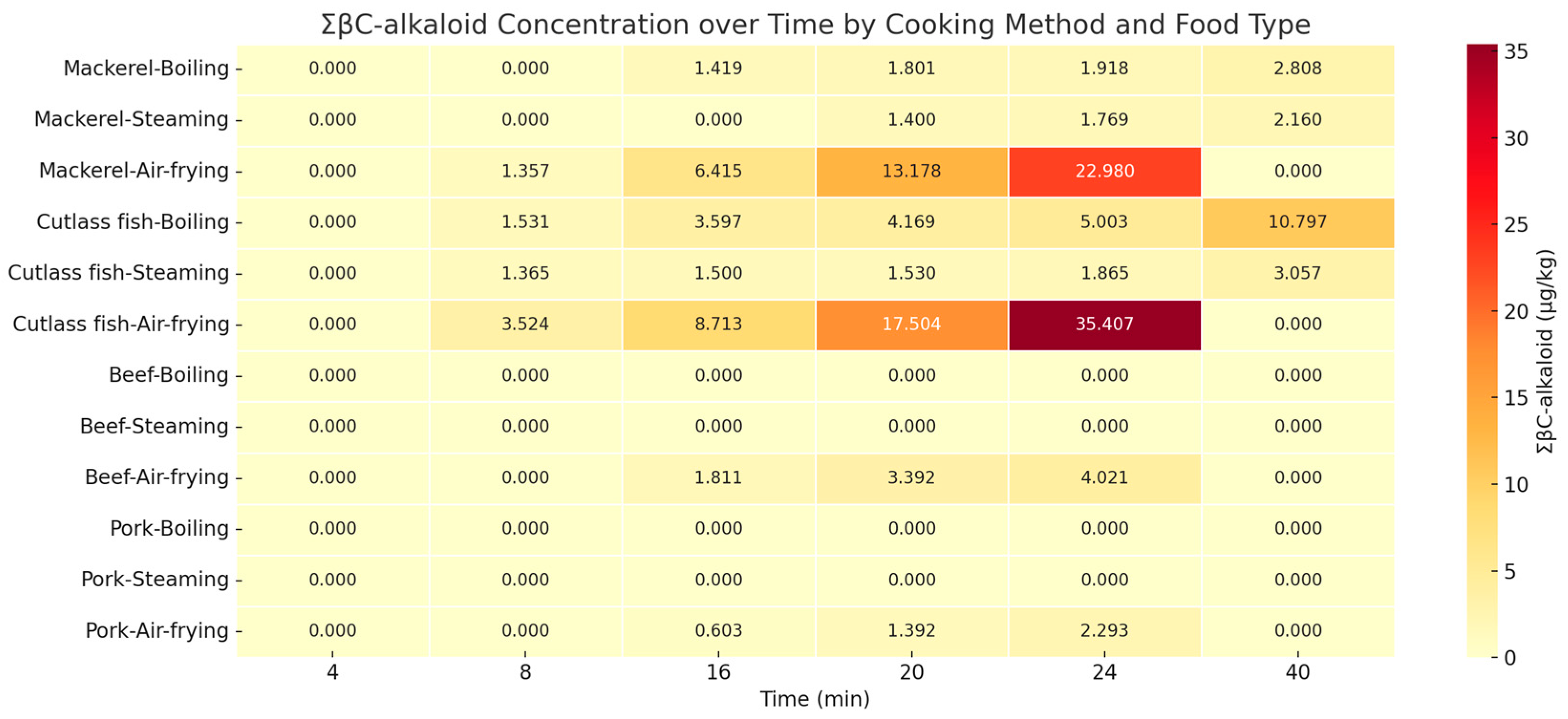
2.2. Effects of Different Cooking Methods on the Formation of βC-Alkaloids in Meat and Seafood Samples
2.3. Effects of Microwave Pretreatment Duration on βC-Alkaloid Levels in Meat and Seafood Samples
3. Discussion
3.1. Effects of Cooking Time and Temperature on the Formation of βC-Alkaloids
3.2. Infulence of Different Cooking Methods on the Formation of Six βC-Alkaloids
3.3. Reduction in βC-Alkaloids in Meat and Seafood via Microwave Pretreatment
4. Conclusions
5. Materials and Methods
5.1. Chemicals and Reagents
5.2. Thermal Processing Conditions Applied to Meat and Seafood Samples Using Various Cooking Methods
5.3. Thermal Treatment with Microwave Pretreatment Followed by Pan-Frying
5.4. Sample Preparation for the Analysis of Six βC-Alkaloids
5.5. Quantitative Analysis of Six βC-Alkaloids by LC-MS/MS
5.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamid, H.A.; Ramli, A.N.; Yusoff, M.M. ΒC-alkaloids from plants as potential leads for antidepressant drugs: A mini review. Front. Pharmacol. 2017, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.H.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199. [Google Scholar] [CrossRef]
- Frison, G.; Favretto, D.; Zancanaro, F.; Fazzin, G.; Ferrara, S.D. A case of β-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Sci. Int. 2008, 179, e37–e43. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, R.; Bharti, R. Pharmacological activities and toxicities of alkaloids on human health. Mater. Today Proc. 2022, 48, 1407–1415. [Google Scholar] [CrossRef]
- Nikam, T.D.; Nitnaware, K.M.; Ahire, M.L. Alkaloids derived from tryptophan: Harmine and related alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 553–574. [Google Scholar]
- Tsen, S.Y.; Ameri, F.; Smith, J. Effects of rosemary extracts on the reduction of heterocyclic amines in beef patties. J. Food Sci. 2006, 71, C469–C473. [Google Scholar] [CrossRef]
- Oz, E.; Aoudeh, E.; Murkovic, M.; Toldra, F.; Gomez-Zavaglia, A.; Brennan, C.; Proestos, C.; Zeng, M.; Oz, F. Heterocyclic aromatic amines in meat: Formation mechanisms, toxicological implications, occurrence, risk evaluation, and analytical methods. Meat Sci. 2023, 205, 109312. [Google Scholar] [CrossRef]
- Bartoszek, A.; Holota, S. Mutagenic and carcinogenic compounds in food. In Chemical and Functional Properties of Food Components; CRC Press: Boca Raton, FL, USA, 2023; pp. 469–496. [Google Scholar]
- Khan, M.R.; Bertus, L.M.; Busquets, R.; Puignou, L. Mutagenic heterocyclic amine content in thermally processed offal products. Food Chem. 2009, 112, 838–843. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, K.S.; Kim, C.-I.; Lee, J.-Y.; Kwon, S.-O.; Park, H.-M. Assessment of dietary exposure to heterocyclic amines based on the Korean total diet study. Food Addit. Contam. Part A 2022, 39, 429–439. [Google Scholar] [CrossRef]
- Herraiz, T. Occurrence of β-carbolines in food. Food Chem. 2008, 107, 842–853. [Google Scholar]
- Bacopoulos, N.G.; Barchas, J.D. Behavioral effects of harman and norharman. Nature 1972, 236, 89–90. [Google Scholar]
- Herraiz, T. Relative exposure to β-carbolines norharman and harman from foods and tobacco smoke. Food Addit. Contam. Part A 2010, 27, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, H.; Zhang, L.; Zhang, Y.; Sheng, Y.; Deng, G.; Wang, C. Subchronic toxicity and concomitant toxicokinetics of long-term oral administration of total alkaloid extracts from seeds of Peganum harmala Linn: A 28-day study in rats. J. Ethnopharmacol. 2019, 238, 111866. [Google Scholar] [CrossRef]
- Jahurul, M.; Jinap, S.; Ang, S.; Abdul-Hamid, A.; Hajeb, P.; Lioe, H.N.; Zaidul, I. Dietary exposure to heterocyclic amines in high-temperature cooked meat and fish in Malaysia. Food Addit. Contam. 2010, 27, 1060–1071. [Google Scholar] [CrossRef]
- Cho, Y.; Jeong, W.Y.; Hwang, S.; Na, S.; Park, H.; Heo, S.; Park, S.; Lim, K.-J.; Shin, H.-S.; Son, J. Development of a simultaneous analytical method for the dietary exposure determination of β-Carboline alkaloids in foods. Food Chem. 2024, 460, 140546. [Google Scholar] [CrossRef]
