Impact of Regulated and Non-Regulated Food-Associated Mycotoxins on the Viability and Proliferation of Enteric Glial Cells
Abstract
1. Introduction
2. Results
2.1. Regulated and Non-Regulated Mycotoxins Alter EGC Proliferation
2.2. Regulated and Non-Regulated Mycotoxins Affect EGC Viability
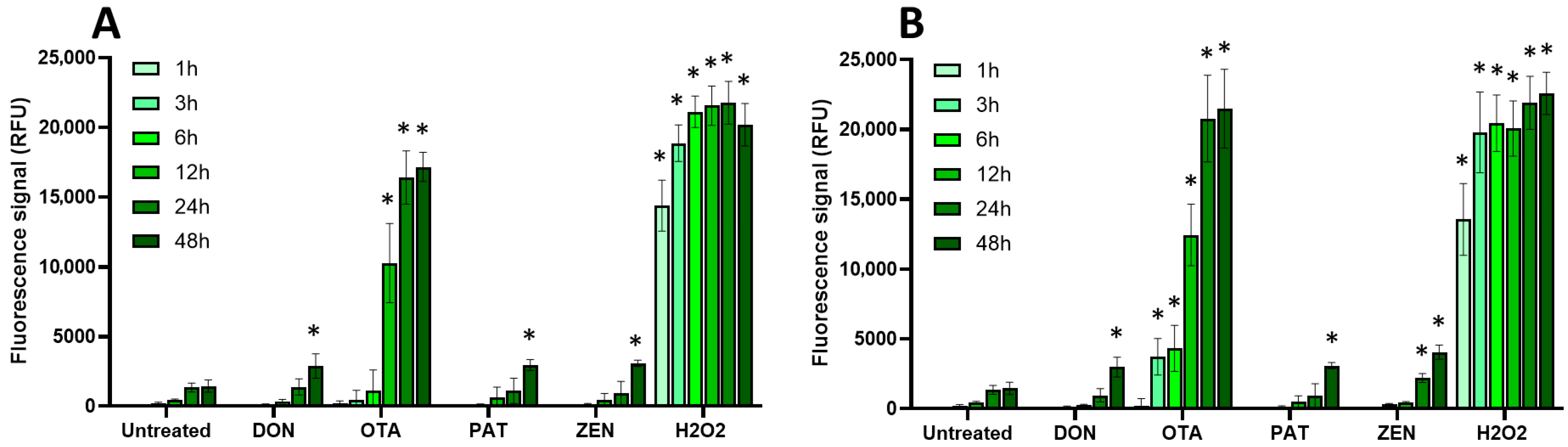
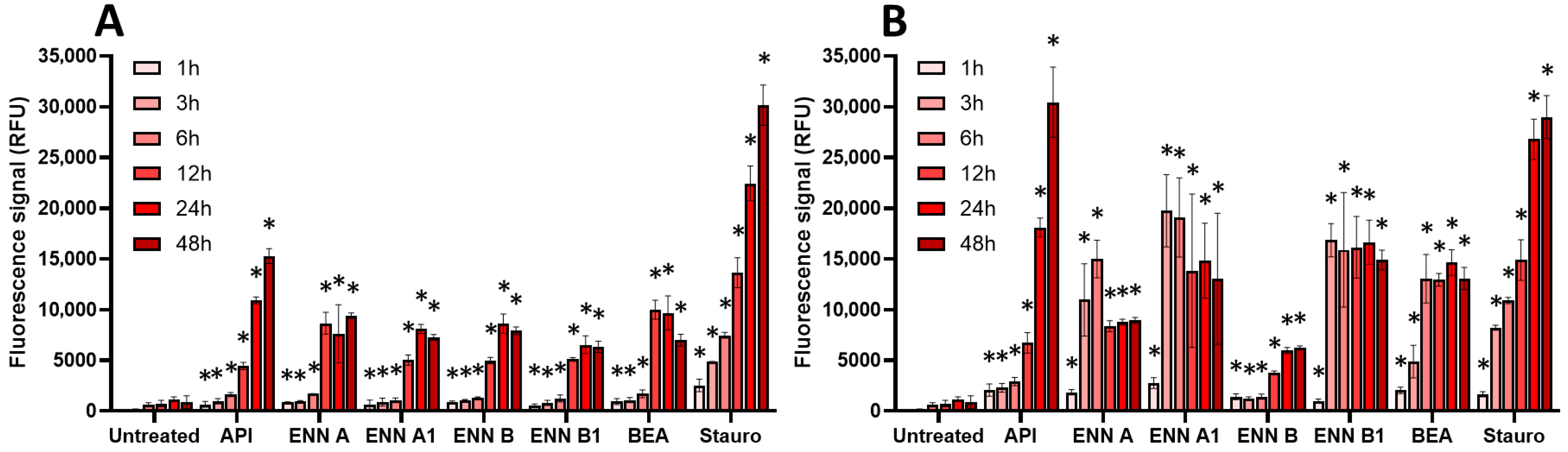
2.3. Oxidative Stress, Membrane Alteration, and/or Apoptosis Induction Are Involved in the Toxic Effect of Regulated and Non-Regulated Mycotoxins on EGCs
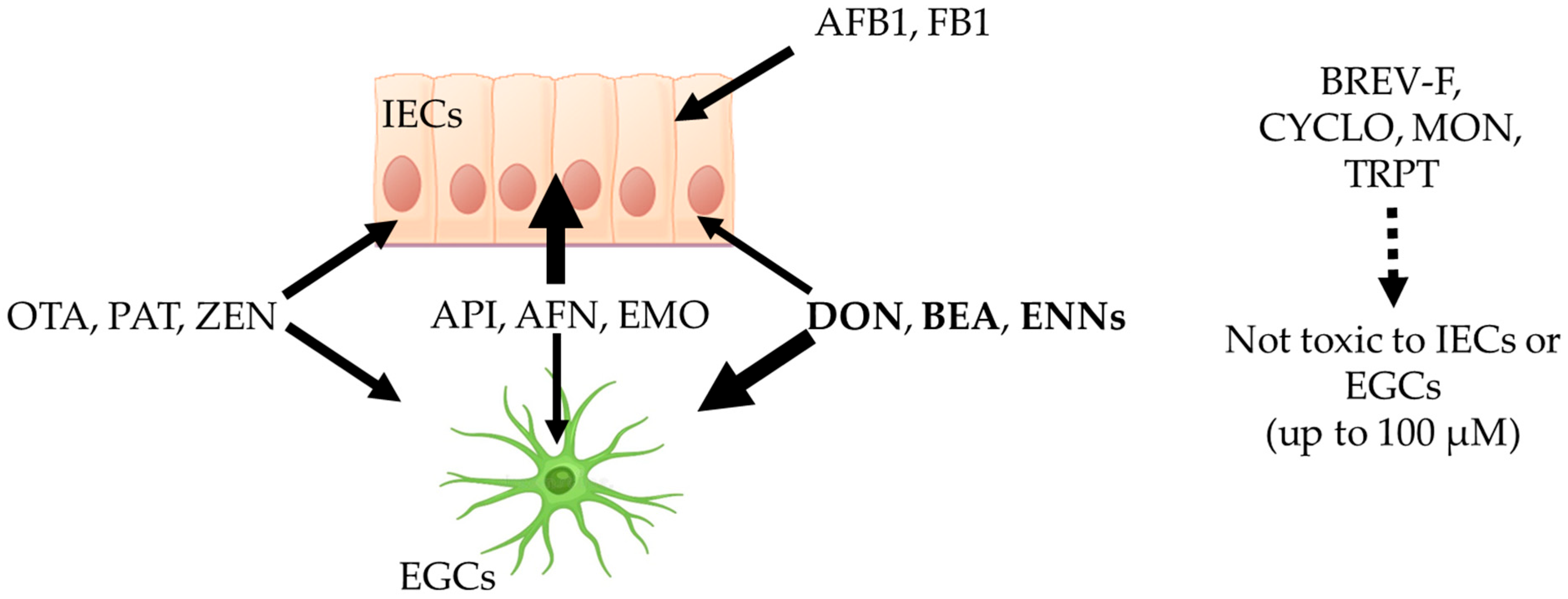
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Mycotoxins
5.2. Cell Culture, Antiproliferative, and Cytotoxicity Assays
5.3. Intracellular ROS Assay
5.4. Propidium Iodide Assay
5.5. Caspase-3/7 Assay
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Thrane, U.; Samson, R.A.; Pitt, J.I. Important mycotoxins and the fungi which produce them. Adv. Exp. Med. Biol. 2006, 571, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D. Foodborne mycotoxicoses, risk assessment and underestimated hazard of masked mycotoxins and joint mycotoxin effects or interaction. Environ. Toxicol. Pharmacol. 2015, 39, 794–809. [Google Scholar] [CrossRef]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Khoshal, A.K.; Novak, B.; Martin, P.G.P.; Jenkins, T.; Neves, M.; Schatzmayr, G.; Oswald, I.P.; Pinton, P. Co-Occurrence of DON and Emerging Mycotoxins in Worldwide Finished Pig Feed and Their Combined Toxicity in Intestinal Cells. Toxins 2019, 11, 727. [Google Scholar] [CrossRef]
- Schothorst, R.C.; van Egmond, H.P. Report from SCOOP task 3.2.10 “collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states”: Subtask: Trichothecenes. Toxicol. Lett. 2004, 153, 133–143. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Effects of mycotoxins on the intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef]
