Variations in “Functional Site” Residues and Classification of Three-Finger Neurotoxins in Snake Venoms
Abstract
1. Introduction
2. Results and Discussion
2.1. Classification of Three-Finger Neurotoxins
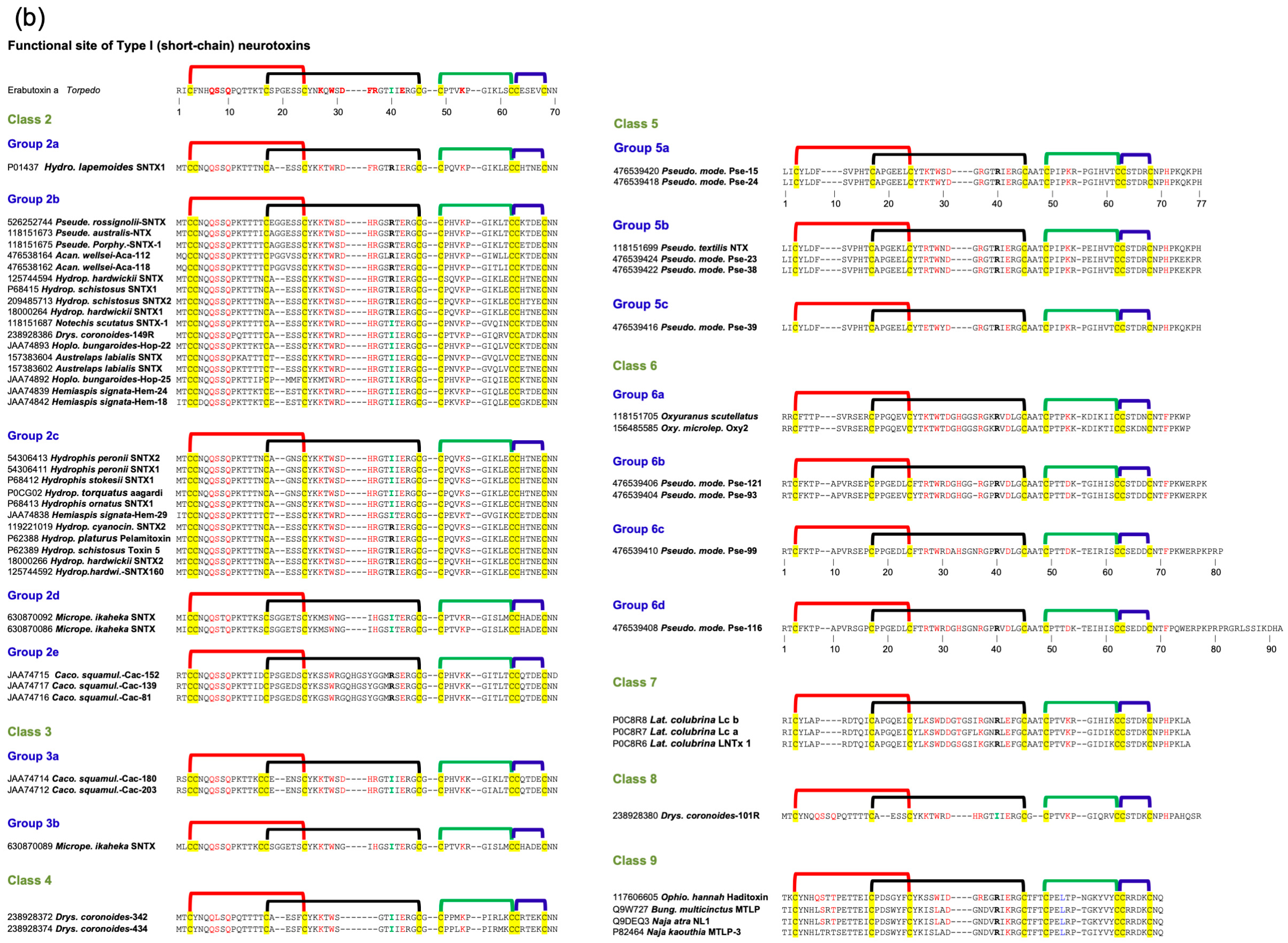
2.2. Type I (Short-Chain) Neurotoxins
2.2.1. Type I Class 1
2.2.2. Type I Class 2
2.2.3. Type I Class 3
2.2.4. Type I Class 4
2.2.5. Type I Class 5 to 8
2.2.6. Type I Class 9
2.2.7. Subgroups in Type I Neurotoxins
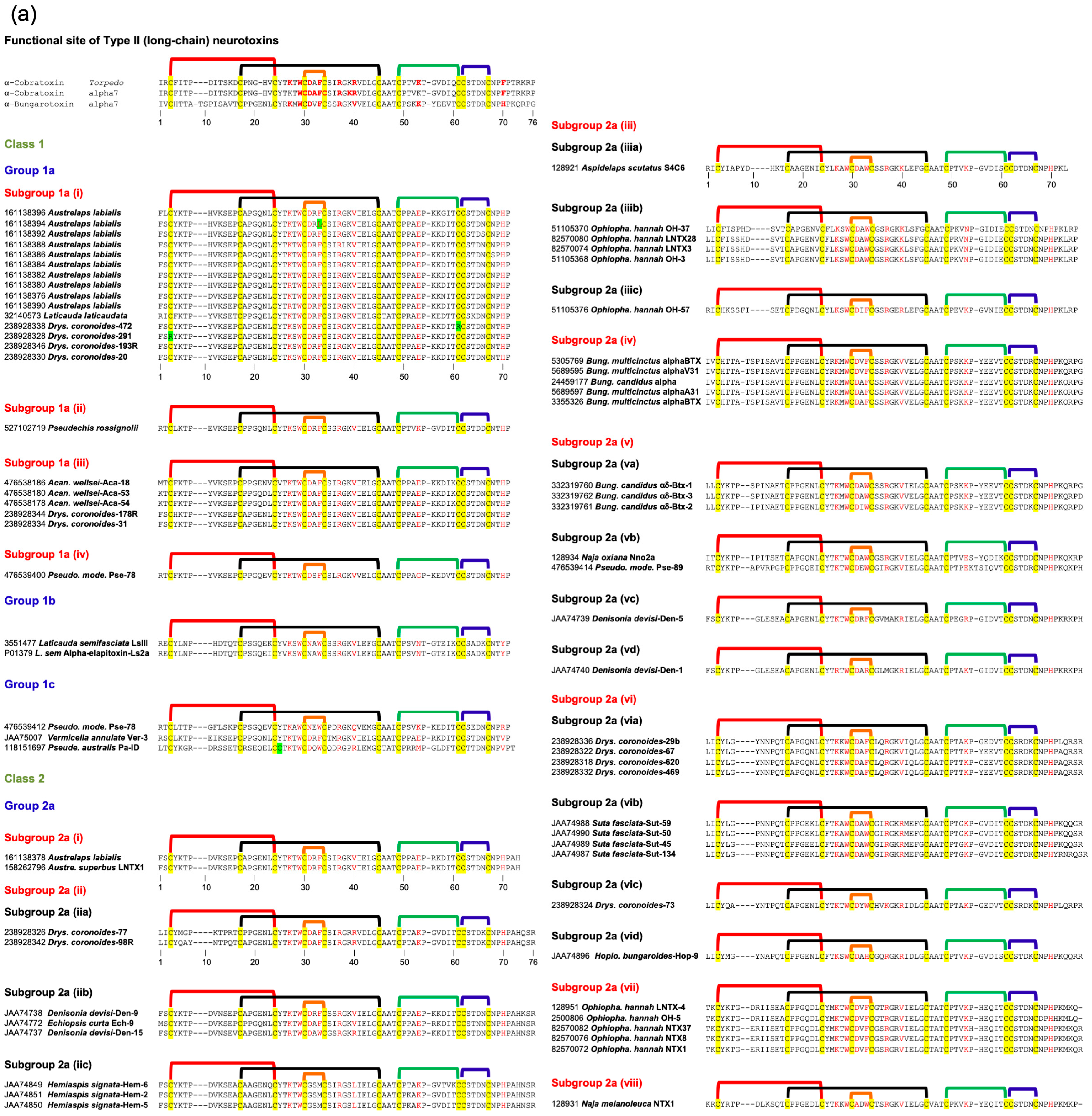
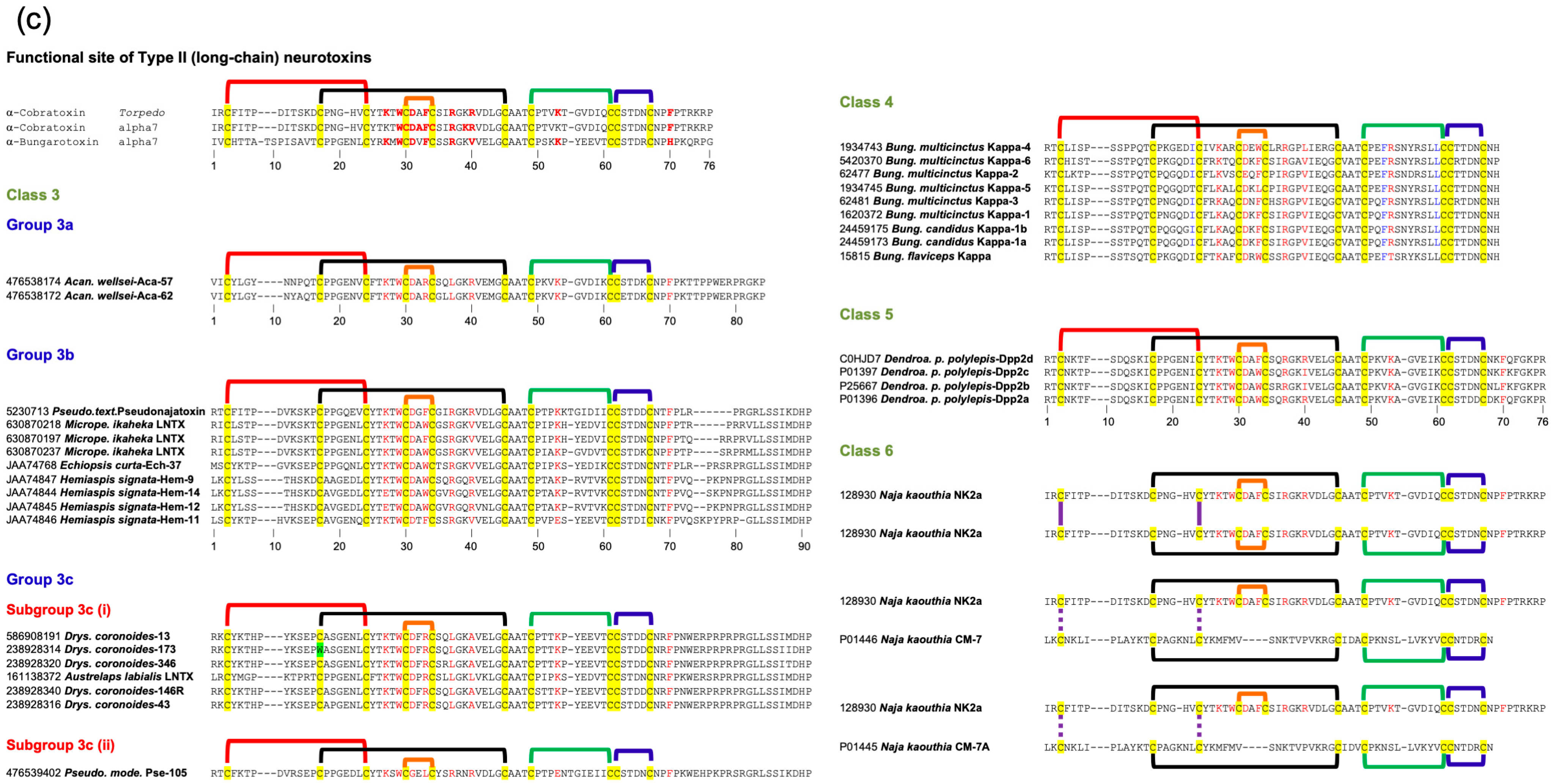
2.3. Type II (Long-Chain) Neurotoxins
2.3.1. Type II Class 1
2.3.2. Type II Class 2
2.3.3. Type II Class 3
2.3.4. Type II Class 4
2.3.5. Type II Class 5
2.3.6. Type II Class 6
2.3.7. Subgroups in Type II Neurotoxins
2.4. Type III Neurotoxins
Subgroups in Type III Neurotoxins
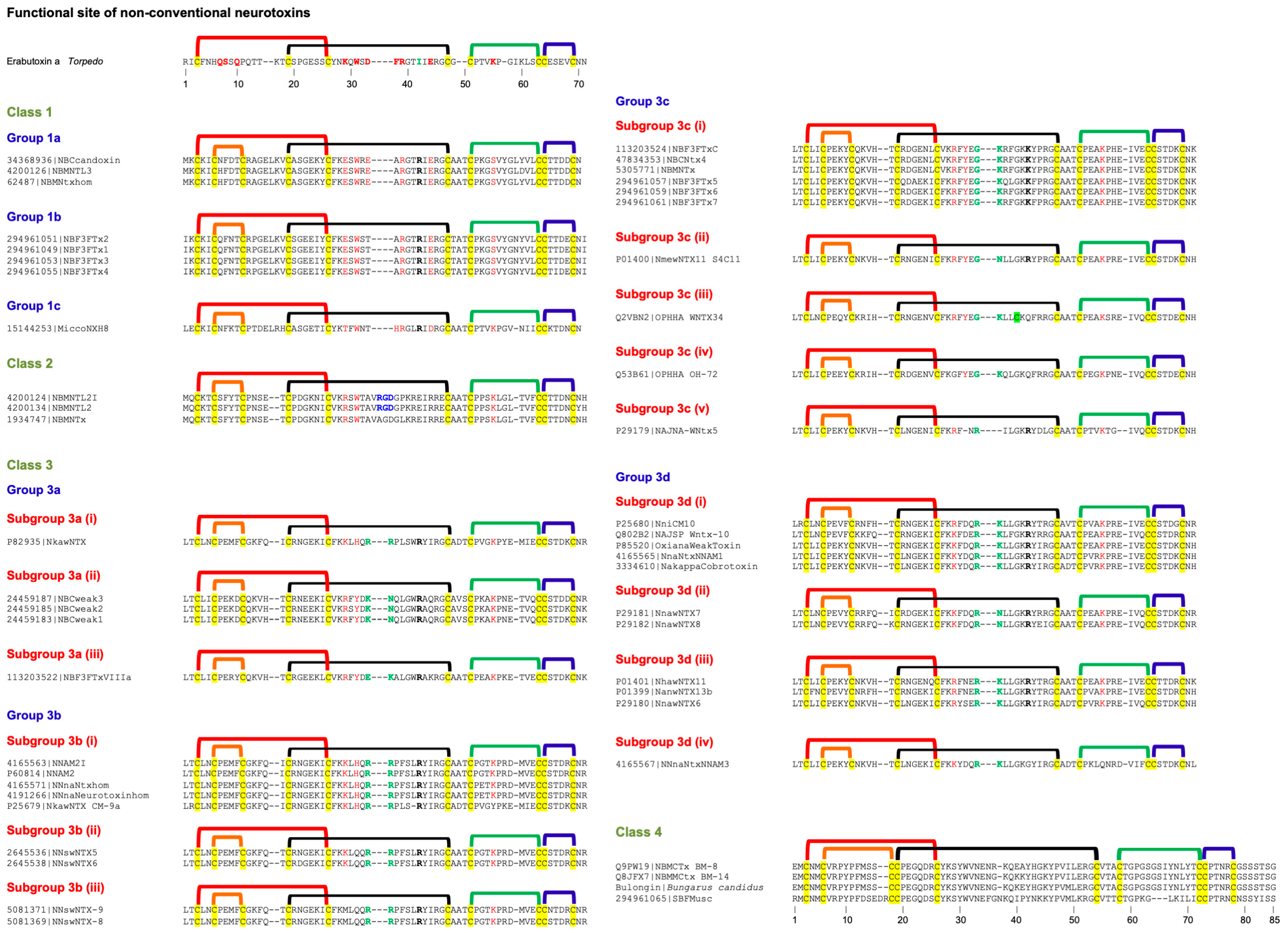
2.5. Non-Conventional Neurotoxins
2.5.1. Non-Conventional Neurotoxins Class 1
2.5.2. Non-Conventional Neurotoxins Class 2
2.5.3. Non-Conventional Neurotoxins Class 3
2.5.4. Non-Conventional Neurotoxins Class 4
2.5.5. Subgroups in Non-Conventional Neurotoxins
2.6. Ω-Neurotoxins
Subgroups in Ω-Neurotoxins
2.7. Σ-Neurotoxins
Subgroups in Σ-Neurotoxins
2.8. Colubrid Neurotoxins
2.8.1. Colubrid Neurotoxins Class 1
2.8.2. Colubrid Neurotoxins Class 2 to 7
2.8.3. Colubrid Neurotoxins Class 8 to 11
2.8.4. Unique Gene Organization of Colubrid Toxins and Role of New Exon 2
2.8.5. Subgroups in Colubrid Neurotoxins
2.9. Endogenous and Exogenous Agonists, Antagonists, and Beyond
2.10. Animal Toxins Inspire the Design of Highly Selective Ligands
2.11. Subtle Variations in Conserved Functional Site Residues in α-Neurotoxins
2.12. Proline Residues Flanking Protein–Protein Interaction Sites
2.13. Predicted 3D Structures of α-Neurotoxins
2.14. Functional Site Residues in Other Neurotoxin Families
2.14.1. Non-Conventional Toxins
2.14.2. Ω-Neurotoxins
2.14.3. Other Neurotoxins
2.15. Dimerization and Neofunctionalization
2.15.1. Non-Covalent Dimers
2.15.2. Covalent Dimers
3. Conclusions
4. Methods
4.1. Messenger RNA and Protein Sequences
4.2. Analysis of Functional Sites of Neurotoxins
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kini, R.M.; Doley, R. Structure, function and evolution of three-finger toxins—Mini proteins with multiple targets. Toxicon 2010, 56, 855–867. [Google Scholar] [CrossRef]
- Kudryavtsev, D.; Shelukhina, I.; Vulfius, C.; Makarieva, T.; Stonik, V.; Zhmak, M.; Ivanov, I.; Kasheverov, I.; Utkin, Y.; Tsetlin, V. Natural compounds interacting with nicotinic acetylcholine receptors: From low-molecular weight ones to peptides and proteins. Toxins 2015, 7, 1683–1701. [Google Scholar] [CrossRef] [PubMed]
- Dutertre, S.; Nicke, A.; Tsetlin, V.I. Nicotinic acetylcholine receptor inhibitors derived from snake and snail venoms. Neuropharmacology 2017, 127, 196–223. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Tamiya, N. Current view on the structure-function relationship of postsynaptic neurotoxins from snake-venoms. Pharmacol. Ther. 1987, 34, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Servent, D.; Antil-Delbeke, S.; Gaillard, C.; Corringer, P.J.; Changeux, J.P.; Menez, A. Molecular characterization of the specificity of interactions of various neurotoxins on two distinct nicotinic acetylcholine receptors. Eur. J. Pharmacol. 2000, 393, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Tsetlin, V.I. Three-finger snake neurotoxins and Ly6 proteins targeting nicotinic acetylcholine receptors: Pharmacological tools and endogenous modulators. Trends Pharmacol. Sci. 2015, 36, 109–123. [Google Scholar] [CrossRef]
- Fry, B.G.; Wüster, W.; Kini, R.M.; Brusic, V.; Khan, A.; Venkataraman, D.; Rooney, A.P. Molecular evolution and phylogeny of elapid snake venom three finger toxins. J. Mol. Evol. 2003, 57, 110–129. [Google Scholar] [CrossRef]
- Chiappinelli, V.A.; Weaver, W.R.; McLane, K.E.; Conti-Fine, B.M.; Fiordalisi, J.J.; Grant, G.A. Binding of native kappa-neurotoxins and site-directed mutants to nicotinic acetylcholine receptors. Toxicon 1996, 34, 1243–1256. [Google Scholar] [CrossRef]
- Gong, N.; Armugam, A.; Jeyaseelan, K. Postsynaptic short-chain neurotoxins from Pseudonaja textilis. cDNA cloning, expression and protein characterization. Eur. J. Biochem. 1999, 265, 982–989. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Kukhtina, V.V.; Kryukova, E.V.; Chiodini, F.; Bertrand, D.; Methfessel, C.; Tsetlin, V.I. “Weak toxin” from Naja kaouthia is a nontoxic antagonist of alpha7 and muscle-type nicotinic acetyl-choline receptors. J. Biol. Chem. 2001, 276, 15810–15815. [Google Scholar] [CrossRef]
- Utkin, Y.N.; Kukhtina, V.V.; Maslennikov, I.V.; Eletsky, A.V.; Starkov, V.G.; Weise, C.; Franke, P.; Hucho, F.; Tsetlin, V.I. First tryptophan-containing weak neurotoxin from cobra venom. Toxicon 2001, 39, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Poh, S.L.; Mourier, G.; Thai, R.; Armugam, A.; Molgo, J.; Servent, D.; Jeyaseelan, K.; Menez, A. A synthetic weak neurotoxin binds with low affinity to Torpedo and chicken alpha7 nicotinic acetyl-choline receptors. Eur. J. Biochem. 2002, 269, 4247–4256. [Google Scholar] [PubMed]
- Nirthanan, S.; Charpantier, E.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Cheah, L.S.; Bertrand, D.; Kini, R.M. Candoxin, a novel toxin from Bungarus candidus, is a reversible antagonist of muscle (abgd) but a poorly reversible antagonist of neuronal alpha7 nicotinic acetylcholine receptors. J. Biol. Chem. 2002, 277, 17811–17820. [Google Scholar] [CrossRef] [PubMed]
- Nirthanan, S.; Gopalakrishnakone, P.; Gwee, M.C.; Khoo, H.E.; Kini, R.M. Non-conventional toxins from Elapid venoms. Toxicon 2003, 41, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Puttaswamy, V.; Adams, D.J.; Kini, R.M. A distinct functional site in W-neurotoxins: Novel antagonists of nicotinic acetylcholine receptors from snake venom. ACS Chem. Biol. 2015, 10, 2805–2815. [Google Scholar] [CrossRef] [PubMed]
- Foo, C.S.; Jobichen, C.; Hassan-Puttaswamy, V.; Dekan, Z.; Tae, H.S.; Bertrand, D.; Adams, D.J.; Alewood, P.F.; Sivaraman, J.; Nirthanan, S.; et al. Fulditoxin, representing a new class of dimeric snake toxins, defines novel pharmacology at nicotinic acetylcholine receptors. Brit. J. Pharmacol. 2020, 177, 1822–1840. [Google Scholar] [CrossRef]
- Weinstein, S.A.; Schmidt, J.J.; Bernheimer, A.W.; Smith, L.A. Characterization and amino acid sequences of two lethal peptides isolated from venom of Wagler’s pit viper, Trimeresurus wagleri. Toxicon 1991, 29, 227–236. [Google Scholar] [CrossRef]
- Bon, C.; Saliou, B. Ceruleotoxin: Identification in the venom of Bungarus fasciatus, molecular properties and importance of phospholipase A2 activity for neurotoxicity. Toxicon 1983, 21, 681–698. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Gorbacheva, E.V.; Starkov, V.G.; Osipov, A.V.; Kasheverov, I.E.; Andreeva, T.V.; Astashev, M.E.; Tsetlin, V.I.; Utkin, Y.N. An unusual phospholipase A2 from puff adder Bitis arietans venom—A novel blocker of nicotinic acetylcholine receptors. Toxicon 2011, 57, 787–793. [Google Scholar] [CrossRef]
- Petrova, S.D.; Atanasov, V.N.; Balashev, K. Vipoxin and its components: Structure-function relationship. Adv. Protein Chem. Struct. Biol. 2012, 87, 117–153. [Google Scholar]