- Felton, J.; Fultz, E.; Dolbeare, F.; Knize, M. Effect of microwave pretreatment on heterocyclic aromatic amine mutagens/carcinogens in fried beef patties. Food Chem. Toxicol. 1994, 32, 897–903. [Google Scholar] [CrossRef]
- Pfau, W.; Skog, K. Exposure to β-carbolines norharman and harman. J. Chromatogr. B 2004, 802, 115–126. [Google Scholar] [CrossRef]
- Zamora, R.; Hidalgo, F.J. Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning. Crit. Rev. Food Sci. Nutr. 2005, 45, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Jia, W.; Zhu, L.; Mao, L.; Zhang, Y. Recent advances in heterocyclic aromatic amines: An update on food safety and hazardous control from food processing to dietary intake. Compr. Rev. Food Sci. Food Saf. 2020, 19, 124–148. [Google Scholar] [CrossRef] [PubMed]
- Jägerstad, M.; Skog, K.; Arvidsson, P.; Solyakov, A. Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Z. Lebensm. Unters. Forsch. A 1998, 207, 419–427. [Google Scholar] [CrossRef]
- Bellmaine, S.; Schnellbaecher, A.; Zimmer, A. Reactivity and degradation products of tryptophan in solution and proteins. Free Radic. Biol. Med. 2020, 160, 696–718. [Google Scholar] [CrossRef]
- Skog, K.; Johansson, M.; Jägerstad, M. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef] [PubMed]
- Zamora, R.; Hidalgo, F.J. The Maillard reaction and lipid oxidation. Lipid Technol. 2011, 23, 59–62. [Google Scholar] [CrossRef]
- Wang, Y.; Dong, M.; Guo, L.; Zhu, Y.; Jiang, Q.; Xiao, J.; Wang, M.; Zhao, Y. Effect of acrolein on the formation of harman and norharman in chemical models and roast beef patties. Food Res. Int. 2023, 164, 112465. [Google Scholar] [CrossRef]
- Senthong, P.; Sirikitputtisak, T.; Wittayasilp, S. Determination of formaldehyde in fresh seafood under different washing and cooking conditions. Int. J. Food Saf. Nutr. Public Health 2021, 6, 158–166. [Google Scholar] [CrossRef]
- Özogul, Y.; Özogul, F.; Alagoz, S. Fatty acid profiles and fat contents of commercially important seawater and freshwater fish species of Turkey: A comparative study. Food Chem. 2007, 103, 217–223. [Google Scholar] [CrossRef]
- McGlory, C.; Calder, P.C.; Nunes, E.A. The influence of omega-3 fatty acids on skeletal muscle protein turnover in health, disuse, and disease. Front. Nutr. 2019, 6, 144. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, C.; Wang, X.; Zhou, H.; Mai, K.; He, G. EPA and DHA promote cell proliferation and enhance activity of the Akt-TOR-S6K anabolic signaling pathway in primary muscle cells of turbot (Scophthalmus maximus L.). Fish Physiol. Biochem. 2024, 50, 1483–1494. [Google Scholar] [CrossRef]
- Herraiz, T. β-Carboline alkaloids. In Bioactive Compounds in Foods, 1st ed.; Springer: Boston, MA, USA, 2008; pp. 199–223. [Google Scholar]
- Chu, J.; Yuan, Y.; Lin, S.; Wang, X.; Zhang, S. A review of heterocyclic aromatic amines in cooked meat: Generation pathways and mitigation mechanism by natural exogenous additives. J. Food Meas. Charact. 2024, 18, 6638–6661. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Seiquer, I.; Haro, A.; Castellano, R.; Navarro, M.P. Development of the Maillard reaction in foods cooked by different techniques. Intake of Maillard-derived compounds. Food Chem. 2010, 122, 145–153. [Google Scholar] [CrossRef]
- Parisi, S.; Luo, W. The importance of Maillard reaction in processed foods. In Chemistry of Maillard Reactions in Processed Foods; Springer: Singapore, 2018; pp. 1–37. [Google Scholar]
- Kwon, J.; Kim, I.; Moon, B.; Lee, K.-W.; Jung, M.; Lee, J. The effects of different cooking methods and spices on the formation of 11 HCAs in chicken wing and pork belly. Food Control 2023, 147, 109572. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Y.; Lin, B.; He, X.; Li, S.; Zhong, C.; Li, S.; Zhou, Y.; Fan, L. The concentration of benzo[a]pyrene in food cooked by air fryer and oven: A comparison study. Toxics 2024, 12, 416. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Lu, R.; Song, H.; Zhang, Y.; Tang, J.; Zhou, N. Effect of different cooking methods on the formation of aroma components and heterocyclic amines in pork loin. J. Food Process. Preserv. 2017, 41, e12981. [Google Scholar] [CrossRef]