- Bertero, A.; Fossati, P.; Tedesco, D.E.A.; Caloni, F. Beauvericin and Enniatins: In Vitro Intestinal Effects. Toxins 2020, 12, 686. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised Intestinal Barrier Induced by Mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the feed contaminant deoxynivalenol on the intestine of monogastric animals: Poultry and swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef]
- Maresca, M.; Fantini, J. Some food-associated mycotoxins as potential risk factors in humans predisposed to chronic intestinal inflammatory diseases. Toxicon 2010, 56, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Guo, C.; Yu, S.; Zhu, L.; Wang, Y.; Hu, H.; Deng, J. Progress in Mycotoxins Affecting Intestinal Mucosal Barrier Function. Int. J. Mol. Sci. 2019, 20, 2777. [Google Scholar] [CrossRef]
- Bonnet, M.S.; Roux, J.; Mounien, L.; Dallaporta, M.; Troadec, J.D. Advances in deoxynivalenol toxicity mechanisms: The brain as a target. Toxins 2012, 4, 1120–1138. [Google Scholar] [CrossRef]
- Doi, K.; Uetsuka, K. Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int. J. Mol. Sci. 2011, 12, 5213–5237. [Google Scholar] [CrossRef]
- Purzycki, C.B.; Shain, D.H. Fungal toxins and multiple sclerosis: A compelling connection. Brain Res. Bull. 2010, 82, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Terciolo, C.; Maresca, M.; Pinton, P.; Oswald, I.P. Review article: Role of satiety hormones in anorexia induction by Trichothecene mycotoxins. Food Chem. Toxicol. 2018, 121, 701–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; You, L.; Wu, W.; Wang, X.; Chrienova, Z.; Nepovimova, E.; Wu, Q.; Kuca, K. The neurotoxicity of trichothecenes T-2 toxin and deoxynivalenol (DON): Current status and future perspectives. Food Chem. Toxicol. 2020, 145, 111676. [Google Scholar] [CrossRef]