- Vulfius, C.A.; Kasheverov, I.E.; Starkov, V.G.; Osipov, A.V.; Andreeva, T.V.; Filkin, S.Y.; Gorbacheva, E.V.; Astashev, M.E.; Tsetlin, V.I.; Utkin, Y.N. Inhibition of nicotinic acetylcholine receptors, a novel facet in the pleiotropic activities of snake venom phospholipases A2. PLoS ONE 2014, 9, e115428. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Kasheverov, I.E.; Kryukova, E.V.; Spirova, E.N.; Shelukhina, I.V.; Starkov, V.G.; Andreeva, T.V.; Faure, G.; Zouridakis, M.; Tsetlin, V.I.; et al. Pancreatic and snake venom presynaptically active phospholipases A2 inhibit nicotinic acetylcholine receptors. PLoS ONE 2017, 12, e0186206. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N.; Weise, C.; Kasheverov, I.E.; Andreeva, T.V.; Kryukova, E.V.; Zhmak, M.N.; Starkov, V.G.; Hoang, N.A.; Bertrand, D.; Ramerstorfer, J.; et al. Azemiopsin from Azemiops feae viper venom, a novel polypeptide ligand of nicotinic acetylcholine receptor. J. Biol. Chem. 2012, 287, 27079–27086. [Google Scholar] [CrossRef] [PubMed]
- Brust, A.; Sunagar, K.; Undheim, E.A.; Vetter, I.; Yang, D.C.; Casewell, N.R.; Jackson, T.N.; Koludarov, I.; Alewood, P.F.; Hodgson, W.C.; et al. Differential evolution and neofunctionalization of snake venom metalloprotease domains. Mol. Cell. Proteom. 2013, 12, 651–663. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Starkov, V.G.; Andreeva, T.V.; Tsetlin, V.I.; Utkin, Y.N. Novel antagonists of nicotinic acetylcholine receptors—Proteins from venoms of Viperidae snakes. Dokl. Biochem. Biophys. 2015, 461, 119–122. [Google Scholar] [CrossRef]
- Vulfius, C.A.; Spirova, E.N.; Serebryakova, M.V.; Shelukhina, I.V.; Kudryavtsev, D.; Kryukova, E.V.; Starkov, V.G.; Kopylova, N.V.; Zhmak, M.N.; Ivanov, I.A.; et al. Peptides from puff adder Bitis arietans venom, novel inhibitors of nicotinic acetylcholine receptors. Toxicon 2016, 121, 70–76. [Google Scholar] [CrossRef]
- Pillet, L.; Tremeau, O.; Ducancel, F.; Drevet, P.; Zinn-Justin, S.; Pinkasfeld, S.; Boulain, J.C.; Menez, A. Genetic engineering of snake toxins. Role of invariant residues in the structural and functional properties of a curaremimetic toxin, as probed by site-directed mutagenesis. J. Biol. Chem. 1993, 268, 909–916. [Google Scholar] [CrossRef]
- Ackermann, E.J.; Taylor, P. Nonidentity of the alpha-neurotoxin binding sites on the nicotinic acetylcholine receptor revealed by modification in alpha-neurotoxin and receptor structures. Biochemistry 1997, 36, 12836–12844. [Google Scholar] [CrossRef] [PubMed]
- Antil, S.; Servent, D.; Menez, A. Variability among the sites by which curaremimetic toxins bind to torpedo acetylcholine receptor, as revealed by identification of the functional residues of alpha-cobratoxin. J. Biol. Chem. 1999, 274, 34851–34858. [Google Scholar] [CrossRef] [PubMed]
- Antil-Delbeke, S.; Gaillard, C.; Tamiya, T.; Corringer, P.J.; Changeux, J.P.; Servent, D.; Menez, A. Molecular determinants by which a long-chain toxin from snake venom interacts with the neuronal alpha7-nicotinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 29594–29601. [Google Scholar] [CrossRef] [PubMed]
- Malany, S.; Osaka, H.; Sine, S.M.; Taylor, P. Orientation of alpha-neurotoxin at the subunit interfaces of the nicotinic acetylcholine receptor. Biochemistry 2000, 39, 15388–15398. [Google Scholar] [CrossRef]
- Chandna, R.; Tae, H.-S.; Seymour, V.A.L.; Chathrath, S.; Adams, D.J.; Kini, R.M. Drysdalin, an antagonist of nicotinic acetylcholine receptors highlights the importance of functional rather than structural conservation of amino acid residues. FASEB BioAdv. 2019, 1, 115–131. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; O’Neill, M.; Reiman, D.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, E.X.; Pereira, E.; Alkondon, M.; Rogers, S.W. Mammalian nicotinic acetylcholine receptors: From structure to function. Physiol. Rev. 2009, 89, 73–120. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A. Overview of nicotinic receptors and their roles in the central nervous system. Biol. Psychiatry. 2001, 49, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Nys, M.; Kesters, D.; Ulens, C. Structural insights into Cys-loop receptor function and ligand recognition. Biochem. Pharmacol. 2013, 86, 1042–1053. [Google Scholar] [CrossRef]
- Lindstrom, J.M. Nicotinic acetylcholine receptors of muscles and nerves: Comparison of their structures, functional roles, and vulnerability to pathology. Ann. N. Y. Acad. Sci. 2003, 998, 41–52. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004, 25, 317–324. [Google Scholar] [CrossRef]
- Kew, J.N.C.; Davies, C.H. Ion Channels: From Structure to Function, 2nd ed.; Oxford University Press: Oxford, UK, 2010; pp. 154–196. [Google Scholar]
- Mok, M.H.; Kew, J.N. Excitation of rat hippocampal interneurons via modulation of endogenous agonist activity at the alpha7 nicotinic ACh receptor. J. Physiol. 2006, 574, 699–710. [Google Scholar]
- Dickinson, J.A.; Kew, J.N.; Wonnacott, S. Presynaptic alpha 7- and beta 2-containing nicotinic acetylcholine receptors modulate excitatory amino acid release from rat prefrontal cortex nerve terminals via distinct cellular mechanisms. Mol. Pharmacol. 2008, 74, 348–359. [Google Scholar] [CrossRef]
- Livingstone, P.D.; Srinivasan, J.; Kew, J.N.; Dawson, L.A.; Gotti, C.; Moretti, M.; Shoaib, M.; Wonnacott, S. Alpha7 and non-alpha7 nicotinic acetylcholine receptors modulate dopamine release in vitro and in vivo in the rat prefrontal cortex. Eur. J. Neurosci. 2009, 29, 539–550. [Google Scholar] [CrossRef]
- Livingstone, P.D.; Dickinson, J.A.; Srinivasan, J.; Kew, J.N.; Wonnacott, S. Glutamate-dopamine crosstalk in the rat prefrontal cortex is modulated by Alpha7 nicotinic receptors and potentiated by PNU-120596. J. Mol. Neurosci. 2010, 40, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, M.D. Insights into nicotinic receptor signaling in nicotine addiction: Implications for prevention and treatment. Curr. Neuropharmacol. 2018, 16, 350–370. [Google Scholar] [CrossRef] [PubMed]
- Murray, T.A.; Bertrand, D.; Papke, R.L.; George, A.A.; Pantoja, R.; Srinivasan, R.; Liu, Q.; Wu, J.; Whiteaker, P.; Lester, H.A.; et al. α7β2 nicotinic acetylcholine receptors assemble, function, and are activated primarily via their α7-α7 interfaces. Mol. Pharmacol. 2012, 81, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lukas, R.J. Naturally-expressed nicotinic acetylcholine receptor subtypes. Biochem. Pharmacol. 2011, 82, 800–807. [Google Scholar] [CrossRef]
- Lindstrom, J. Neuronal nicotinic acetylcholine receptors. Ion Channels 1996, 4, 377–450. [Google Scholar]
- Gotti, C.; Moretti, M.; Gaimarri, A.; Zanardi, A.; Clementi, F.; Zoli, M. Heterogeneity and complexity of native brain nicotinic receptors. Biochem. Pharmacol. 2007, 74, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Flores, C.M.; Rogers, S.W.; Pabreza, L.A.; Wolfe, B.B.; Kellar, K.J. A subtype of nicotinic cholinergic receptor in rat brain is composed of alpha 4 and beta 2 subunits and is up-regulated by chronic nicotine treatment. Mol. Pharmacol. 1992, 41, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Dohgu, S.; Takata, F.; Matsumoto, J.; Kawahara, Y.; Nishihira, M.; Sakada, S.; Saisho, T.; Yamauchi, A.; Kataoka, Y. Activation of the α7 nicotinic acetylcholine receptor upregulates blood-brain barrier function through increased claudin-5 and occludin expression in rat brain endothelial cells. Neurosci. Lett. 2019, 694, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Du, W.; Guo, C.; Geng, P.; Liu, W.; Jin, X. α7nACh receptor, a promising target to reduce BBB damage by regulating inflammation and autophagy after ischemic stroke. Biomed. Pharmacother. 2024, 179, 117337. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Vijayaraghavan, S. Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 2002, 53, 524–534. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L. Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem. Pharmacol. 2014, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Stokes, C.; Treinin, M.; Papke, R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015, 36, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Fucile, S. Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium 2004, 35, 1–8. [Google Scholar] [CrossRef]