- Xue, W.; Shen, X.; Wu, L. Microwave pretreatment of hemp seeds changes the flavor and quality of hemp seed oil. Ind. Crops Prod. 2024, 213, 118396. [Google Scholar] [CrossRef]
- Oliveira, M.; Franca, A. Microwave heating of foodstuffs. J. Food Eng. 2002, 53, 347–359. [Google Scholar] [CrossRef]
- Hoa, V.B.; Seol, K.H.; Seo, H.W.; Seong, P.N.; Kang, S.M.; Kim, Y.S.; Cho, S.H. Meat quality characteristics of pork bellies in relation to fat level. Anim. Biosci. 2021, 34, 1663. [Google Scholar] [CrossRef]
- Zhang, C.X.; Xi, J.; Wang, S.T.; Ma, Y.X.; Wang, X.D. Effects of deep fat frying conditions on the formation of heterocyclic aromatic amines in chicken meat. Food Sci. Technol. 2021, 42, e67321. [Google Scholar] [CrossRef]
- Ackman, R.; Eaton, C. Mackerel lipids and fatty acids. Can. Inst. Food Technol. J. 1971, 4, 169–174. [Google Scholar] [CrossRef]
- Jiang, H.; Liu, Z.; Wang, S. Microwave processing: Effects and impacts on food components. Crit. Rev. Food Sci. Nutr. 2018, 58, 2476–2489. [Google Scholar] [CrossRef]
- Yin, W.-T.; Shi, R.; Li, K.; Wang, A.-N.; Zhao, Y.-H.; Zhai, Z.-Q. Effect of microwave pretreatment of sunflower kernels on the aroma-active composition, sensory quality, lipid oxidation, tocopherols, heterocyclic amines and polycyclic aromatic hydrocarbons of sunflower oil. LWT 2022, 170, 114077. [Google Scholar] [CrossRef]
- Arimi, J.; Duggan, E.; O’Sullivan, M.; Lyng, J.; O’Riordan, E. Effect of moisture content and water mobility on microwave expansion of imitation cheese. Food Chem. 2010, 121, 509–516. [Google Scholar] [CrossRef]
- Paciulli, M.; Rinaldi, M.; Cavazza, A.; Younce, F.; Chiavaro, E. Influence of microwave heating on food bioactives. In Retention of Bioactives in Food Processing; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Z.; Shi, L.; Cui, Z.; Li, Y. Degradation of β-carbolines harman and norharman in edible oils during heating. Molecules 2021, 26, 7018. [Google Scholar] [CrossRef] [PubMed]
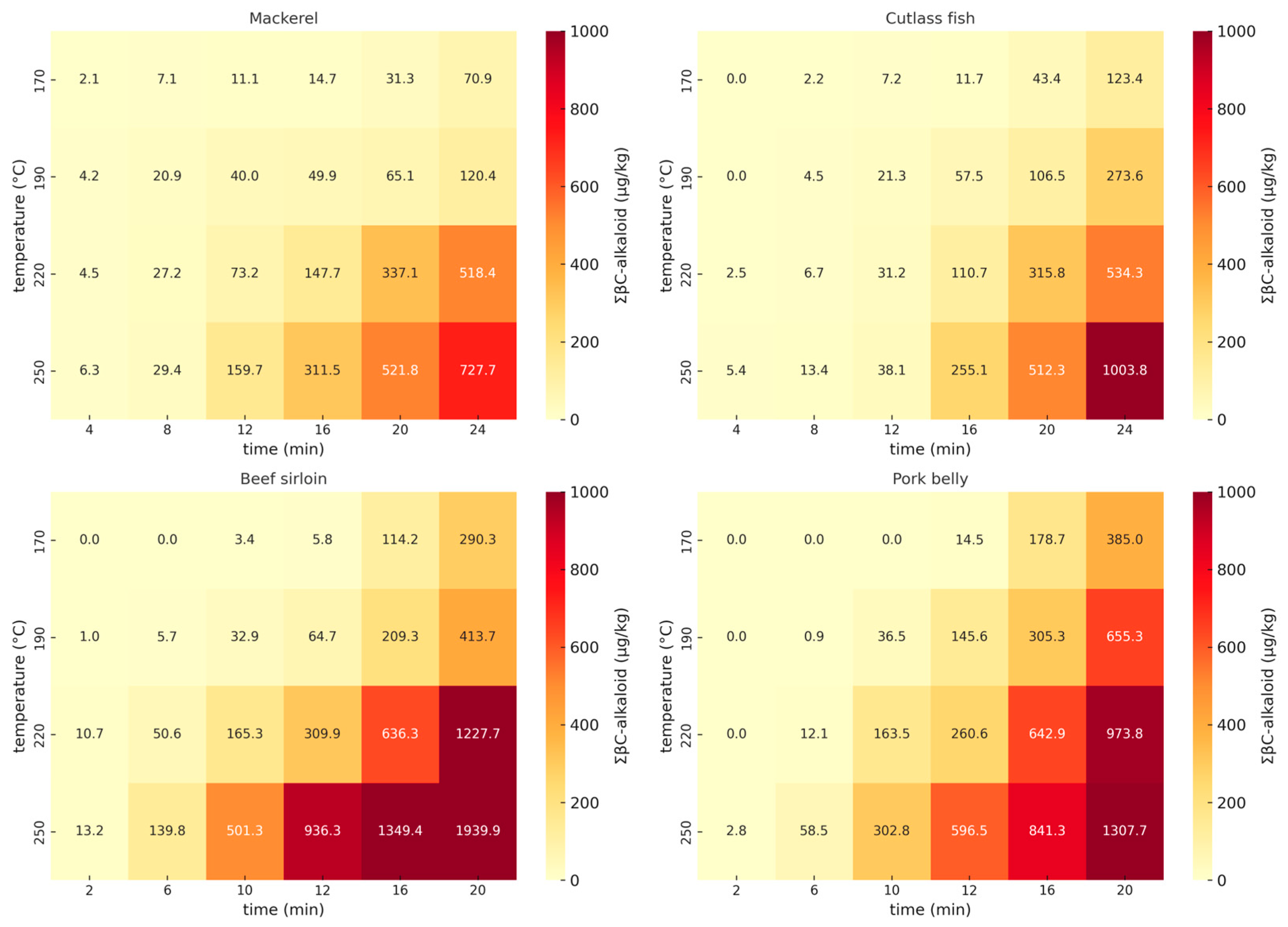
| Temp (°C) | βC-Alkaloids (µg/kg) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | ||
| 170 | Norharman | 2.114 ± 0.207 A,a | 4.431 ± 0.114 A,b | 5.417 ± 0.103 A,bc | 6.304 ± 0.231 A,cd | 7.126 ± 0.202 A,d | 9.045 ± 0.165 A,e | 24.702 ± 0.304 A,f | 35.075 ± 1.350 A,g |
| Harman | ND | ND | 1.662 ± 0.147 A,a | 1.817 ± 0.179 A,a | 2.401 ± 0.136 A,b | 2.858 ± 0.240 A,c | 3.374 ± 0.160 A,d | 16.224 ± 0.195 A,e | |
| Harmol | ND | ND | ND | ND | 0.798 ± 0.014 A,a | 1.174 ± 0.118 A,ab | 1.486 ± 0.026 A,b | 5.187 ± 0.418 A,c | |
| Harmalol | - | ND | ND | 0.363 ± 0.081 A,a | 0.733 ± 0.019 A,b | 1.004 ± 0.028 A,bc | 1.076 ± 0.040 A,c | 3.367 ± 0.297 A,d | |
| Harmine | - | - | ND | ND | ND | 0.601 ± 0.048 A,a | 0.703 ± 0.031 A,a | 3.900 ± 0.172 A,b | |
| Harmaline | - | - | - | ND | ND | ND | ND | 7.164 ± 0.285 A,a | |
| 190 | Norharman | 2.219 ± 0.044 A,a | 9.593 ± 0.199 B,b | 14.291 ± 0.330 B,c | 18.276 ± 1.429 B,d | 21.649 ± 0.097 B,e | 23.599 ± 0.619 B,e | 30.933 ± 1.111 B,f | 56.195 ± 0.967 B,g |
| Harman | 1.967 ± 0.200 A,a | 2.411 ± 0.140 A,ab | 3.900 ± 0.066 B,bc | 4.749 ± 0.140 B,c | 8.993 ± 0.118 B,d | 11.629 ± 0.808 B,e | 15.746 ± 0.746 B,f | 26.77 ± 1.374 B,g | |
| Harmol | ND | ND | 1.736 ± 0.149 A,a | 2.058 ± 0.153 A,ab | 2.654 ± 0.180 A,b | 4.493 ± 0.219 AB,c | 6.514 ± 0.273 B,d | 11.451 ± 0.771 B,e | |
| Harmalol | ND | ND | 1.016 ± 0.050 A,a | 1.51 ± 0.051 B,ab | 1.779 ± 0.071 B,b | 2.027 ± 0.167 AB,b | 3.041 ± 0.275 B,c | 8.700 ± 0.397 B,d | |
| Harmine | - | - | ND | 1.533 ± 0.095 A,a | 2.110 ± 0.144 A,a | 3.641 ± 0.150 B,b | 3.783 ± 0.226 B,b | 7.663 ± 0.868 B,c | |
| Harmaline | - | - | ND | 1.429 ± 0.091 A,a | 2.854 ± 0.114 A,b | 4.509 ± 0.120 A,c | 5.131 ± 0.171 A,d | 9.602 ± 0.140 B,e | |
| 220 | Norharman | 2.858 ± 0.199 B,a | 10.107 ± 0.356 B,b | 16.342 ± 1.269 C,c | 24.163 ± 0.928 C,d | 27.845 ± 1.269 C,d | 47.333 ± 2.705 C,e | 83.021 ± 2.289 C,f | 122.522 ± 0.477 C,g |