- Makowska, K.; Obremski, K.; Gonkowski, S. The impact of T-2 toxin on vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nerve structures in the wall of the porcine stomach and duodenum. Toxins 2018, 10, 138. [Google Scholar] [CrossRef]
- Makowska, K.; Obremski, K.; Zielonka, L.; Gonkowski, S. The influence of low doses of zearalenone and T-2 toxin on calcitonin gene related peptide-like immunoreactive (CGRP-LI) neurons in the ENS of the porcine descending colon. Toxins 2017, 9, 98. [Google Scholar] [CrossRef]
- Obremski, K.; Gonkowski, S.; Wojtacha, P. Zearalenone-induced changes in the lymphoid tissue and mucosal nerve fibers in the porcine ileum. Pol. J. Vet. Sci. 2015, 18, 357–365. [Google Scholar] [CrossRef]
- Gonkowski, S.; Gajęcka, M.; Makowska, K. Mycotoxins and the Enteric Nervous System. Toxins 2020, 12, 461. [Google Scholar] [CrossRef]
- Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Bernardini, N.; Blandizzi, C.; Fornai, M. Enteric Glia at the Crossroads between Intestinal Immune System and Epithelial Barrier: Implications for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 9199. [Google Scholar] [CrossRef]
- Neunlist, M.; Rolli-Derkinderen, M.; Latorre, R.; Van Landeghem, L.; Coron, E.; Derkinderen, P.; De Giorgio, R. Enteric glial cells: Recent developments and future directions. Gastroenterology 2014, 147, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Cortes, F.; Turco, F.; Linan-Rico, A.; Soghomonyan, S.; Whitaker, E.; Wehner, S.; Cuomo, R.; Christofi, F.L. Enteric Glial Cells: A New Frontier in Neurogastroenterology and Clinical Target for Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 433–449. [Google Scholar] [CrossRef]