- Papke, R.L.; Bencherif, M.; Lippiello, P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha7 subtype. Neurosci. Lett. 1996, 213, 201–204. [Google Scholar] [CrossRef]
- Chavez-Noriega, L.E.; Crona, J.H.; Washburn, M.S.; Urrutia, A.; Elliott, K.J.; Johnson, E.C. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors hα2β2, hα2β4, hα3β2, hα3β4, hα4β2, hα4β4 and hα7 Expressed in Xenopus oocytes. J. Pharmacol. Exp. Ther. 1997, 280, 346–356. [Google Scholar] [CrossRef]
- Nelson, M.E.; Kuryatov, A.; Choi, C.H.; Zhou, Y.; Lindstrom, J. Alternate stoichiometries of α4β2 nicotinic acetylcholine receptors. Mol. Pharmacol. 2003, 63, 332–341. [Google Scholar] [CrossRef]
- Moroni, M.; Bermudez, I. Stoichiometry and pharmacology of two human alpha4beta2 nicotinic receptor types. J. Mol. Neurosci. 2006, 30, 95–96. [Google Scholar] [CrossRef]
- Carbone, A.L.; Moroni, M.; Groot-Kormelink, P.J.; Bermudez, I. Pentameric concatenated (α4)(2) (β2)(3) and (α4)(3) (β2)(2) nicotinic acetylcholine receptors: Subunit arrangement determines functional expression. Br. J. Pharmacol. 2009, 156, 970–981. [Google Scholar] [CrossRef]
- Lukas, R.J.; Changeux, J.P.; Le Novère, N.; Albuquerque, E.X.; Balfour, D.J.; Berg, D.K.; Bertrand, D.; Chiappinelli, V.A.; Clarke, P.B.; Collins, A.C.; et al. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol. Rev. 1999, 51, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Arneric, S.P.; Holladay, M.; Williams, M. Neuronal nicotinic receptors: A perspective on two decades of drug discovery research. Biochem. Pharmacol. 2007, 74, 1092–1101. [Google Scholar] [CrossRef]
- Hurst, R.; Rollema, H.; Bertrand, D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol. Ther. 2013, 137, 22–54. [Google Scholar] [CrossRef] [PubMed]
- Woodruff-Pak, D.S.; Gould, T.J. Neuronal nicotinic acetylcholine receptors: Involvement in Alzheimer’s disease and schizophrenia. Behav. Cogn. Neurosci. Rev. 2002, 1, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Fontana, I.C.; Kumar, A.; Nordberg, A. The role of astrocytic α7 nicotinic acetylcholine receptors in Alzheimer disease. Nat. Rev. Neurol. 2023, 19, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Hone, A.J.; McIntosh, J.M. Nicotinic acetylcholine receptors: Therapeutic targets for novel ligands to treat pain and inflammation. Pharmacol. Res. 2023, 190, 106715. [Google Scholar] [CrossRef] [PubMed]
- Elgoyhen, A.B. The α9α10 nicotinic acetylcholine receptor: A compelling drug target for hearing loss? Expert Opin. Ther. Targets 2022, 26, 291–302. [Google Scholar] [CrossRef]
- Slika, E.; Fuchs, P.A. Genetic tools for studying cochlear inhibition. Front. Cell. Neurosci. 2024, 18, 1372948. [Google Scholar] [CrossRef]
- Leslie, F.M.; Mojica, C.Y.; Reynaga, D.D. Nicotinic receptors in addiction pathways. Mol. Pharmacol. 2013, 83, 753–758. [Google Scholar] [CrossRef]
- Cohen, C.; Bergis, O.E.; Galli, F.; Lochead, A.W.; Jegham, S.; Biton, B.; Leonardon, J.; Avenet, P.; Sgard, F.; Besnard, F.; et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J. Pharmacol. Exp. Ther. 2003, 306, 407–420. [Google Scholar] [CrossRef]
- Kurzen, H.; Wessler, I.; Kirkpatrick, C.J.; Kawashima, K.; Grando, S.A. The non-neuronal cholinergic system of human skin. Horm. Metab. Res. 2007, 39, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Weis, M.; Aicher, A.; Dimmeler, S.; Cooke, J.P. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J. Clin. Investig. 2002, 110, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Chellappan, S.P. Nicotine-mediated cell proliferation and angiogenesis: New twists to an old story. Cell Cycle 2006, 5, 2324–2328. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S. Application of natural killer T-cells to post-transplantation immunotherapy. Int. J. Hematol. 2005, 81, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Russo, P.; Del Bufalo, A.; Milic, M.; Salinaro, G.; Fini, M.; Cesario, A. Cholinergic receptors as target for cancer therapy in a systems medicine perspective. Curr. Mol. Med. 2014, 14, 1126–1138. [Google Scholar] [CrossRef]
- Hecht, S.S. Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst. 1999, 91, 1194–1210. [Google Scholar] [CrossRef]
- Schuller, H.M.; Orloff, M. Tobacco-specific carcinogenic nitrosamines. Ligands for nicotinic acetylcholine receptors in human lung cancer cells. Biochem. Pharmacol. 1998, 55, 1377–1384. [Google Scholar] [CrossRef]
- Plummer, H.K., 3rd; Dhar, M.; Schuller, H.M. Expression of the alpha7 nicotinic acetylcholine receptor in human lung cells. Respir. Res. 2005, 6, 29. [Google Scholar] [CrossRef]
- Brognard, J.; Clark, A.S.; Ni, Y.; Dennis, P.A. Akt/protein kinase B is constitutively active in non-small cell lung cancer cells and promotes cellular survival and resistance to chemotherapy and radiation. Cancer Res. 2001, 61, 3986–3997. [Google Scholar]
- Dasgupta, P.; Rastogi, S.; Pillai, S.; Ordonez-Ercan, D.; Morris, M.; Haura, E.; Chellappan, S. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J. Clin. Investig. 2006, 116, 2208–2217. [Google Scholar] [CrossRef]
- Improgo, M.R.; Soll, L.G.; Tapper, A.R.; Gardner, P.D. Nicotinic acetylcholine receptors mediate lung cancer growth. Front. Physiol. 2013, 4, 251. [Google Scholar] [CrossRef]
- Hung, R.J.; McKay, J.D.; Gaborieau, V.; Boffetta, P.; Hashibe, M.; Zaridze, D.; Mukeria, A.; Szeszenia-Dabrowska, N.; Lissowska, J.; Rudnai, P.; et al. A susceptibility locus for lung cancer maps to nicotinic acetylcholine receptor subunit genes on 15q25. Nature 2008, 452, 633–637. [Google Scholar] [CrossRef]
- Ulloa, L. The vagus nerve and the nicotinic anti-inflammatory pathway. Nat. Rev. Drug Discov. 2005, 4, 673–684. [Google Scholar] [CrossRef]
- Horenstein, N.A.; Papke, R.L. Anti-inflammatory Silent Agonists. ACS Med. Chem. Lett. 2017, 8, 989–991. [Google Scholar] [CrossRef]
- Quadri, M.; Bagdas, D.; Toma, W.; Stokes, C.; Horenstein, N.A.; Damaj, M.I.; Papke, R.L. The antinociceptive and anti-inflammatory properties of the α7 nAChR weak partial agonist p-CF3 N,N-diethyl-N′-phenylpiperazine. J. Pharmacol. Exp. Ther. 2018, 367, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Dani, J.A.; Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef] [PubMed]
- Trémeau, O.; Lemaire, C.; Drevet, P.; Pinkasfeld, S.; Ducancel, F.; Boulain, J.C.; Ménez, A. Genetic engineering of snake toxins. The functional site of Erabutoxin a, as delineated by site-directed mutagenesis, includes variant residues. J. Biol. Chem. 1995, 270, 9362–9369. [Google Scholar] [CrossRef] [PubMed]
- Groebe, D.R.; Gray, W.R.; Abramson, S.N. Determinants involved in the affinity of alpha-conotoxins GI and SI for the muscle subtype of nicotinic acetylcholine receptors. Biochemistry 1997, 36, 6469–6474. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M.; Evans, H.J. A hypothetical structural role for proline residues in the flanking segments of protein-protein interaction sites. Biochem. Biophys. Res. Commun. 1995, 212, 1115–1124. [Google Scholar] [CrossRef]