| Harman | 1.680 ± 0.184 A,a | 2.852 ± 0.164 B,a | 5.344 ± 0.140 C,ab | 11.819 ± 0.382 C,bc | 15.185 ± 0.913 C,c | 25.425 ± 0.589 C,d | 49.545 ± 3.850 C,e | 105.316 ± 6.421 C,f | |
| Harmol | ND | ND | 2.105 ± 0.099 B,a | 2.440 ± 0.209 A,ab | 3.201 ± 0.120 A,b | 5.263 ± 0.634 B,c | 13.594 ± 0.672 C,d | 20.616 ± 0.140 C,e | |
| Harmalol | ND | 0.381 ± 0.043 A,a | 1.356 ± 0.055 B,b | 3.131 ± 0.145 C,c | 10.806 ± 0.130 D,d | 21.056 ± 1.071 C,e | 27.078 ± 0.420 D,f | 40.576 ± 1.285 D,g | |
| Harmine | - | ND | 0.535 ± 0.058 A,ab | 2.076 ± 0.140 B,b | 4.760 ± 0.060 C,c | 10.939 ± 0.459 D,d | 35.489 ± 0.874 D,e | 58.084 ± 1.183 D,f | |
| Harmaline | ND | ND | 1.504 ± 0.242 A,a | 4.025 ± 0.234 B,a | 11.452 ± 0.682 B,b | 37.670 ± 2.235 B,c | 128.327 ± 2.445 C,d | 171.241 ± 4.215 C,e | |
| 250 | Norharman | 3.971 ± 0.244 D,a | 11.931 ± 0.859 D,b | 17.889 ± 0.189 D,b | 28.454 ± 1.259 D,c | 63.971 ± 2.580 D,d | 90.514 ± 1.408 D,e | 120.478 ± 2.847 D,f | 174.191 ± 5.320 D,g |
| Harman | 2.367 ± 0.130 B,a | 4.010 ± 0.007 C,a | 5.788 ± 0.064 D,a | 16.293 ± 0.743 D,b | 54.024 ± 1.571 D,c | 125.627 ± 2.690 D,d | 176.627 ± 4.875 D,e | 233.994 ± 1.852 D,f | |
| Harmol | ND | ND | 2.368 ± 0.069 D,ab | 5.736 ± 0.451 B,b | 14.719 ± 1.862 B,c | 25.718 ± 2.766 C,d | 33.760 ± 2.890 D,e | 49.633 ± 0.750 D,f | |
| Harmalol | ND | 0.381 ± 0.043 A,a | 1.356 ± 0.055 B,b | 3.131 ± 0.145 C,c | 10.806 ± 0.130 D,d | 21.056 ± 1.071 C,e | 27.078 ± 0.420 D,f | 40.576 ± 1.285 D,g | |
| Harmine | - | ND | 0.535 ± 0.058 A,ab | 2.076 ± 0.140 B,b | 4.760 ± 0.060 C,c | 10.939 ± 0.459 D,d | 35.489 ± 0.874 D,e | 58.084 ± 1.183 D,f | |
| Harmaline | ND | ND | 1.504 ± 0.242 A,a | 4.025 ± 0.234 B,a | 11.452 ± 0.682 B,b | 37.670 ± 2.235 B,c | 128.327 ± 2.445 C,d | 171.241 ± 4.215 C,e | |
| Temp (°C) | βC-Alkaloids (µg/kg) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | ||
| 170 | Norharman | ND | 2.144 ± 0.149 A,ab | 2.241 ± 0.075 A,ab | 3.510 ± 0.401 A,bc | 5.711 ± 0.250 A,c | 9.787 ± 0.367 A,d | 33.806 ± 1.863 A,e | 104.191 ± 2.764 A,f |
| Harman | ND | ND | ND | 1.167 ± 0.014 A,a | 1.501 ± 0.058 A,ab | 1.921 ± 0.106 A,b | 5.886 ± 0.475 A,c | 10.970 ± 0.466 A,d | |
| Harmol | ND | ND | ND | ND | ND | ND | 1.535 ± 0.222 A,a | 2.923 ± 0.420 A,b | |
| Harmalol | ND | ND | ND | ND | ND | ND | 0.628 ± 0.058 A,a | 0.943 ± 0.035 A,b | |
| Harmine | ND | ND | ND | ND | ND | ND | ND | 0.965 ± 0.085 A,a | |
| Harmaline | ND | ND | ND | ND | ND | ND | 1.592 ± 0.092 A,a | 3.396 ± 0.214 A,b | |
| 190 | Norharman | ND | 2.943 ± 0.156 B,ab | 3.255 ± 0.424 B,b | 4.226 ± 0.353 A,b | 13.71 ± 0.858 B,c | 37.231 ± 1.041 B,d | 61.077 ± 1.211 B,e | 145.75 ± 2.556 B,f |
| Harman | ND | ND | 1.261 ± 0.072 A,ab | 1.385 ± 0.083 A,ab | 2.535 ± 0.042 B,b | 10.571 ± 0.313 B,c | 28.645 ± 0.659 B,d | 70.066 ± 1.248 B,e | |
| Harmol | ND | ND | ND | ND | 1.094 ± 0.090 A,a | 2.561 ± 0.100 A,b | 4.581 ± 0.081 A,c | 8.834 ± 0.144 B,d | |
| Harmalol | ND | ND | ND | ND | 0.508 ± 0.073 A,a | 1.553 ± 0.142 A,b | 2.962 ± 0.112 B,c | 5.113 ± 0.441 B,d | |
| Harmine | ND | ND | ND | ND | 0.473 ± 0.041 A,a | 0.902 ± 0.094 A,b | 2.357 ± 0.205 A,c | 12.005 ± 0.241 B,d | |
| Harmaline | ND | ND | ND | 1.417 ± 0.034 A,ab | 2.985 ± 0.081 A,bc | 4.663 ± 0.494 A,cd | 6.874 ± 0.437 B,d | 31.828 ± 2.519 B,e | |
| 220 | Norharman | 2.518 ± 0.072 A,a | 3.827 ± 0.264 C,a | 4.971 ± 0.182 C,a | 6.315 ± 0.832 B,a | 19.686 ± 0.770 C,b | 63.05 ± 0.368 C,c | 147.296 ± 2.150 C,d | 268.828 ± 5.540 C,e |
| Harman | ND | 0.958 ± 0.024 A,ab | 1.716 ± 0.060 A,ab | 2.347 ± 0.123 B,ab | 3.685 ± 0.337 C,b | 22.055 ± 0.200 C,c | 88.663 ± 1.756 C,d | 132.718 ± 2.516 C,e | |
| Harmol | ND | ND | ND | ND | 1.258 ± 0.112 A,a | 5.843 ± 0.424 B,b | 16.246 ± 2.058 B,c | 27.803 ± 1.072 C,d | |
| Harmalol | ND | ND | ND | ND | 0.688 ± 0.074 A,a | 2.552 ± 0.404 B,b | 4.917 ± 0.469 C,c | 10.641 ± 0.707 C,d | |
| Harmine | ND | ND | ND | ND | 0.766 ± 0.111 B,a | 1.266 ± 0.070 B,a | 9.898 ± 0.374 B,b | 17.307 ± 0.412 C,c | |
| Harmaline | ND | ND | ND | 1.983 ± 0.067 B,ab | 5.087 ± 0.339 B,b | 15.888 ± 1.430 B,c | 48.828 ± 2.076 C,d | 76.969 ± 3.285 C,e | |