- Pochard, C.; Coquenlorge, S.; Freyssinet, M.; Naveilhan, P.; Bourreille, A.; Neunlist, M.; Rolli-Derkinderen, M. The multiple faces of inflammatory enteric glial cells: Is Crohn’s disease a gliopathy? Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G1–G11. [Google Scholar] [CrossRef]
- Savidge, T.C.; Sofroniew, M.V.; Neunlist, M. Starring roles for astroglia in barrier pathologies of gut and brain. Lab. Investig. 2007, 87, 731–736. [Google Scholar] [CrossRef]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Rissato, D.F.; de Santi Rampazzo, A.P.; Borges, S.C.; Sousa, F.C.; Busso, C.; Buttow, N.C.; Natali, M.R.M. Chronic ingestion of deoxynivalenol-contaminated diet dose-dependently decreases the area of myenteric neurons and gliocytes of rats. Neurogastroenterol. Motil. 2020, 32, e13770. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure-Activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Coulet, F.; Hymery, N.; Coton, E.; Coton, M. Enniatin Mycotoxins in Food: A Systematic Review of Global Occurrence, Biosynthesis, and Toxicological Impacts on In Vitro Human Cell Models. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70270. [Google Scholar] [CrossRef]
- Assunção, R.; Alvito, P.; Kleiveland, C.R.; Lea, T.E. Characterization of in vitro effects of patulin on intestinal epithelial and immune cells. Toxicol. Lett. 2016, 250, 47. [Google Scholar] [CrossRef]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.; Duarte-Hospital, C.; Vignard, J.; Boutet-Robinet, E.; Sulyok, M.; Snini, S.P.; Alassane-Kpembi, I.; Lippi, Y.; Puel, S.; Oswald, I.P.; et al. Versicolorin A, a precursor in aflatoxins biosynthesis, is a food contaminant toxic for human intestinal cells. Environ. Int. 2020, 137, 105568. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martínez, M.; Martínez-Larrañaga, M.R.; Anadón, A.; Martínez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353, 21. [Google Scholar] [CrossRef]