- Eipper, B.A.; Mains, R.E. Peptide alpha-amidation. Annu. Rev. Physiol. 1988, 50, 333–344. [Google Scholar] [CrossRef]
- Maeda, N.; Tamiya, N. Three neurotoxins from venom of a sea snake Astrotia stokesii, Including two long-chain neurotoxic proteins with amidated C-termini. Biochem. J. 1978, 175, 507–517. [Google Scholar] [CrossRef]
- Correa-Netto, C.; Junqueira-de-Azevedo, I.D.M.; Silva, D.A.; Ho, P.L.; Leitao-de-Araujo, M.; Alves, M.L.M.; Sanz, L.; Foguel, D.; Zingali, R.B.; Calvete, J.J. Snake venomics and venom gland transcriptomic analysis of Brazilian coral snakes, Micrurus altirostris and M. corallinus. J. Proteom. 2011, 74, 1795–1809. [Google Scholar] [CrossRef]
- Wang, C.I.; Reeks, T.; Vetter, I.; Vergara, I.; Kovtun, O.; Lewis, R.J.; Alewood, P.F.; Durek, T. Isolation and structural and pharmacological characterization of α-elapitoxin-Dpp2d, an amidated three finger toxin from black mamba venom. Biochemistry 2014, 53, 3758–3766. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Elzinga, M.; Tu, A.T. Amino acid sequence of a snake neurotoxin from the venom of Lapemis hardwickii and the detection of a sulfhydryl group by laser Raman spectroscopy. FEBS Lett. 1977, 80, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, H.; Allen, M.; Tu, A.T. Effect of sulfhydryl group modification on the neurotoxic action of a sea snake toxin. J. Pharm. Pharmacol. 1984, 36, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Zhou, X.; Chong, M.Z.; D’hoedt, D.; Foo, C.S.; Rajagopalan, N.; Nirthanan, S.; Bertrand, D.; Sivaraman, J.; Kini, R.M. Structural and functional characterization of a novel homodimeric three-finger neurotoxin from the venom of Ophiophagus hannah (king cobra). J. Biol. Chem. 2010, 285, 8302–8315. [Google Scholar] [CrossRef] [PubMed]
- Strydom, A.J. Snake venom toxins. The amino acid sequences of two toxins from Dendroaspis jamesoni kaimosae (Jameson’s mamba) venom. Biochim. Biophys. Acta 1973, 328, 491–509. [Google Scholar] [CrossRef]
- Banks, B.E.; Miledi, R.; Shipolini, R.A. The primary sequences and neuromuscular effects of three neurotoxic polypeptides from the venom of Dendroaspis viridis. Eur. J. Biochem. 1974, 45, 457–468. [Google Scholar] [CrossRef]
- Bechis, G.; Granier, C.; Van Rietschoten, J.; Jover, E.; Rochat, H.; Miranda, F. Purification of six neurotoxins from the venom of Dendroaspis viridis. Primary structure of two long toxins. Eur. J. Biochem. 1976, 68, 445–456. [Google Scholar] [CrossRef]
- Blacklow, B.; Kornhauser, R.; Hains, P.G.; Loiacono, R.; Escoubas, P.; Graudins, A.; Nicholson, G.M. α-Elapitoxin-Aa2a, a long-chain snake α-neurotoxin with potent actions on muscle (α1)(2)βγδ nicotinic receptors, lacks the classical high affinity for neuronal α7 nicotinic receptors. Biochem. Pharmacol. 2011, 81, 314–325. [Google Scholar] [CrossRef]
- Chandna, R.; Kaczanowska, K.; Taylor, P.; Kini, R.M. Drysdalin, a snake neurotoxin with higher affinity for soluble acetylcholine binding protein from Aplysia californica than from Lymnaea stagnalis. Toxicon. 2020, 187, 86–92. [Google Scholar] [CrossRef]
- Loring, R.H. The molecular basis of curaremimetic snake neurotoxin specificity for neuronal nicotinic receptor subtypes. J. Toxic. Toxin Rev. 1993, 12, 105–153. [Google Scholar] [CrossRef]
- Papke, R.L.; Duvoisin, R.M.; Heinemann, S.F. The amino terminal half of the nicotinic beta-subunit extracellular domain regulates the kinetics of inhibition by neuronal-bungarotoxin. Proc. R. Soc. Lond. Ser. B 1993, 252, 141–147. [Google Scholar]
- Dewan, J.C.; Grant, G.A.; Sacchettini, J.C. Crystal structure of kappa-bungarotoxin at 2.3-Å resolution. Biochemistry 1994, 33, 13147–13154. [Google Scholar] [CrossRef]
- Osipov, A.V.; Kasheverov, I.E.; Makarova, Y.V.; Starkov, V.G.; Vorontsova, O.V.; Ziganshin, R.K.h.; Andreeva, T.V.; Serebryakova, M.V.; Benoit, A.; Hogg, R.C.; et al. Naturally occurring disulfide-bound dimers of three-fingered toxins: A paradigm for biological activity diversification. J. Biol. Chem. 2008, 283, 14571–14580. [Google Scholar] [CrossRef]
- Osipov, A.V.; Rucktooa, P.; Kasheverov, I.E.; Filkin, S.Y.; Starkov, V.G.; Andreeva, T.V.; Sixma, T.K.; Bertrand, D.; Utkin, Y.N.; Tsetlin, V.I. Dimeric α-cobratoxin X-ray structure: Localization of intermolecular disulfides and possible mode of binding to nicotinic acetylcholine receptors. J. Biol. Chem. 2012, 287, 6725–6734. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, F.H. Snake venom toxins. The primary structure of protein S4C11. A neurotoxin homologue from the venom of forest cobra (Naja melanoleuca). Biochim. Biophys. Acta 1975, 400, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Joubert, F.J. The purification and amino acid sequence of toxin CM-13b from Naja haje annulifera (Egyptian cobra) venom. Hoppe-Seylers Z. Physiol. Chem. 1975, 356, 1901–1908. [Google Scholar] [PubMed]
- Joubert, F.J.; Taljaard, N. Snake venoms. The amino acid sequences of two Melanoleuca-type toxins. Hoppe-Seylers Z. Physiol. Chem. 1980, 361, 425–436. [Google Scholar] [CrossRef]
- Shafqat, J.; Siddiqi, A.R.; Zaidi, Z.H.; Jörnvall, H. Extensive multiplicity of the miscellaneous type of neurotoxins from the venom of the cobra Naja naja naja and structural characterization of major components. FEBS Lett. 1991, 284, 70–72. [Google Scholar] [CrossRef]
- Aird, S.D.; Womble, G.C.; Yates, J.R., 3rd; Griffin, P.R. Primary structure of gamma-bungarotoxin, a new postsynaptic neurotoxin from venom of Bungarus multicinctus. Toxicon 1999, 37, 609–625. [Google Scholar] [CrossRef]
- Servent, D.; Menez, A. Snake neurotoxins that interact with nicotinic acetylcholine receptors. In Handbook of Neurotoxicology; Massaro, E.J., Ed.; Humana: Totowa, NJ, USA, 2001; Volume 1, pp. 385–425. [Google Scholar]
- Pawlak, J.; Mackessy, S.P.; Fry, B.G.; Bhatia, M.; Mourier, G.; Fruchart-Gaillard, C.; Servant, D.; Ménez, R.; Stura, E.; Ménez, A.; et al. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 2006, 281, 29030–29041. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, J.; Mackessy, S.P.; Sixberry, N.M.; Stura, E.A.; Le Du, M.H.; Ménez, R.; Foo, C.S.; Ménez, A.; Nirthanan, S.; Kini, R.M. Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity. FASEB J. 2009, 23, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Pahari, S.; Mackessy, S.P.; Kini, R.M. The venom gland transcriptome of the Desert Massasauga rattlesnake (Sistrurus catenatus edwardsii): Towards an understanding of venom composition among advanced snakes (Superfamily Colubroidea). BMC Mol. Biol. 2007, 8, 115. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, P.; Deacon, A.M.; Comoso, S.; Rajaseger, G.; Kini, R.M.; Usón, I.; Kolatkar, P.R. The atomic resolution structure of bucandin, a novel toxin isolated from the Malayan krait, determined by direct methods. Acta Crystallogr. D Biol. Crystallogr. 2000, 56, 1401–1407. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.M.; Kini, R.M.; Selvanayagam, N.; Kuchel, P.W. NMR structure of bucandin, a neurotoxin from the venom of the Malayan krait (Bungarus candidus). Biochem. J. 2001, 360, 539–548. [Google Scholar] [CrossRef]
- Chang, L.S.; Liou, J.C.; Lin, S.R.; Huang, H.B. Purification and characterization of a neurotoxin from the venom of Ophiophagus hannah (king cobra). Biochem. Biophys. Res. Commun. 2002, 294, 574–578. [Google Scholar] [CrossRef]