| 250 | Norharman | 4.410 ± 0.257 B,a | 5.314 ± 0.345 D,a | 9.204 ± 0.534 D,ab | 14.905 ± 0.368 C,bc | 20.171 ± 0.488 C,c | 164.718 ± 2.072 D,d | 285.481 ± 3.060 D,e | 533.008 ± 5.122 D,f |
| Harman | 0.962 ± 0.067 A,a | 1.016 ± 0.094 A,a | 3.113 ± 0.356 B,a | 3.353 ± 0.230 c,ab | 7.190 ± 0.336 D,b | 41.992 ± 1.765 D,c | 121.814 ± 1.784 D,d | 212.438 ± 2.867 D,e | |
| Harmol | ND | ND | 1.076 ± 0.100 A,a | 1.484 ± 0.124 A,a | 1.850 ± 0.118 B,a | 12.827 ± 1.204 C,b | 23.835 ± 1.169 C,c | 58.255 ± 1.179 D,d | |
| Harmalol | ND | ND | ND | ND | 1.191 ± 0.093 B,a | 4.965 ± 0.567 C,b | 10.216 ± 0.757 D,c | 18.619 ± 1.082 D,d | |
| Harmine | ND | ND | ND | ND | 0.834 ± 0.028 B,a | 3.089 ± 0.054 C,b | 12.645 ± 0.074 C,c | 33.796 ± 0.716 D,d | |
| Harmaline | ND | ND | ND | 2.115 ± 0.104 B,ab | 6.819 ± 0.259 C,b | 27.506 ± 2.182 C,c | 58.311 ± 2.969 D,d | 147.642 ± 4.124 D,e | |
| Temp (°C) | βC-Alkloids (µg/kg) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 16 | 20 | ||
| 170 | Norharman | ND | ND | ND | 1.413 ± 0.025 A,a | 1.567 ± 0.020 A,a | 2.894 ± 0.062 A,b | 36.472 ± 0.278 A,c | 62.819 ± 0.253 A,d |
| Harman | ND | ND | ND | 1.153 ± 0.016 A,ab | 1.796 ± 0.038 A,bc | 2.943 ± 0.046 A,c | 40.342 ± 0.837 A,d | 108.955 ± 1.066 A,e | |
| Harmol | - | - | ND | ND | ND | ND | 8.725 ± 0.355 A,a | 41.698 ± 1.338 A,b | |
| Harmalol | - | - | - | - | ND | ND | 8.859 ± 0.178 A,a | 26.217 ± 0.439 A,b | |
| Harmine | - | - | - | - | - | ND | 3.777 ± 0.121 A,a | 16.736 ± 0.880 A,b | |
| Harmaline | - | - | - | - | ND | ND | 16.005 ± 0.461 A,a | 33.916 ± 0.334 A,b | |
| 190 | Norharman | ND | 1.070 ± 0.015 A,ab | 1.686 ± 0.049 A,ab | 2.651 ± 0.010 B,b | 12.420 ± 0.292 B,c | 21.876 ± 0.337 B,d | 62.965 ± 1.588 B,e | 84.801 ± 1.330 B,f |
| Harman | 1.001 ± 0.004 A,a | 2.197 ± 0.031 A,ab | 4.009 ± 0.090 A,bc | 4.629 ± 0.040 B,c | 13.491 ± 0.176 B,d | 24.369 ± 0.384 B,e | 75.105 ± 0.911 B,f | 135.765 ± 1.869 B,g | |
| Harmol | - | ND | ND | ND | 2.649 ± 0.053 A,a | 8.036 ± 0.178 A,b | 14.416 ± 0.312 B,c | 53.502 ± 1.298 B,d | |
| Harmalol | - | - | ND | 0.325 ± 0.029 A,a | 1.308 ± 0.046 A,b | 3.061 ± 0.118 A,c | 20.030 ± 0.445 B,d | 50.402 ± 0.466 B,e | |
| Harmine | - | - | - | ND | 0.915 ± 0.025 A,a | 2.108 ± 0.041 A,b | 4.140 ± 0.006 B,c | 19.872 ± 0.764 B,d | |
| Harmaline | - | - | ND | ND | 2.117 ± 0.035 A,a | 5.212 ± 0.142 A,b | 32.659 ± 0.505 B,c | 69.336 ± 1.006 B,d | |
| 220 | Norharman | 3.138 ± 0.259 A,a | 6.385 ± 0.127 B,b | 17.700 ± 0.438 B,c | 22.092 ± 0.652 C,d | 56.987 ± 1.131 C,e | 87.854 ± 0.799 C,f | 163.199 ± 0.690 C,f | 197.632 ± 1.536 C,g |
| Harman | 4.380 ± 0.291 B,a | 5.565 ± 0.051 B,a | 16.354 ± 0.624 B,b | 22.812 ± 0.992 C,c | 57.700 ± 1.178 C,d | 102.869 ± 1.291 C,e | 245.632 ± 0.783 C,f | 441.524 ± 1.632 C,g | |
| Harmol | 0.847 ± 0.026 A,a | 1.500 ± 0.051 A,a | 5.244 ± 0.145 A,b | 9.865 ± 0.041 A,c | 15.505 ± 0.279 B,d | 32.998 ± 0.708 B,e | 58.110 ± 0.592 C,f | 79.494 ± 1.221 C,g | |
| Harmalol | 0.495 ± 0.012 A,a | 1.195 ± 0.012 A,a | 3.056 ± 0.062 A,b | 5.775 ± 0.169 B,c | 9.908 ± 0.350 B,d | 27.816 ± 0.360 B,e | 47.483 ± 0.819 C,f | 60.263 ± 0.637 C,g | |
| Harmine | 0.459 ± 0.029 A,a | 0.614 ± 0.015 A,a | 1.486 ± 0.033 A,a | 2.775 ± 0.109 A,b | 5.359 ± 0.077 B,c | 10.815 ± 0.246 B,d | 16.573 ± 0.606 C,e | 53.757 ± 0.801 C,f | |
| Harmaline | 1.392 ± 0.013 A,a | 1.843 ± 0.049 A,a | 6.786 ± 0.136 A,b | 10.37 ± 0.150 A,c | 19.861 ± 0.224 B,d | 47.519 ± 0.588 B,e | 105.267 ± 1.185 C,f | 395.026 ± 1.558 C,g | |
| 250 | Norharman | 4.553 ± 0.104 B,a | 21.342 ± 1.236 c,b | 40.122 ± 0.963 C,c | 52.635 ± 0.390 D,d | 113.850 ± 1.176 D,e | 166.368 ± 0.531 D,f | 184.383 ± 1.647 D,g | 217.055 ± 1.833 D,h |
| Harman | 4.047 ± 0.104 B,a | 16.981 ± 0.541 C,b | 44.294 ± 1.668 C,c | 66.087 ± 0.554 D,d | 218.813 ± 0.579 D,e | 349.656 ± 0.925 D,f | 454.306 ± 1.747 D,g | 534.629 ± 2.444 D,h | |
| Harmol | 1.060 ± 0.079 B,a | 7.471 ± 0.409 B,b | 14.26 ± 0.352 B,c | 19.292 ± 0.304 B,d | 35.243 ± 0.408 C,e | 43.164 ± 0.566 C,f | 72.951 ± 1.155 D,g | 113.104 ± 1.591 D,h | |
| Harmalol | 1.401 ± 0.068 B,a | 10.217 ± 0.261 B,b | 12.997 ± 0.524 B,c | 21.994 ± 0.558 C,d | 26.409 ± 0.756 C,e | 34.484 ± 0.461 C,f | 54.199 ± 0.711 D,g | 75.612 ± 0.863 D,h | |