- Fan, W.; Shen, T.; Ding, Q.; Lv, Y.; Li, L.; Huang, K.; Yan, L.; Song, S. Zearalenone induces ROS-mediated mitochondrial damage in porcine IPEC-J2 cells. J. Biochem. Mol. Toxicol. 2017, 31, 10. [Google Scholar] [CrossRef]
- Bony, S.; Carcelen, M.; Olivier, L.; Devaux, A. Genotoxicity assessment of deoxynivalenol in the Caco-2 cell line model using the Comet assay. Toxicol. Lett. 2006, 166, 67–76. [Google Scholar] [CrossRef]
- Pinton, P.; Terciolo, C.; Payros, D.; Oswald, I.P. Mycotoxins hazard: The European view. Curr. Opin. Food Sci. 2025, 63, 101306. [Google Scholar] [CrossRef]
- Novak, B.; Rainer, V.; Sulyok, M.; Haltrich, D.; Schatzmayr, G.; Mayer, E. Twenty-Eight Fungal Secondary Metabolites Detected in Pig Feed Samples: Their Occurrence, Relevance and Cytotoxic Effects In Vitro. Toxins 2019, 11, 537. [Google Scholar] [CrossRef] [PubMed]
- Sergent, T.; Parys, M.; Garsou, S.; Pussemier, L.; Schneider, Y.J.; Larondelle, Y. Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations. Toxicol. Lett. 2006, 164, 167–176. [Google Scholar] [CrossRef]
- Razafimanjato, H.; Garmy, N.; Guo, X.J.; Varini, K.; di Scala, C.; di Pasquale, E.; Taïeb, N.; Maresca, M. The food-associated fungal neurotoxin ochratoxin A inhibits the absorption of glutamate by astrocytes through a decrease in cell surface expression of the excitatory amino-acid transporters GLAST and GLT-1. Neurotoxicology 2010, 31, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Razafimanjato, H.; Benzaria, A.; Taïeb, N.; Guo, X.J.; Vidal, N.; Di Scala, C.; Varini, K.; Maresca, M. The ribotoxin deoxynivalenol affects