- Plow, E.F.; Pierschbacher, M.D.; Ruoslahti, E.; Marguerie, G.; Ginsberg, M.H. Arginyl-glycyl-aspartic acid sequences and fibrinogen binding to platelets. Blood 1987, 70, 110–115. [Google Scholar] [CrossRef]
- Hawiger, J.; Kloczewiak, M.; Bednarek, M.A.; Timmons, S. Platelet receptor recognition domains on the alpha chain of human fibrinogen: Structure-function analysis. Biochemistry 1989, 28, 2909–2914. [Google Scholar] [CrossRef]
- Yamada, K.M. Adhesive recognition sequences. J. Biol. Chem. 1991, 266, 12809–12812. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shulepko, M.A.; Shenkarev, Z.O.; Kasheverov, I.E.; Chugunov, A.O.; Kulbatskii, D.S.; Myshkin, M.Y.; Utkin, Y.N.; Efremov, R.G.; Tsetlin, V.I.; et al. Central loop of non-conventional toxin WTX from Naja kaouthia is important for interaction with nicotinic acetylcholine receptors. Toxicon 2016, 119, 274–279. [Google Scholar] [CrossRef]
- Lyukmanova, E.N.; Shenkarev, Z.O.; Shulepko, M.A.; Paramonov, A.S.; Chugunov, A.O.; Janickova, H.; Dolejsi, E.; Dolezal, V.; Utkin, Y.N.; Tsetlin, V.I.; et al. Structural Insight into specificity of interactions between nonconventional three-finger weak toxin from Naja kaouthia (WTX) and muscarinic acetylcholine receptors. J. Biol. Chem. 2015, 290, 23616–23630. [Google Scholar] [CrossRef]
- Starkov, V.G.; Poliak, Y.L.; Vulfius, E.A.; Kriukova, E.V.; Tsetlin, V.I.; Utkin, Y.N. New weak toxins from the cobra venom. Bioorg. Khim. 2009, 35, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.C.; Fan, C.Y.; Gong, Y.; Yang, S.L. cDNA sequence analysis of a novel member of the three loop protein family from the Chinese continental banded krait. Biosci. Biotechnol. Biochem. 1999, 63, 940–942. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.; Wu, B.N.; Yang, C.C.; Chang, L.S. Muscarinic toxin-like proteins from Taiwan banded krait (Bungarus multicinctus) venom: Purification, characterization and gene organization. Biol. Chem. 2002, 383, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N.; Kasheverov, I.E.; Kudryavtsev, D.S.; Andreeva, T.V.; Starkov, V.G.; Ziganshin, R.H.; Kuznetsov, D.V.; Anh, H.N.; Thao, N.T.; Khoa, N.C.; et al. Nonconventional three-finger toxin BMLCL from krait Bungarus multicinctus venom with high affinity interacts with nicotinic acetylcholine receptors. Dokl. Biochem. Biophys. 2015, 464, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Siang, A.S.; Doley, R.; Vonk, F.J.; Kini, R.M. Transcriptomic analysis of the venom gland of the red-headed krait (Bungarus flaviceps) using expressed sequence tags. BMC Mol. Biol. 2010, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Osipov, A.V.; Meshcheryakova, A.V.; Starkov, V.G.; Ziganshin, R.K.; Oustitch, T.L.; Peters, L.E.; Tsetlin, V.I.; Utkin, Y.N. New paradoxical three-finger toxin from the cobra Naja kaouthia venom: Isolation and characterization. Dokl. Biochem. Biophys. 2017, 475, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.E.; Mackessy, S.P. Venom yields from several species of colubrid snakes and differential effects of ketamine. Toxicon 1997, 35, 671–678. [Google Scholar] [CrossRef]
- Vest, D.K.; Mackessy, S.P.; Kardong, K.V. The unique Duvernoy’s secretion of the brown tree snake (Boiga irregularis). Toxicon 1991, 29, 532–535. [Google Scholar] [CrossRef]
- Hill, R.E.; Mackessy, S.P. Characterization of venom (Duvernoy’s secretion) from twelve species of colubrid snakes and partial sequence of four venom proteins. Toxicon 2000, 38, 1663–1687. [Google Scholar] [CrossRef] [PubMed]
- Kamiguti, A.S.; Theakston, R.D.; Sherman, N.; Fox, J.W. Mass spectrophotometric evidence for P-III/P-IV metalloproteinases in the venom of the Boomslang. Toxicon 2000, 38, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Biochemistry and pharmacology of colubrid snake venoms. J. Toxicol. Toxin Rev. 2002, 21, 43–83. [Google Scholar] [CrossRef]
- Fry, B.G.; Lumsden, N.G.; Wüster, W.; Wickramaratna, J.C.; Hodgson, W.C.; Kini, R.M. Isolation of a neurotoxin (alpha-colubritoxin) from a nonvenomous colubrid: Evidence for early origin of venom in snakes. J. Mol. Evol. 2003, 57, 446–452. [Google Scholar] [CrossRef]
- Fry, B.G.; Wüster, W.; Ramjan, S.F.R.; Jackson, T.; Martelli, P.; Kini, R.M. Analysis of Colubroidea snake venoms by liquid chromatography with mass spectrometry: Evolutionary and toxinological implications. Rapid Commun. Mass Spectrom. 2003, 17, 2047–2062. [Google Scholar] [CrossRef]
- Lumsden, N.G.; Fry, B.G.; Ventura, S.; Kini, R.M.; Hodgson, W.C. The in vitro and in vivo pharmacological activity of Boiga dendrophila (mangrove catsnake) venom. Auton. Autacoid Pharmacol. 2004, 24, 107–113. [Google Scholar] [CrossRef]
- Pawlak, J.; Kini, R.M. Unique gene organization of colubrid three-finger toxins: Complete cDNA and gene sequences of denmotoxin, a bird-specific toxin from colubrid snake Boiga dendrophila (Mangrove Catsnake). Biochimie 2008, 90, 868–877. [Google Scholar] [CrossRef]
- Pawlak, J.; Kini, R.M. Snake venom glutaminyl cyclase. Toxicon 2006, 48, 278–286. [Google Scholar] [CrossRef]
- Modahl, C.M.; Mackessy, S.P. Venoms of rear-fanged snakes: New proteins and novel activities. Front. Ecol. Evol. 2019, 7, 279. [Google Scholar] [CrossRef]
- Fry, B.G.; Scheib, H.; van der Weerd, L.; Young, B.; McNaughtan, J.; Ramjan, S.F.R.; Vidal, N.; Poelmann, R.E.; Norman, J.A. Evolution of an arsenal: Structural and functional diversification of the venom system in the advanced snakes (Caenophidia). Mol. Cell. Proteom. 2008, 7, 215. [Google Scholar] [CrossRef]
- McGivern, J.J.; Wray, K.P.; Margres, M.J.; Couch, M.E.; Mackessy, S.P.; Rokyta, D.R. RNA-seq and high-definition mass spectrometry reveal the complex and divergent venoms of two rear-fanged colubrid snakes. BMC Genom. 2014, 15, 1061. [Google Scholar] [CrossRef]
- Modahl, C.M.; Mackessy, S.P. Full-length venom protein cDNA sequences from venom-derived mRNA: Exploring compositional variation and adaptive multigene evolution. PLoS Negl. Trop. Dis. 2016, 10, e0004587. [Google Scholar] [CrossRef]
- Pla, D.; Petras, D.; Saviola, A.J.; Modahl, C.M.; Sanz, L.; Pérez, A.; Juárez, E.; Frietze, S.; Dorrestein, P.C.; Mackessy, S.P.; et al. Transcriptomics-guided bottom-up and top-down venomics of neonate and adult specimens of the arboreal rear-fanged Brown Treesnake, Boiga irregularis, from Guam. J. Proteom. 2018, 174, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, D.; Debono, J.; Rokyta, D.; Nouwens, A.; Josh, P.; Fry, B.G. Three-finger toxin diversification in the venoms of cat-eye snakes (Colubridae: Boiga). J. Mol. Evol. 2018, 86, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.M.; Mrinalini; Frietze, S.; Mackessy, S.P. Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proc. Biol. Sci. 2018, 285, 1003. [Google Scholar]
- Mackessy, S.P.; Bryan, W.; Smith, C.F.; Lopez, K.; Fernández, J.; Bonilla, F.; Camacho, E.; Sasa, M.; Lomonte, B. Venomics of the central American lyre snake Trimorphodon quadruplex (Colubridae: Smith, 1941) from Costa Rica. J. Proteom. 2020, 220, 103778. [Google Scholar] [CrossRef]
- Heyborne, W.H.; Mackessy, S.P. Identification and characterization of a taxon-specific three-finger toxin from the venom of the Green Vinesnake (Oxybelis fulgidus; family Colubridae). Biochimie 2013, 95, 1923–1932. [Google Scholar] [CrossRef]