| Harmine | 0.538 ± 0.015 B,a | 2.103 ± 0.031 B,b | 7.810 ± 0.037 B,c | 9.280 ± 0.088 B,d | 17.635 ± 0.343 C,e | 43.271 ± 0.417 C,f | 66.184 ± 0.519 D,g | 113.229 ± 0.900 D,h | |
| Harmaline | 1.576 ± 0.093 B,a | 7.605 ± 0.279 B,b | 20.296 ± 0.181 B,c | 45.554 ± 0.327 B,d | 89.375 ± 1.438 C,e | 299.406 ± 1.440 C,f | 517.417 ± 1.485 D,g | 886.260 ± 1.488 D,h | |
| Temp (°C) | βC-Alkloids (µg/kg) | Time (min) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2 | 4 | 6 | 8 | 10 | 12 | 16 | 20 | ||
| 170 | Norharman | ND | ND | ND | ND | ND | 3.683 ± 0.287 A,a | 44.343 ± 0.630 A,b | 91.228 ± 1.205 A,c |
| Harman | ND | ND | ND | ND | ND | 2.116 ± 0.104 A,a | 65.905 ± 0.583 A,b | 160.948 ± 1.141 A,c | |
| Harmol | - | - | - | - | ND | 0.930 ± 0.007 A,a | 21.752 ± 0.637 A,b | 28.018 ± 0.538 A,c | |
| Harmalol | - | - | - | - | - | ND | 8.406 ± 0.193 A,a | 10.818 ± 0.361 A,b | |
| Harmine | - | - | - | - | ND | 5.965 ± 0.450 A,a | 16.437 ± 0.319 A,b | ||
| Harmaline | - | - | - | - | ND | 1.841 ± 0.162 A,a | 21.877 ± 0.188 A,b | 93.981 ± 1.815 A,c | |
| 190 | Norharman | ND | ND | ND | 3.164 ± 0.072 A,a | 12.857 ± 0.101 A,b | 54.423 ± 0.723 B,c | 79.478 ± 0.706 B,d | 107.496 ± 0.596 B,e |
| Harman | ND | ND | 0.932 ± 0.043 A,a | 2.933 ± 0.230 A,b | 14.974 ± 0.270 A,c | 59.796 ± 1.000 B,d | 148.833 ± 1.048 B,e | 303.693 ± 1.040 B,f | |
| Harmol | - | ND | ND | ND | 3.269 ± 0.042 A,a | 13.051 ± 0.154 B,b | 26.183 ± 0.725 B,c | 57.929 ± 0.509 B,d | |
| Harmalol | - | - | - | - | 2.130 ± 0.067 A,a | 6.589 ± 0.010 A,b | 11.964 ± 0.101 B,c | 25.000 ± 0.435 B,d | |
| Harmine | - | - | - | ND | ND | 3.104 ± 0.114 A,a | 12.552 ± 0.524 B,b | 25.606 ± 0.387 B,c | |
| Harmaline | - | - | - | 1.579 ± 0.206 A,a | 3.298 ± 0.334 A,b | 8.622 ± 0.140 B,c | 26.333 ± 0.314 B,d | 135.59 ± 1.157 B,e | |
| 220 | Norharman | ND | 0.820 ± 0.017 A,a | 5.281 ± 0.144 A,b | 37.719 ± 0.753 B,c | 60.438 ± 0.565 B,d | 79.735 ± 0.677 C,e | 133.857 ± 1.407 C,f | 169.837 ± 1.781 C,g |
| Harman | ND | ND | 3.714 ± 0.037 B,a | 28.730 ± 0.475 B,b | 59.749 ± 1.364 B,c | 98.009 ± 0.623 C,d | 278.264 ± 1.828 C,e | 404.763 ± 1.448 C,f | |
| Harmol | ND | ND | 1.745 ± 0.037 A,a | 8.244 ± 0.180 A,b | 12.485 ± 0.648 B,c | 22.793 ± 0.978 C,d | 52.898 ± 0.893 C,e | 77.422 ± 1.462 C,f | |
| Harmalol | - | - | ND | 4.769 ± 0.248 A,a | 6.465 ± 0.437 B,b | 11.801 ± 0.387 B,c | 28.095 ± 0.794 C,d | 51.353 ± 1.150 C,e | |
| Harmine | - | - | ND | 2.034 ± 0.052 A,a | 5.924 ± 0.252 A,b | 12.650 ± 0.169 B,c | 25.051 ± 0.804 C,d | 38.405 ± 1.114 C,e | |
| Harmaline | - | - | 1.357 ± 0.122 A,a | 13.59 ± 0.494 B,b | 18.409 ± 0.853 B,c | 35.603 ± 0.860 C,d | 124.777 ± 0.817 C,e | 232.057 ± 1.302 C,f | |
| 250 | Norharman | 1.025 ± 0.034 A,a | 13.810 ± 0.314 B,b | 23.782 ± 1.017 B,c | 57.838 ± 0.841 C,d | 83.563 ± 0.943 C,e | 125.03 ± 0.772 D,f | 167.82 ± 1.663 D,g | 195.105 ± 1.061 D,h |
| Harman | 1.766 ± 0.226 A,a | 16.623 ± 0.475 A,b | 19.619 ± 0.896 C,b | 77.722 ± 1.213 C,c | 131.238 ± 1.439 C,d | 283.772 ± 1.411 D,e | 338.825 ± 2.871 D,f | 502.671 ± 2.972 D,g | |
| Harmol | ND | 2.663 ± 0.217 A,a | 5.195 ± 0.201 B,b | 19.825 ± 1.230 B,c | 28.291 ± 0.503 C,d | 39.756 ± 0.646 D,e | 60.901 ± 0.661 D,f | 131.548 ± 1.390 D,g | |
| Harmalol | - | 1.115 ± 0.016 A,a | 2.615 ± 0.025 A,a | 8.619 ± 0.072 B,b | 15.303 ± 0.422 C,c | 27.433 ± 1.393 C,d | 39.788 ± 1.491 D,e | 64.380 ± 1.674 D,f | |
| Harmine | - | 1.049 ± 0.030 A,a | 1.624 ± 0.171 A,a | 3.681 ± 0.183 B,b | 7.051 ± 0.300 B,c | 19.656 ± 0.620 C,d | 33.349 ± 0.540 D,e | 78.894 ± 1.706 D,f | |
| Harmaline | ND | 3.899 ± 0.061 A,a | 5.664 ± 0.127 B,b | 29.485 ± 0.842 C,c | 37.345 ± 1.063 C,d | 100.892 ± 0.705 D,e | 200.614 ± 1.531 D,f | 335.061 ± 2.449 D,g | |
| Cooking Method | βC-Alkaloids (µg/kg) | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | 40 | ||
| Boiling | Norharman | ND | ND | ND | 0.960 ± 0.096 a | 1.124 ± 0.194 ab | 1.419 ± 0.150 b | 1.801 ± 0.144 c | 1.918 ± 0.110 c | 2.808 ± 0.123 d |