the viability and functions of glial cells. Glia 2011, 59, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Reale, O.; Huguet, A.; Fessard, V. Novel Insights on the Toxicity of Phycotoxins on the Gut through the Targeting of Enteric Glial Cells. Mar. Drugs 2019, 17, 429. [Google Scholar] [CrossRef]
- Olleik, H.; Yacoub, T.; Hoffer, L.; Gnansounou, S.M.; Benhaiem-Henry, K.; Nicoletti, C.; Mekhalfi, M.; Pique, V.; Perrier, J.; Hijazi, A.; et al. Synthesis and Evaluation of the Antibacterial Activities of 13-Substituted Berberine Derivatives. Antibiotics 2020, 9, 381. [Google Scholar] [CrossRef]
- Peña, Q.; Rodríguez-Calado, S.; Simaan, A.J.; Capdevila, M.; Bayón, P.; Palacios, O.; Lorenzo, J.; Iranzo, O. Cell-penetrating peptide-conjugated copper complexes for redox-mediated anticancer therapy. Front. Pharmacol. 2022, 13, 1060827. [Google Scholar] [CrossRef]
- Di Pasquale, E.; Salmi-Smail, C.; Brunel, J.M.; Sanchez, P.; Fantini, J.; Maresca, M. Biophysical studies of the interaction of squalamine and other cationic amphiphilic molecules with bacterial and eukaryotic membranes: Importance of the distribution coefficient in membrane selectivity. Chem. Phys. Lipids 2010, 163, 131–140. [Google Scholar] [CrossRef]
- Huang, T.C.; Lee, J.F.; Chen, J.Y. Pardaxin, an antimicrobial peptide, triggers caspase-dependent and ROS-mediated apoptosis in HT-1080 cells. Mar. Drugs 2011, 9, 1995–2009. [Google Scholar] [CrossRef] [PubMed]
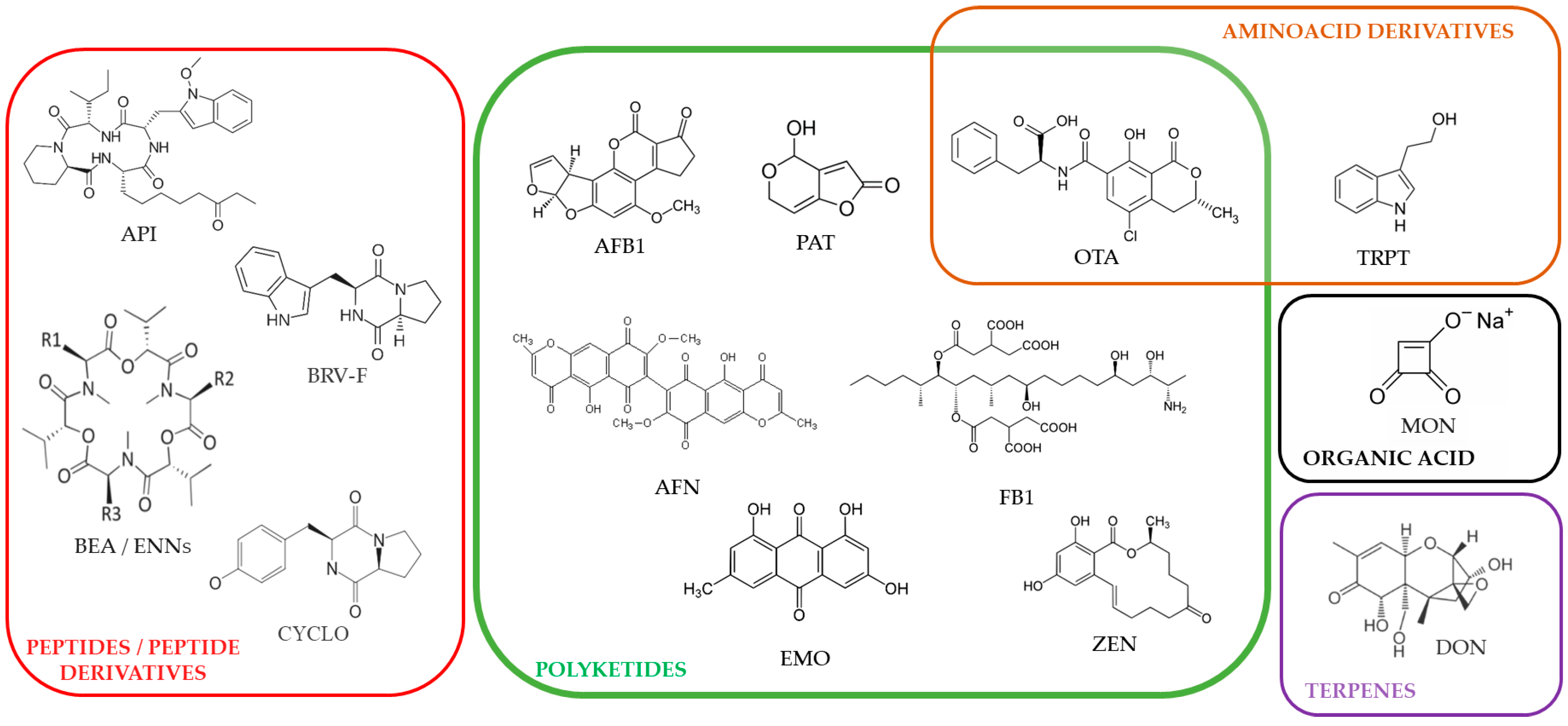
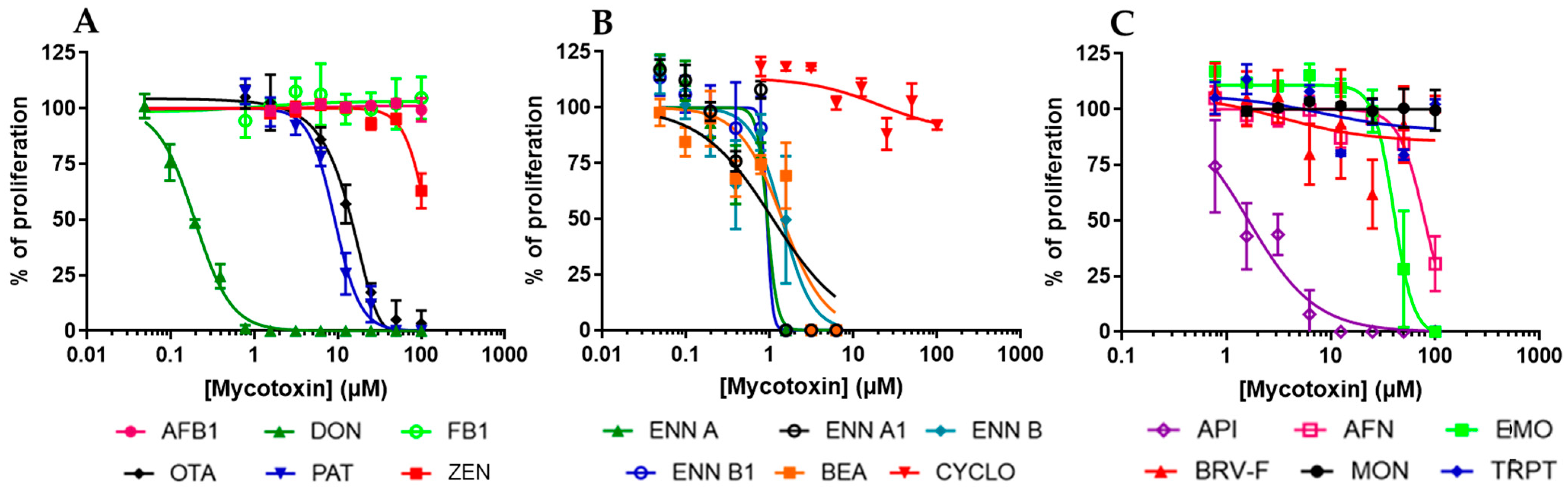
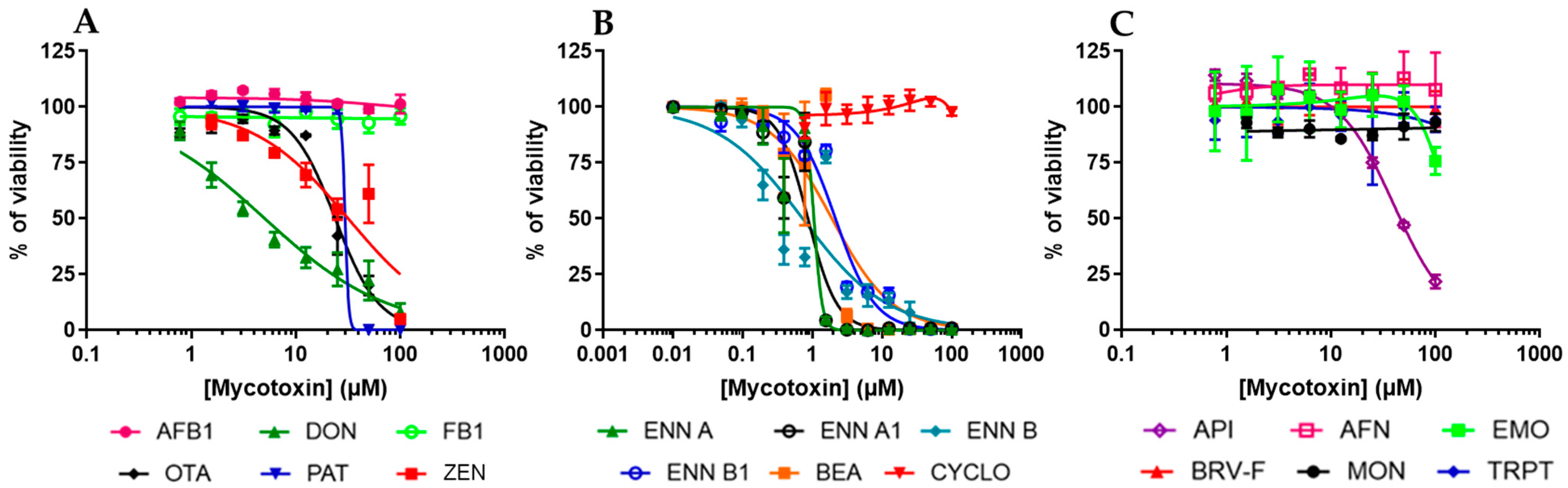
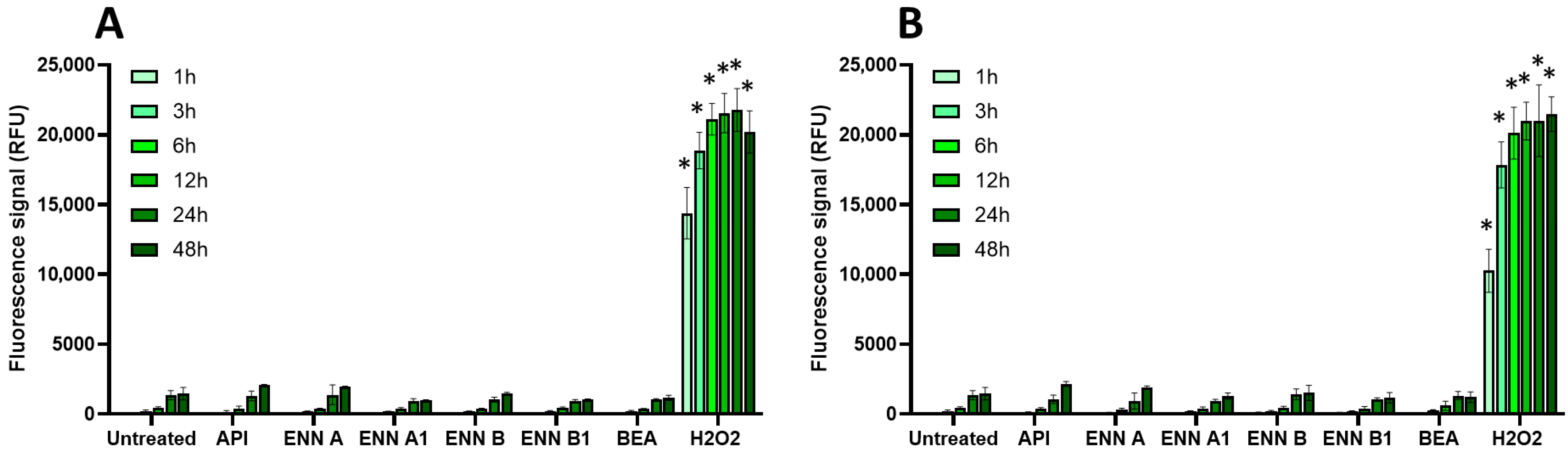
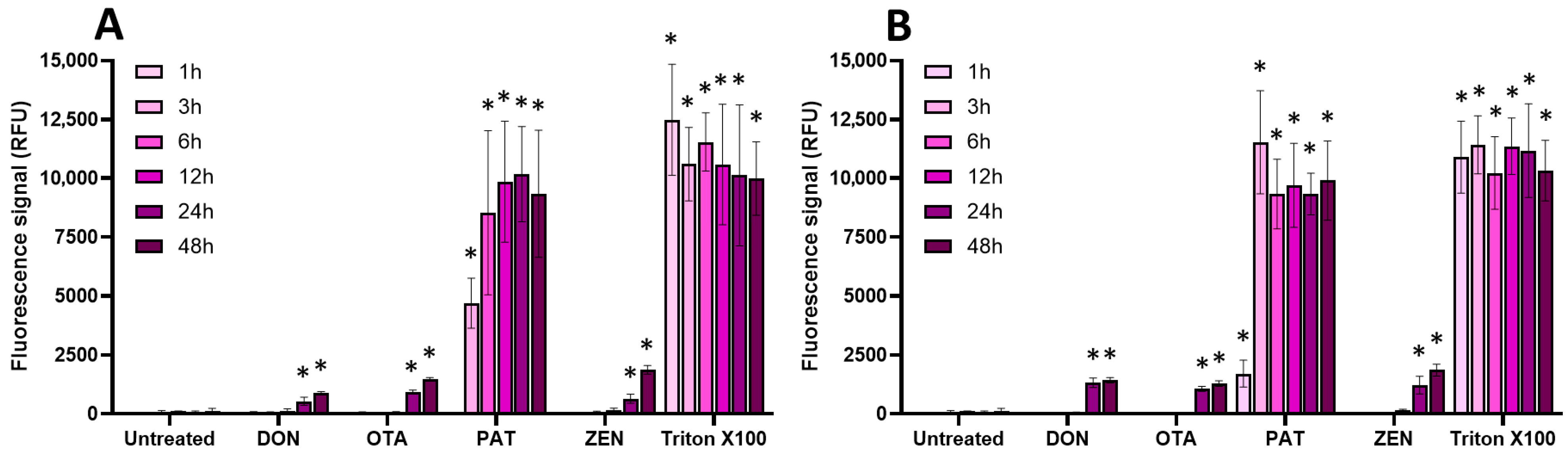
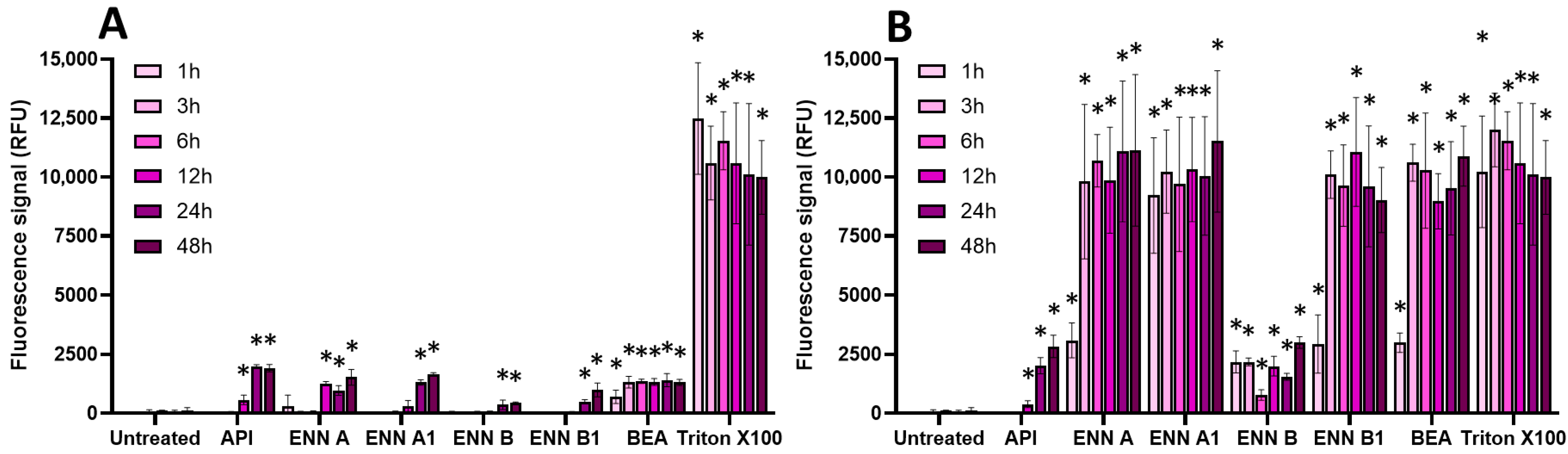
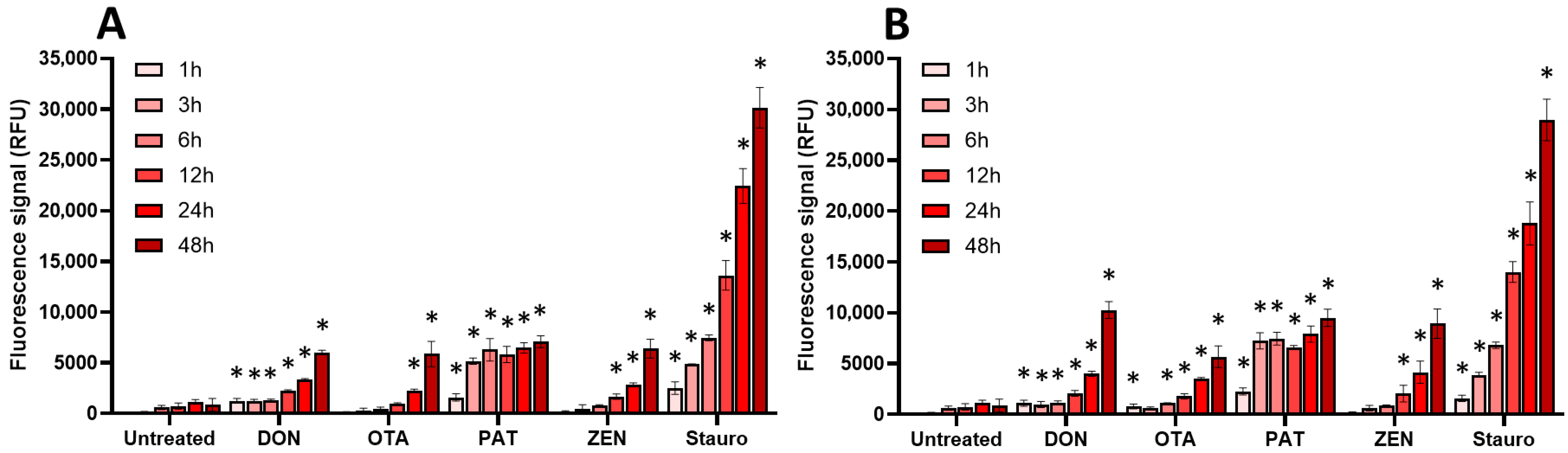
| AFB1 | DON | FB1 | OTA | PAT | ZEN | |