- Murayama, N.; Hayashi, M.A.; Ohi, H.; Ferreira, L.A.; Hermann, V.V.; Saito, H.; Fujita, Y.; Higuchi, S.; Fernandes, B.L.; Yamane, T.; et al. Cloning and sequence analysis of a Bothrops jararaca cDNA encoding a precursor of seven bradykinin-potentiating peptides and a C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 1997, 94, 1189–1193. [Google Scholar] [CrossRef]
- Higuchi, S.; Murayama, N.; Saguchi, K.; Ohi, H.; Fujita, Y.; Camargo, A.C.; Ogawa, T.; Deshimaru, M.; Ohno, M. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology 1999, 44, 129–135. [Google Scholar] [CrossRef]
- Whittington, A.C.; Mason, A.J.; Rokyta, D.R. A single mutation unlocks cascading exaptations in the origin of a potent pitviper neurotoxin. Mol. Biol. Evol. 2018, 35, 887–898. [Google Scholar] [CrossRef]
- Seger, M.A.; Bennett, H.P. Structure and bioactivity of the amino-terminal fragment of pro-opiomelanocortin. J. Steroid Biochem. 1986, 25, 703–710. [Google Scholar] [CrossRef]
- Rholam, M.; Fahy, C. Processing of peptide and hormone precursors at the dibasic cleavage sites. Cell. Mol. Life Sci. 2009, 66, 2075–2091. [Google Scholar] [CrossRef]
- Solovyeva, N.I.; Gureeva, T.A.; Timoshenko, O.S.; Moskvitina, T.A.; Kugaevskaya, E.V. Furin as proprotein convertase and its role in normal and pathological biological processes. Biomed. Khim. 2016, 62, 609–621. [Google Scholar] [CrossRef]
- Małuch, I.; Walewska, A.; Sikorska, E.; Prahl, A. Proprotein convertases—Family of serine proteases with a broad spectrum of physiological functions. Postepy Biochem. 2016, 62, 472–481. [Google Scholar] [PubMed]
- Klein-Szanto, A.J.; Bassi, D.E. Proprotein convertase inhibition: Paralyzing the cell’s master switches. Biochem. Pharmacol. 2017, 140, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Remacle, A.G.; Shiryaev, S.A.; Oh, E.S.; Cieplak, P.; Srinivasan, A.; Wei, G.; Liddington, R.C.; Ratnikov, B.I.; Parent, A.; Desjardins, R.; et al. Substrate cleavage analysis of furin and related proprotein convertases. A comparative study. J. Biol. Chem. 2008, 283, 20897–20906. [Google Scholar] [CrossRef] [PubMed]
- Fuse, N.; Tsuchiya, T.; Nonomura, Y.; Menez, A.; Tamiya, T. Structure of the snake short-chain neurotoxin, erabutoxin c, precursor gene. Eur. J. Biochem. 1990, 193, 629–633. [Google Scholar] [CrossRef]
- Afifiyan, F.; Armugam, A.; Tan, C.H.; Gopalakrishnakone, P.; Jeyaseelan, K. Postsynaptic alpha-neurotoxin gene of the spitting cobra, Naja naja sputatrix: Structure, organization, and phylogenetic analysis. Genome Res. 1999, 9, 259–266. [Google Scholar] [CrossRef]
- Chang, L.S.; Lin, J.; Chou, Y.C.; Hong, E. Genomic structures of cardiotoxin 4 and cobrotoxin from Naja naja atra (Taiwan cobra). Biochem. Biophys. Res. Commun. 1997, 239, 756–762. [Google Scholar] [CrossRef]
- Fujimi, T.J.; Nakajyo, T.; Nishimura, E.; Ogura, E.; Tsuchiya, T.; Tamiya, T. Molecular evolution and diversification of snake toxin genes, revealed by analysis of intron sequences. Gene 2003, 313, 111–118. [Google Scholar] [CrossRef]
- Doley, R.; Pahari, S.; Mackessy, S.P.; Kini, R.M. Accelerated exchange of exon segments in Viperid three-finger toxin genes (Sistrurus catenatus edwardsii; Desert Massasauga). BMC Evol. Biol. 2008, 8, 196. [Google Scholar] [CrossRef]
- Ogawa, T.; Oda, N.; Nakashima, K.; Sasaki, H.; Hattori, M.; Sakaki, Y.; Kihara, H.; Ohno, M. Unusually high conservation of untranslated sequences in cDNAs for Trimeresurus flavoviridis phospholipase A2 isozymes. Proc. Natl. Acad. Sci. USA 1992, 89, 8557–8561. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ogawa, T.; Oda, N.; Hattori, M.; Sakaki, Y.; Kihara, H.; Ohno, M. Accelerated evolution of Trimeresurus flavoviridis venom gland phospholipase A2 isozymes. Proc. Natl. Acad. Sci. USA 1993, 90, 5964–5968. [Google Scholar] [CrossRef] [PubMed]
- Nobuhisa, I.; Nakashima, K.; Deshimaru, M.; Ogawa, T.; Shimohigashi, Y.; Fukumaki, Y.; Sakaki, Y.; Hattori, S.; Kihara, H.; Ohno, M. Accelerated evolution of Trimeresurus okinavensis venom gland phospholipase A2 isozyme-encoding genes. Gene 1996, 172, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Lynch, V.J. Inventing an arsenal: Adaptive evolution and neofunctionalization of snake venom phospholipase A2 genes. BMC Evol. Biol. 2007, 7, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juárez, P.; Comas, I.; González-Candelas, F.; Calvete, J.J. Evolution of snake venom disintegrins by positive Darwinian selection. Mol. Biol. Evol. 2008, 25, 2391–2407. [Google Scholar] [CrossRef] [PubMed]
- Doley, R.; Mackessy, S.P.; Kini, R.M. Role of accelerated segment switch in exons to alter targeting (ASSET) in the molecular evolution of snake venom proteins. BMC Evol. Biol. 2009, 9, 146. [Google Scholar] [CrossRef]
- Sunagar, K.; Jackson, T.N.; Undheim, E.A.; Ali, S.A.; Antunes, A.; Fry, B.G. Three-fingered RAVERs: Rapid accumulation of variations in exposed residues of snake venom toxins. Toxins 2013, 5, 2172–2208. [Google Scholar] [CrossRef]
- Kini, R.M. Accelerated evolution of toxin genes: Exonization and intronization in snake venom disintegrin/metalloprotease genes. Toxicon 2018, 148, 16–25. [Google Scholar] [CrossRef]
- Sine, S.M. End-plate acetylcholine receptor: Structure, mechanism, pharmacology, and disease. Physiol. Rev. 2012, 92, 1189–1234. [Google Scholar] [CrossRef]
- Zoli, M.; Pistillo, F.; Gotti, C. Diversity of native nicotinic receptor subtypes in mammalian brain. Neuropharmacology 2015, 96, 302–311. [Google Scholar] [CrossRef]
- Dellisanti, C.D.; Yao, Y.; Stroud, J.C.; Wang, Z.Z.; Chen, L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 Å resolution. Nat. Neurosci. 2007, 10, 953–962. [Google Scholar] [CrossRef]
- Dellisanti, C.D.; Yao, Y.; Stroud, J.C.; Wang, Z.Z.; Chen, L. Structural determinants for alpha-neurotoxin sensitivity in muscle nAChR and their implications for the gating mechanism. Channels 2007, 1, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Zhong, W.; Gallivan, J.P.; Zhang, Y.; Li, L.; Lester, H.A.; Dougherty, D.A. From ab initio quantum mechanics to molecular neurobiology: A cation–p binding site in the nicotinic receptor. Proc. Natl. Acad. Sci. USA 1998, 95, 12088–12093. [Google Scholar] [CrossRef] [PubMed]
- Karlin, A. Emerging structure of the nicotinic acetylcholine receptors. Nat. Rev. Neurosci. 2002, 3, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.; Séquéla, P.; Vernino, S.; Amador, M.; Luetje, C.; Dani, J.A. Functional diversity of neuronal nicotinic acetylcholine receptors. Prog. Brain Res. 1993, 98, 113–120. [Google Scholar] [PubMed]
- Clements, J.D. Transmitter timecourse in the synaptic cleft: Its role in central synaptic function. Trends Neurosci. 1996, 19, 163–171. [Google Scholar] [CrossRef]
- Savtchenko, L.P.; Antropov, S.N.; Korogod, S.M. Effect of voltage drop within the synaptic cleft on the current and voltage generated at a single synapse. Biophys. J. 2000, 78, 1119–1125. [Google Scholar] [CrossRef]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4 Å resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Rayes, D.; De Rosa, M.J.; Sine, S.M.; Bouzat, C. Number and locations of agonist binding sites required to activate homomeric Cys-loop receptors. J. Neurosci. 2009, 29, 6022–6032. [Google Scholar] [CrossRef]
- Chojnacka, K.; Papke, R.L.; Horenstein, N.A. Synthesis and evaluation of a conditionally-silent agonist for the α7 nicotinic acetylcholine receptor. Bioorg. Med. Chem. Lett. 2013, 23, 4145–4149. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Bagdas, D.; Kulkarni, A.R.; Gould, T.; AlSharari, S.D.; Thakur, G.A.; Damaj, M.I. The analgesic-like properties of the alpha7 nAChR silent agonist NS6740 is associated with non-conducting conformations of the receptor. Neuropharmacology 2015, 91, 34–42. [Google Scholar] [CrossRef] [PubMed]