| Harman | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmol | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Steaming | Norharman | ND | ND | ND | ND | ND | ND | 1.400 ± 0.049 a | 1.769 ± 0.097 b | 2.160 ± 0.063 c |
| Harman | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmol | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Air- frying (180 °C) | Norharman | ND | 0.765 ± 0.106 ab | 1.357 ± 0.136 b | 1.511 ± 0.184 b | 4.921 ± 0.290 c | 6.415 ± 0.116 d | 13.178 ± 0.642 e | 22.980 ± 1.728 f | |
| Harman | ND | ND | ND | ND | 1.297 ± 0.126 a | 2.471 ± 0.059 b | 5.406 ± 0.509 c | 10.430 ± 0.202 d | ||
| Harmol | ND | ND | ND | ND | ND | ND | 0.854 ± 0.029 a | 1.876 ± 0.120 b | ||
| Harmalol | - | - | - | - | - | ND | 0.629 ± 0.017 a | 0.961 ± 0.118 b | ||
| Harmine | - | - | - | - | ND | ND | 0.324 ± 0.019 a | 0.514 ± 0.019 b | ||
| Harmaline | - | - | - | - | - | - | ND | ND | ||
| Cooking Method | βC-Alkaloids (µg/kg) | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | 40 | ||
| Boiling | Norharman | ND | 1.441 ± 0.252 a | 1.531 ± 0.066 a | 2.379 ± 0.098 b | 2.555 ± 0.178 b | 3.597 ± 0.132 c | 4.169 ± 0.258 c | 5.003 ± 0.270 d | 10.797 ± 0.616 e |
| Harman | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmol | - | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Steaming | Norharman | ND | 1.300 ± 0.064 ab | 1.365 ± 0.061 ab | 1.385 ± 0.141 ab | 1.453 ± 0.117 ab | 1.500 ± 0.102 abc | 1.530 ± 0.077 bc | 1.865 ± 0.093 b | 3.057 ± 0.220 d |
| Harman | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmol | ND | ND | ND | ND | ND | ND | ND | ND | ND | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Air- frying (180 °C) | Norharman | ND | 1.568 ± 0.194 ab | 3.524 ± 0.326 bc | 4.3 ± 0.084 bc | 5.058 ± 0.329 c | 8.713 ± 0.250 d | 17.504 ± 1.244 e | 35.407 ± 2.648 f | |
| Harman | ND | ND | ND | ND | ND | ND | ND | 6.423 ± 0.229 a | ||
| Harmol | ND | ND | ND | ND | ND | ND | ND | 2.879 ± 0.331 a | ||
| Harmalol | - | - | - | - | - | - | - | 0.542 ± 0.133 a | ||
| Harmine | - | - | - | - | - | - | - | 0.673 ± 0.029 a | ||
| Harmaline | - | - | - | - | - | - | - | 1.896 ± 0.160 a | ||
| Cooking Method | βC-Alkaloids (µg/kg) | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | 40 | ||
| Boiling | Norharman | - | - | - | - | - | - | - | - | - |
| Harman | - | - | - | - | - | - | - | - | - | |
| Harmol | - | - | - | - | - | - | - | - | - | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Steam | Norharman | - | - | - | - | - | - | - | - | - |
| Harman | - | - | - | - | - | - | - | - | - | |
| Harmol | - | - | - | - | - | - | - | - | - | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Air- frying (180 °C) | Norharman | - | - | - | - | ND | 1.147 ± 0.057 a | 2.208 ± 0.262 b | 2.685 ± 0.049 c | |
| Harman | - | - | - | - | ND | 0.664 ± 0.091 a | 1.184 ± 0.093 b | 1.336 ± 0.097 c | ||
| Harmol | - | - | - | - | - | ND | ND | ND | ||
| Harmalol | - | - | - | - | - | ND | ND | ND | ||
| Harmine | - | - | - | - | - | - | - | - | ||
| Harmaline | - | - | - | - | - | - | - | - | ||
| Cooking Method | βC-Alkaloids (µg/kg) | Time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 6 | 8 | 10 | 12 | 16 | 20 | 24 | 40 | ||
| Boiling | Norharman | - | - | - | - | - | - | - | - | - |
| Harman | - | - | - | - | - | - | - | - | - | |
| Harmol | - | - | - | - | - | - | - | - | - | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Steaming | Norharman | - | - | - | - | - | - | - | - | - |
| Harman | - | - | - | - | - | - | - | - | - | |
| Harmol | - | - | - | - | - | - | - | - | - | |
| Harmalol | - | - | - | - | - | - | - | - | - | |
| Harmine | - | - | - | - | - | - | - | - | - | |
| Harmaline | - | - | - | - | - | - | - | - | - | |
| Air- frying (180 °C) | Norharman | - | - | - | - | ND | 0.603 ± 0.178 a | 1.392 ± 0.236 b | 1.541 ± 0.192 b | |
| Harman | - | - | - | - | - | ND | ND | 0.752 ± 0.106 a | ||
| Harmol | ND | ND | ND | ND | ND | ND | ND | ND | ||
| Harmalol | - | - | - | - | - | - | - | - | ||
| Harmine | - | - | - | - | - | - | - | - | ||
| Harmaline | - | - | - | - | - | - | - | ND | ||
| Food Sample | βC-Alkaloids (µg/kg) | Time (min) | |||||
|---|---|---|---|---|---|---|---|