| IC50 on proliferation | >100 | 0.19 ± 0.07 | >100 | 13.79 ± 1.13 | 9.19 ± 0.96 | >100 |
| CC50 on viability | >100 | 5.06 ± 0.48 | >100 | 23.88 ± 1.36 | 38.02 ± 11.37 | 31.75 ± 4.94 |
| BEA | ENN A | ENN A1 | ENN B | ENN B1 | CYCLO | |
| IC50 on proliferation | 1.41 ± 0.20 | 0.93 ± 0.13 | 1.08 ± 0.34 | 1.40 ± 0.18 | 0.92 ± 1.07 | >100 |
| CC50 on viability | 1.91 ± 0.45 | 1.05 ± 0.11 | 0.86 ± 0.09 | 0.72 ± 0.16 | 2.14 ± 0.17 | >100 |
| AFN | API | BRV-F | EMO | MON | TRPT | |
| IC50 on proliferation | 79.51 ± 3.68 | 1.63 ± 0.21 | >100 | 40.01 ± 2.70 | >100 | >100 |
| CC50 on viability | >100 | 59.59 ± 10.27 | >100 | >100 | >100 | >100 |
| Mycotoxin | Toxicological Threshold | Value µg/kg bw/Day | Gastrointestinal Concentration at the BMDL/NOAEL Level (µM) * | Blood Concentration at the BMDL/NOAEL Level (µM) ** | Antiproliferative (IC50)/Cytotoxic (CC50) Concentrations on EGCs (µM) |
|---|---|---|---|---|---|
| DON | NOAEL | 100 | 23.6 | 3.31 | 0.19/5.06 |
| OTA | Non-neoplastic effects BMDL10 | 14.5 | 2.5 | 0.43 | 13.79/23.88 |
| PAT | NOAEL | 40 | 19.5 | 0.08 | 9.19/38.02 |
| ZEN | NOAEL | 100 | 22 | 3.5 | >100/31.75 |
| ENNs, BEA | LOAEL *** | 17,000 | 18 | 2.9 | 0.92/0.72 |
| Name | Abbreviation | Solvent Used | Purity | Supplier |
|---|---|---|---|---|
| Aflatoxin B1 | AFB1 | DMSO | >98% | Sigma Aldrich, Lyon, France |
| Apicidin | API | DMSO | >98% | Sigma Aldrich |
| Aurofusarin | AFN | DMSO | >97% | Santa Cruz Biotechnology, Dallas, TX, USA |
| Beauvericin | BEA | Ethanol | >97% | Sigma Aldrich |
| Brevianamide-F | BRV-F | Ethanol | >95% | BioAustralis, Smithfield, Australia |
| Cyclo-(L-Pro-L-Tyr) | CYCLO | Ethanol | >98% | BioAustralis |
| Deoxynivalenol | DON | Ethanol | >98% | Sigma Aldrich |
| Emodin | EMO | DMSO | >90% | Sigma Aldrich |