- van Maanen, M.A.; Papke, R.L.; Koopman, F.A.; Koepke, J.; Bevaart, L.; Clark, R.; Lamppu, D.; Elbaum, D.; LaRosa, G.J.; Tak, P.P.; et al. Two Novel α7 Nicotinic Acetylcholine Receptor Ligands. In Vitro Properties and Their Efficacy in Collagen-Induced Arthritis in Mice. PLoS ONE 2015, 10, e0116227. [Google Scholar] [CrossRef] [PubMed]
- Quadri, M.; Papke, R.L.; Horenstein, N.A. Dissection of N, N-diethyl-N′-phenylpiperazines as α7 nicotinic receptor silent agonists. Bioorg. Med. Chem. 2016, 24, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Papke, R.L.; Quadri, M.; Gulsevin, A. Silent agonists for alpha7 nicotinic acetylcholine receptors. Pharmacol. Res. 2023, 190, 106736. [Google Scholar] [CrossRef] [PubMed]
- Neher, E. The charge carried by single-channel currents of rat cultured muscle cells in the presence of local anaesthetics. J. Physiol. 1983, 339, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Giraudat, J.; Dennis, M.; Heidmann, T.; Chang, J.Y.; Changeux, J.P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: Serine-262 of the d subunit is labeled by [3H]chlorpromazine. Proc. Natl. Acad. Sci. USA 1986, 83, 2719–2723. [Google Scholar] [CrossRef]
- Giraudat, J.; Dennis, M.; Heidmann, T.; Haumont, P.Y.; Lederer, F.; Changeux, J.P. Structure of the high-affinity binding site for noncompetitive blockers of the acetylcholine receptor: [3H]chlorpromazine labels homologous residues in the b and d chains. Biochemistry 1987, 26, 2410–2418. [Google Scholar] [CrossRef]
- Pelzer, D.; Grant, A.O.; Cavalié, A.; Pelzer, S.; Sieber, M.; Hofmann, F.; Trautwein, W. Calcium channels reconstituted from the skeletal muscle dihydropyridine receptor protein complex and its alpha 1 peptide subunit in lipid bilayers. Ann. N. Y. Acad. Sci. 1989, 560, 138–154. [Google Scholar] [CrossRef]
- Leonard, R.J.; Charnet, P.; Labarca, C.; Vogelaar, N.J.; Czyzyk, L.; Gouin, A.; Davidson, N.; Lester, H.A. Reverse pharmacology of the nicotinic acetylcholine receptor. Mapping the local anesthetic binding site. Ann. N. Y. Acad. Sci. 1991, 625, 588–599. [Google Scholar] [CrossRef]
- Changeux, J.-P. The TiPS lecture. The nicotinic acetylcholine receptor: An allosteric protein prototype of ligand-gated ion channels. Trends Pharmacol. Sci. 1990, 11, 485–492. [Google Scholar] [CrossRef]
- Bouzat, C.; Barrantes, F.J. Assigning functions to residues in the acetylcholine receptor channel region (review). Mol. Membr. Biol. 1997, 14, 167–177. [Google Scholar] [CrossRef]
- Manetti, D.; Dei, S.; Arias, H.R.; Braconi, L.; Gabellini, A.; Teodori, E.; Romanelli, M.N. Recent Advances in the Discovery of Nicotinic Acetylcholine Receptor Allosteric Modulators. Molecules 2023, 28, 1270. [Google Scholar] [CrossRef] [PubMed]
- Sanders, V.R.; Millar, N.S. Potentiation and allosteric agonist activation of alpha7 nicotinic acetylcholine receptors: Binding sites and hypotheses. Pharmacol. Res. 2023, 191, 106759. [Google Scholar] [CrossRef] [PubMed]
- Braunscheidel, K.M.; Voren, G.; Fowler, C.D.; Lu, Q.; Kuryatov, A.; Cameron, M.D.; Ibañez-Tallon, I.; Lindstrom, J.M.; Kamenecka, T.M.; Kenny, P.J. SR9883 is a novel small-molecule enhancer of alpha4beta2* nicotinic acetylcholine receptor signaling that decreases intravenous nicotine self-administration in rats. Front. Mol. Neurosci. 2024, 17, 1459098. [Google Scholar] [CrossRef] [PubMed]
- ElNebrisi, E.; Lozon, Y.; Oz, M. The Role of alpha7-Nicotinic Acetylcholine Receptors in the Pathophysiology and Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 3210. [Google Scholar] [CrossRef]
- Papke, R.L.; Lindstrom, J.M. Nicotinic acetylcholine receptors: Conventional and unconventional ligands and signaling. Neuropharmacology 2020, 168, 108021. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V., Jr.; Callahan, P.M. Alpha7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 2020, 170, 108053. [Google Scholar] [CrossRef] [PubMed]
- Cecchini, M.; Changeux, J.P. Nicotinic receptors: From protein allostery to computational neuropharmacology. Mol. Asp. Med. 2022, 84, 101044. [Google Scholar] [CrossRef] [PubMed]
- Hampsey, E.; Perkins, A.; Young, A.H. BNC210: An investigational alpha7-nicotinic acetylcholine receptor modulator for the treatment of anxiety disorders. Expert Opin. Investig. Drugs 2023, 32, 277–282. [Google Scholar] [CrossRef]
- Taylor, P.; Osaka, H.; Molles, B.E.; Sugiyama, N.; Marchot, P.; Ackermann, E.J.; Malany, S.; McArdle, J.J.; Sine, S.M.; Tsigelny, I. Toxins selective for subunit interfaces as probes of nicotinic acetylcholine receptor structure. J. Physiol. Paris. 1998, 92, 79–83. [Google Scholar] [CrossRef]
- Taylor, P.; Malany, S.; Molles, B.E.; Osaka, H.; Tsigelny, I. Subunit interface selective toxins as probes of nicotinic acetylcholine receptor structure. Pflügers Arch. 2000, 440 (Suppl. S1), R115–R117. [Google Scholar] [CrossRef] [PubMed]
- Kini, R.M. Toxins for decoding interface selectivity in nicotinic acetylcholine receptors. Biochem. J. 2019, 476, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Marchot, P.; Kawanishi, C.; Osaka, H.; Molles, B.; Sine, S.M.; Taylor, P. Residues at the subunit interfaces of the nicotinic acetylcholine receptor that contribute to alpha-conotoxin M1 binding. Mol. Pharmacol. 1998, 53, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Sine, S.M.; Kreienkamp, H.J.; Bren, N.; Maeda, R.; Taylor, P. Molecular dissection of subunit interfaces in the acetylcholine receptor: Identification of determinants of alpha-conotoxin M1 selectivity. Neuron 1995, 15, 205–211. [Google Scholar] [CrossRef]
- Molles, B.E.; Rezai, P.; Kline, E.F.; McArdle, J.J.; Sine, S.M.; Taylor, P. Identification of residues at the alpha and epsilon subunit interfaces mediating species selectivity of Waglerin-1 for nicotinic acetylcholine receptors. J. Biol. Chem. 2002, 277, 5433–5440. [Google Scholar] [CrossRef]
- Molles, B.E.; Tsigelny, I.; Nguyen, P.D.; Gao, S.X.; Sine, S.M.; Taylor, P. Residues in the epsilon subunit of the nicotinic acetylcholine receptor interact to confer selectivity of waglerin-1 for the alpha-epsilon subunit interface site. Biochemistry 2002, 41, 7895–7906. [Google Scholar] [CrossRef] [PubMed]
- Osaka, H.; Malany, S.; Molles, B.E.; Sine, S.M.; Taylor, P. Pairwise electrostatic interactions between alpha-neurotoxins and gamma, delta, and epsilon subunits of the nicotinic acetylcholine receptor. J. Biol. Chem. 2000, 275, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, E.J.; Ang, E.T.; Kanter, J.R.; Tsigelny, I.; Taylor, P. Identification of pairwise interactions in the alpha-neurotoxin-nicotinic acetylcholine receptor complex through double mutant cycles. J. Biol. Chem. 1998, 273, 10958–10964. [Google Scholar] [CrossRef] [PubMed]
- Shiu, J.H.; Chen, C.Y.; Chang, L.S.; Chen, Y.C.; Chen, Y.C.; Lo, Y.H.; Liu, Y.C.; Chuang, W.J. Solution structure of gamma-bungarotoxin: The functional significance of amino acid residues flanking the RGD motif in integrin binding. Proteins 2004, 57, 839–849. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Sun, C.; Dong, M.; Zhao, N.; Wang, Y.; Yu, K.; Zhang, J.; Xu, N.; Liu, W. Gamma-Bungarotoxin impairs the vascular endothelial barrier function by inhibiting integrin alpha5. Toxicol. Lett. 2023, 383, 177–191. [Google Scholar] [CrossRef]