| Con. | 1 | 2 | 3 | 4 | 5 | ||
| Pork belly | Norharman | 61.432 ± 1.483 e | 49.530 ± 1.193 d | 42.482 ± 0.871 c | 24.983 ± 0.677 a | 30.765 ± 1.008 b | 69.850 ± 1.521 f |
| Harman | 60.876 ± 0.634 e | 55.219 ± 0.286 d | 48.371 ± 0.342 c | 15.707 ± 0.211 a | 19.819 ± 0.621 b | 70.234 ± 0.968 f | |
| Harmol | 12.897 ± 0.454 e | 6.568 ± 0.351 c | 5.250 ± 0.215 b | 2.721 ± 0.137 a | 6.023 ± 0.286 bc | 8.473 ± 0.308 d | |
| Harmalol | 6.749 ± 0.309 e | 2.568 ± 0.221 c | 1.858 ± 0.027 ab | 1.456 ± 0.047 a | 2.255 ± 0.110 bc | 4.177 ± 0.162 d | |
| Harmine | 6.593 ± 0.326 f | 3.260 ± 0.041 d | 2.534 ± 0.138 c | 0.454 ± 0.035 a | 1.126 ± 0.172 b | 3.727 ± 0.060 e | |
| Harmaline | 18.171 ± 0.375 e | 10.177 ± 0.398 d | 8.99 ± 0.185 c | 2.439 ± 0.103 a | 4.145 ± 0.083 b | 17.921 ± 0.481 e | |
| Beef sirloin | Norharman | 59.516 ± 0.887 e | 38.805 ± 1.273 d | 12.882 ± 0.480 a | 15.859 ± 0.407 b | 34.935 ± 1.141 c | 97.448 ± 0.800 f |
| Harman | 60.131 ± 0.557 d | 29.458 ± 0.205 c | 11.865 ± 0.506 a | 13.838 ± 0.150 b | 29.653 ± 1.150 c | 84.902 ± 0.832 e | |
| Harmol | 16.067 ± 0.234 c | 6.665 ± 0.136 b | 1.178 ± 0.018 a | 2.097 ± 0.222 a | 6.209 ± 0.189 b | 15.099 ± 3.204 c | |
| Harmalol | 11.146 ± 0.307 e | 5.540 ± 0.174 c | 0.922 ± 0.060 a | 1.373 ± 0.151 a | 2.910 ± 0.164 b | 7.335 ± 0.110 d | |
| Harmine | 6.422 ± 0.225 d | 2.866 ± 0.221 b | 0.253 ± 0.019 a | 0.391 ± 0.038 a | 0.648 ± 0.070 a | 4.548 ± 0.166 c | |
| Harmaline | 20.348 ± 0.321 d | 4.056 ± 0.243 b | 0.715 ± 0.053 a | 0.884 ± 0.023 a | 1.528 ± 0.036 a | 15.070 ± 0.802 c | |
| Mackerel | Norharman | 29.749 ± 0.533 f | 26.591 ± 0.152 e | 19.664 ± 0.298 c | 17.531 ± 0.155 b | 13.414 ± 0.183 a | 23.722 ± 0.306 d |
| Harman | 15.899 ± 0.096 f | 13.652 ± 0.150 e | 6.399 ± 0.093 c | 5.059 ± 0.021 b | 4.164 ± 0.055 a | 7.040 ± 0.167 d | |
| Harmol | 3.976 ± 0.057 e | 2.533 ± 0.107 d | 1.702 ± 0.049 c | 1.029 ± 0.056 b | 0.828 ± 0.047 a | 1.580 ± 0.048 c | |
| Harmalol | 2.436 ± 0.029 e | 1.864 ± 0.034 d | 0.665 ± 0.026 c | 0.553 ± 0.025 b | 0.427 ± 0.009 a | 0.698 ± 0.023 c | |
| Harmine | 3.069 ± 0.204 d | 1.986 ± 0.085 c | 0.351 ± 0.037 a | 0.238 ± 0.058 a | 0.404 ± 0.171 a | 0.915 ± 0.089 b | |
| Harmaline | 3.368 ± 0.088 e | 2.800 ± 0.033 d | 0.809 ± 0.042 c | 0.577 ± 0.044 b | 0.314 ± 0.027 a | 0.930 ± 0.012 c | |
| Cutlassfish | Norharman | 22.294 ± 0.436 e | 10.105 ± 0.317 d | 5.909 ± 0.098 b | 4.040 ± 0.127 a | 4.683 ± 0.141 a | 6.813 ± 0.197 c |
| Harman | 4.241 ± 0.111 e | 1.839 ± 0.081 d | 0.905 ± 0.035 b | 0.601 ± 0.033 a | 0.785 ± 0.034 b | 1.126 ± 0.057 c | |
| Harmol | 1.379 ± 0.033 b | 1.111 ± 0.064 a | ND | - | ND | ND | |
| Harmalol | 0.821 ± 0.060 c | 0.354 ± 0.016 a | ND | - | ND | 0.442 ± 0.019 b | |
| Harmine | 0.998 ± 0.019 e | 0.549 ± 0.007 c | 0.41 ± 0.008 b | ND | 0.268 ± 0.008 a | 0.636 ± 0.009 d | |
| Harmaline | 5.766 ± 0.142 d | 3.686 ± 0.152 c | 2.731 ± 0.136 b | 1.228 ± 0.035 a | 1.397 ± 0.036 a | 2.678 ± 0.109 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, K.-J.; Lee, D.-K.; Shin, H.-S. Evaluation of the Formation of Six Beta-Carboline Alkaloids, a Class of Natural Toxins, in Meat Products Using Liquid Chromatography Tandem Mass Spectrometry. Toxins 2025, 17, 266. https://doi.org/10.3390/toxins17060266
Lim K-J, Lee D-K, Shin H-S. Evaluation of the Formation of Six Beta-Carboline Alkaloids, a Class of Natural Toxins, in Meat Products Using Liquid Chromatography Tandem Mass Spectrometry. Toxins. 2025; 17(6):266. https://doi.org/10.3390/toxins17060266
Chicago/Turabian StyleLim, Kyung-Jik, Do-Kyeong Lee, and Han-Seung Shin. 2025. "Evaluation of the Formation of Six Beta-Carboline Alkaloids, a Class of Natural Toxins, in Meat Products Using Liquid Chromatography Tandem Mass Spectrometry" Toxins 17, no. 6: 266. https://doi.org/10.3390/toxins17060266
APA StyleLim, K.-J., Lee, D.-K., & Shin, H.-S. (2025). Evaluation of the Formation of Six Beta-Carboline Alkaloids, a Class of Natural Toxins, in Meat Products Using Liquid Chromatography Tandem Mass Spectrometry. Toxins, 17(6), 266. https://doi.org/10.3390/toxins17060266