| Enniatins | ENNs | Ethanol | >99% | Sigma Aldrich |
| Fumonisin B1 | FB1 | DMSO | >98% | Sigma Aldrich |
| Moniliformin | MON | Ethanol | >95% | Sigma Aldrich |
| Ochratoxin A | OTA | Ethanol | >95% | Sigma Aldrich |
| Patulin | PAT | Ethanol | >98% | Sigma Aldrich |
| Tryptophol | TRPT | DMSO | >97% | Sigma Aldrich |
| Zearalenone | ZEN | DMSO | >98% | Sigma Aldrich |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, M.; Olleik, H.; Di Maio, A.; Kadri, A.; Camps, V.; Perrier, J.; Ajandouz, E.H.; Pinton, P.; Santos, R.R.; Oswald, I.P.; et al. Impact of Regulated and Non-Regulated Food-Associated Mycotoxins on the Viability and Proliferation of Enteric Glial Cells. Toxins 2025, 17, 587. https://doi.org/10.3390/toxins17120587
Dąbrowski M, Olleik H, Di Maio A, Kadri A, Camps V, Perrier J, Ajandouz EH, Pinton P, Santos RR, Oswald IP, et al. Impact of Regulated and Non-Regulated Food-Associated Mycotoxins on the Viability and Proliferation of Enteric Glial Cells. Toxins. 2025; 17(12):587. https://doi.org/10.3390/toxins17120587
Chicago/Turabian StyleDąbrowski, Michał, Hamza Olleik, Attilio Di Maio, Amine Kadri, Valérie Camps, Josette Perrier, El Hassan Ajandouz, Philippe Pinton, Regiane R. Santos, Isabelle P. Oswald, and et al. 2025. "Impact of Regulated and Non-Regulated Food-Associated Mycotoxins on the Viability and Proliferation of Enteric Glial Cells" Toxins 17, no. 12: 587. https://doi.org/10.3390/toxins17120587
APA StyleDąbrowski, M., Olleik, H., Di Maio, A., Kadri, A., Camps, V., Perrier, J., Ajandouz, E. H., Pinton, P., Santos, R. R., Oswald, I. P., Zielonka, Ł., & Maresca, M. (2025). Impact of Regulated and Non-Regulated Food-Associated Mycotoxins on the Viability and Proliferation of Enteric Glial Cells. Toxins, 17(12), 587. https://doi.org/10.3390/toxins17120587