- Kessler, P.; Marchot, P.; Silva, M.; Servent, D. The three-finger toxin fold: A multifunctional structural scaffold able to modulate cholinergic functions. J. Neurochem. 2017, 142 (Suppl. S2), 7–18. [Google Scholar] [CrossRef] [PubMed]
- Servent, D.; Winckler-Dietrich, V.; Hu, H.Y.; Kessler, P.; Drevet, P.; Bertrand, D.; Menez, A. Only snake curaremimetic toxins with a fifth disulfide bond have high affinity for the neuronal a7 nicotinic receptor. J. Biol. Chem. 1997, 272, 24279–24286. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Lee, C.Y. Isolation of neurotoxins from the venom of Bungarus multicinctus and their modes of neuromuscular blocking action. Arch. Int. Pharmacodyn. Ther. 1963, 144, 241–257. [Google Scholar] [PubMed]
- Chang, C.C. Looking back on the discovery of α-bungarotoxin. J. Biomed. Sci. 1999, 6, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Carvalho, L.P.; Chan, M.Y.; Kini, R.M.; Kang, T.S. Fasxiator, a novel factor XIa inhibitor from snake venom, and its site-specific mutagenesis to improve potency and selectivity. J. Thromb. Haemost. 2015, 13, 248–261. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.S.; Li, A.W.L.; Lim, J.J.M.; Leong, E.J.E.; Amran, F.S.; Kini, R.M.; Chan, M.Y.Y.; Koh, C.Y. A Factor XIa inhibitor engineered from banded krait venom toxin: Efficacy and safety in rodent models of arterial and venous thrombosis. Biomedicines 2022, 10, 1679. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, M.; Yu, S.; Huang, Q.; Chen, R.; Xu, S.; Huang, Y.; Yu, Y.; Liao, M.; Dai, Q. A single amino acid replacement boosts the analgesic activity of alpha-conotoxin AuIB through the inhibition of the GABA(B)R-coupled N-type calcium channel. Mar. Drugs 2022, 20, 750. [Google Scholar] [CrossRef]
- Osmakov, D.I.; Khasanov, T.A.; Maleeva, E.E.; Pavlov, V.M.; Palikov, V.A.; Belozerova, O.A.; Koshelev, S.G.; Korolkova, Y.V.; Dyachenko, I.A.; Kozlov, S.A.; et al. Two amino acid substitutions improve the pharmacological profile of the snake venom peptide mambalgin. Toxins 2025, 17, 101. [Google Scholar] [CrossRef]
- Mourier, G.; Servent, D.; Zinn-Justin, S.; Ménez, A. Chemical engineering of a three-fingered toxin with anti-a7 neuronal acetylcholine receptor activity. Protein Eng. 2000, 13, 217–225. [Google Scholar] [CrossRef]
- Fruchart-Gaillard, C.; Mourier, G.; Blanchet, G.; Vera, L.; Gilles, N.; Ménez, R.; Marcon, E.; Stura, E.A.; Servent, D. Engineering of three-finger fold toxins creates ligands with original pharmacological profiles for muscarinic and adrenergic receptors. PLoS ONE 2012, 7, e39166. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Snake venom three-finger toxins and their potential in drug development targeting cardiovascular diseases. Biochem. Pharmacol. 2020, 181, 114105. [Google Scholar] [CrossRef]
- Xu, J.; Lei, X.; Li, A.; Li, J.; Li, S.; Chen, L. Scalable production of recombinant three-finger proteins: From inclusion bodies to high quality molecular probes. Microb. Cell Fact. 2024, 23, 48. [Google Scholar] [CrossRef]









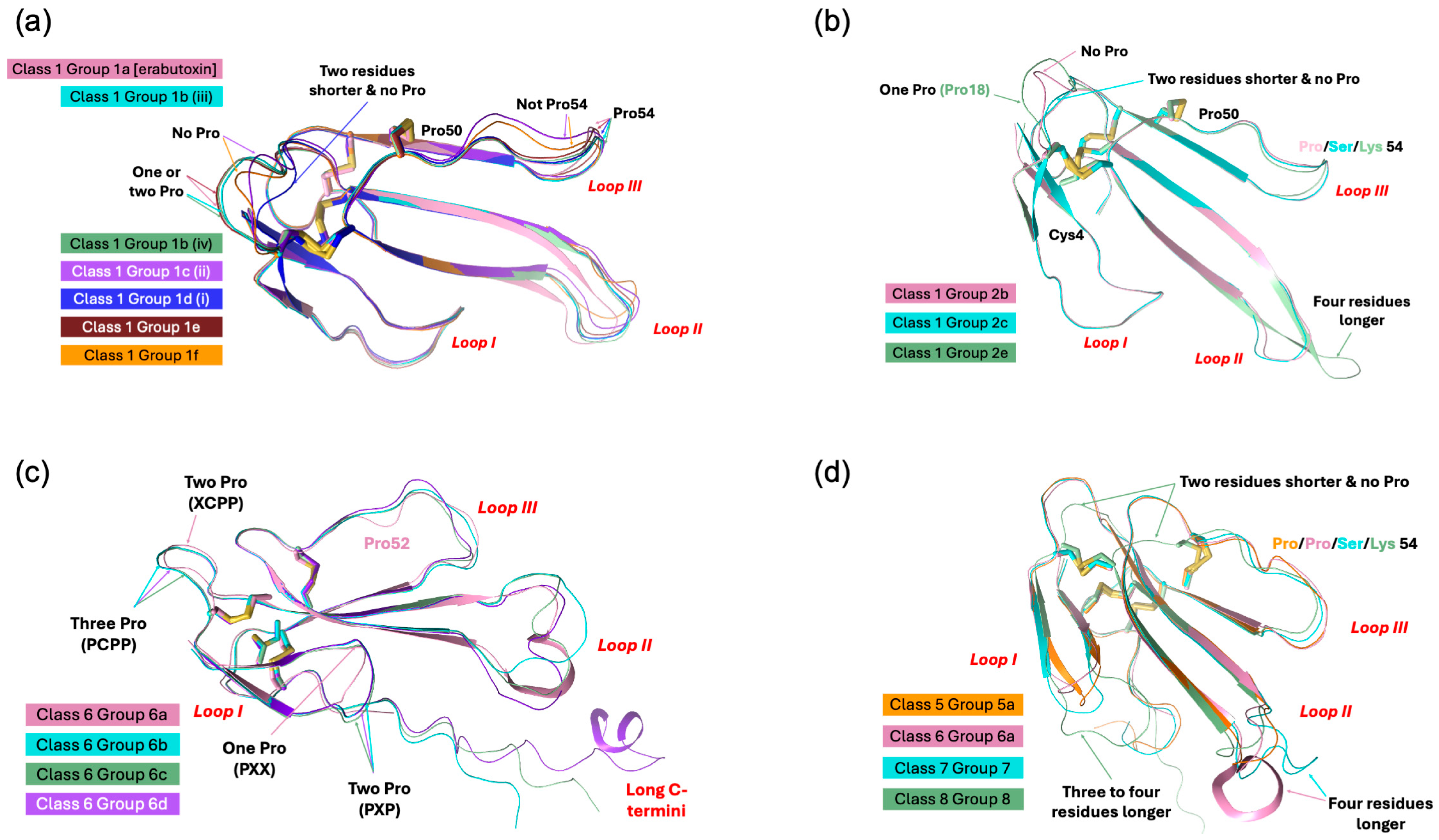
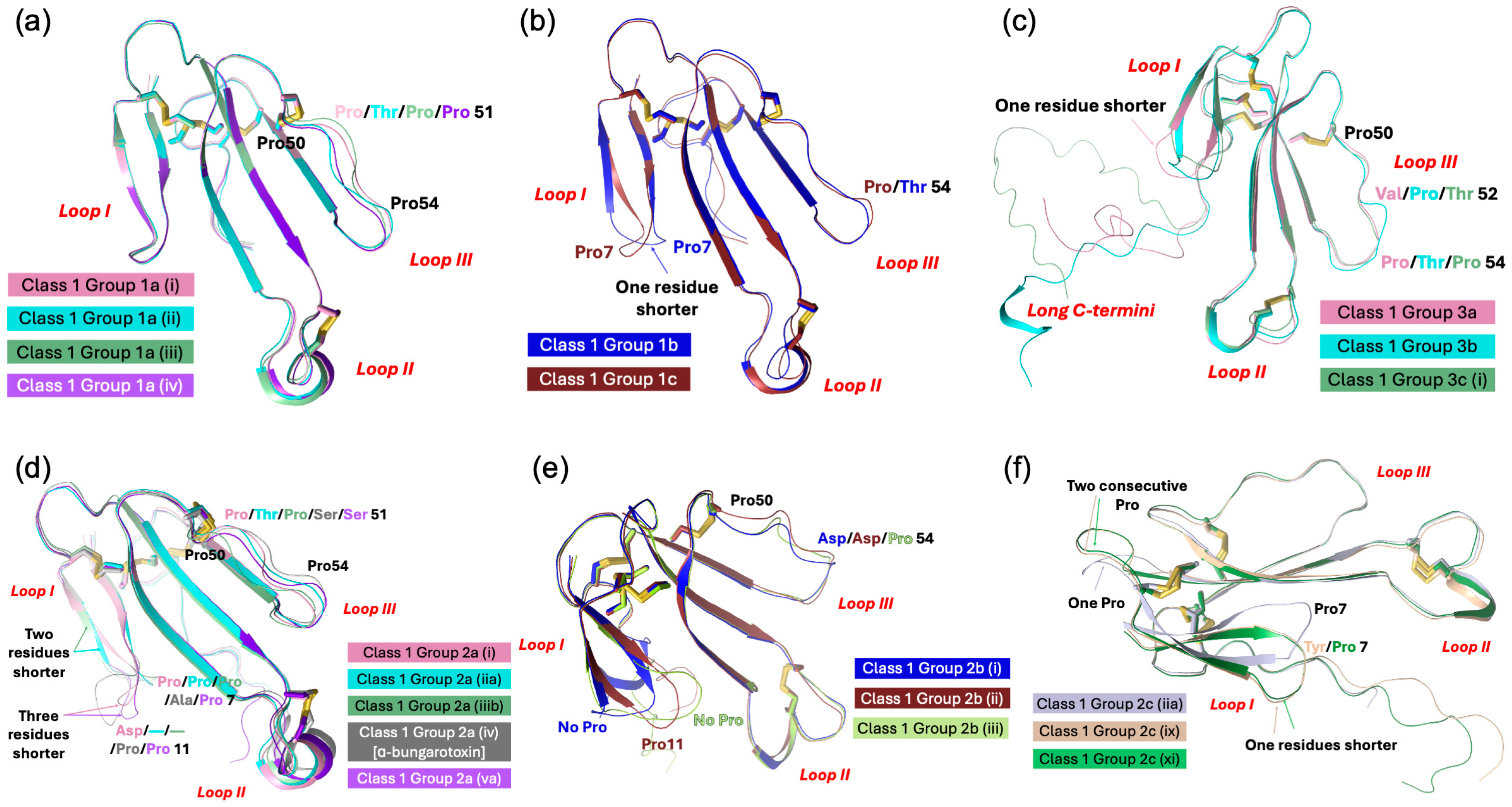

| Name | Class | Group | Total Number of Sequences | Subgroups | Main Grouping Criteria (Refer to Text for Details) |
|---|---|---|---|---|---|
| Type I, Short-chain neurotoxins | 1 | 1a | 11 | Erabutoxin and closely related toxins | |
| 1b | 39 | i–iv | Limited and conserved substitutions in functional residues | ||
| 1c | 22 | i–iii | Pro12 instead of Pro11 | ||
| 1d | 10 | i–v | Pro11 and Pro12 | ||
| 1e | 8 | Pro7 and Pro11 | |||
| 1f | 5 | Only Pro44(50) | |||
| 1g | 3 | Gln10Thr substitution | |||
| 1h | 4 | Only 4 functional residues are retained | |||
| 1i | 16 | Only a few functional residues are conserved | |||
| 2 | 2a | 1 | Cys4 and Phe32(36) | ||
| 2b | 17 | Cys4 and His32(36) | |||
| 2c | 11 | Cys4 and Ser/Thr/His/Arg48(54) | |||
| 2d | 2 | Cys4, Lys27Met, Asp31Gly, Phe32(36)Ile, and Arg33(37)His | |||
| 2e | 3 | Cys4 and long Gly/Ser-rich loop II | |||
| 3 | 3a | 2 | Cys4 and Cys16 | ||
| 3b | 1 | 7 out of 10 functional residues conserved | |||
| 4 | 2 | Asp31, Phe/His32(36), and Arg33(37) deleted | |||
| 5 | 5a | 2 | Shorter loop I, longer C-terminus, all functional residues except Phe/His32(36) are conserved | ||
| 5b | 3 | ||||
| 5c | 1 | ||||
| 6 | 6a | 2 | Shorter loop I, longer loop II and C-terminus, no fifth disulfide in loop II unlike long-chain α-neurotoxins | ||
| 6b | 2 | ||||
| 6c | 1 | ||||
| 6d | 1 | ||||
| 7 | 3 | Shorter loop I, longer loop II and C-terminus, no fifth disulfide in loop II unlike long-chain α-neurotoxins, His32(36)Thr/Ser | |||
| 8 | 1 | Loop I and II are characteristic of short-chain α-neurotoxins but has longer C-terminus like long-chain α-neurotoxins | |||
| 9 | 4 | Covalent homodimer, few conserved functional residues | |||
| Type II, Long-chain neurotoxins | 1 | 1a | 22 | i–iv | α-bungarotoxin and closely related toxins |
| 1b | 2 | Asp27(31)Asn, Phe29(33)Trp, Lys49(53)Asn, His65(70)Tyr | |||
| 1c | 3 | Additional substitutions | |||
| 2 | 2a | 47 | i, iia–c, iiia–c, iv, va–d, via–d, vii–viii | Longer C-terminus (6–13 residues), conserved His65(70), various other substitution among functional residues | |
| 2b | 6 | i–iii | Longer C-terminus (6–13 residues), His/Phe65(70) is non-conserved | ||
| 2c | 43 | i, iia–b, iiia–b, iva–b, v, via–c, viia–b, via–b, ix, xa–b, xi–xiii | Longer C-terminus (7–13 residues), conserved Phe65(70), varying substitutions among functional residues | ||
| 3 | 3a | 2 | Long C-terminus (17–24 residues), variable C-terminus sequences | ||
| 3b | 9 | ||||
| 3c | 7 | i–ii | |||
| 4 | 9 | κ-neurotoxins, non-covalent homodimers | |||
| 5 | 4 | Non-covalent homodimers, functionally similar to α-neurotoxins, C-terminal amidation | |||
| 6 | 6 | α-cobratoxin, covalent homo/hetero-dimers | |||
| Type III neurotoxins | 1 | 1a | 35 | Each loop is shorter, and none of the functional residues are conserved | |
| 1b | 2 | ||||
| 2 | 1 | Longer C-terminus | |||
| Non-conventional neurotoxins | 1 | 1a | 3 | Candoxin and related toxins, 5th disulfide in loop I, many short-chain α-neurotoxin functional residues in loop II are conserved | |
| 1b | 4 | ||||
| 1c | 1 | ||||
| 2 | 3 | γ-bungarotoxin and related toxins, Arg27(29), Trp29(31), and Lys47(55) | |||
| 3 | 3a | 5 | i–iii | WTX and related toxins, Arg31(33), Arg32(37), and Arg37(42) as important functional residues | |
| 3b | 9 | i–iii | |||
| 3c | 10 | i–v | |||
| 3d | 11 | i–iv | |||
| 4 | 4 | Longer C-terminus, slightly altered location of the 5th disulfide | |||
| Ω-neurotoxins | 1 | 1a | 3 | Short loop I and III, functional residues in short- and long-chain α-neurotoxins are not conserved | |
| 1b | 4 | ||||
| 1c | 2 | ||||
| 1d | 2 | ||||
| 2 | 1 | ||||
| Σ-neurotoxins | 1 | 1a | 8 | Non-covalent dimer, five-residue extension at C-terminus, functional residues in α-neurotoxins and Ω-neurotoxins are not conserved | |
| 1b | 10 | ||||
| 1c | 1 | ||||
| 2 | 2a | 10 | Shortest C-terminus among Σ-neurotoxins, conserved residues at dimer interface | ||
| 2b | 1 | ||||
| 2c | 2 | ||||
| 3 | 1 | Two residues extension at C-terminus compared to class 2, conserved residues at dimer interface | |||
| Colubrid FTxs | 1 | 1a | 58 | i–iv | N-terminal Gln modified to pyroglutamic acid, seven-residue N-terminal extension compared to typical 3FTxs, fifteen-residue propeptide with Xaa-Gln processing site |
| 1b | 6 | Tetrabasic propetide processing site | |||
| 1c | 9 | Dibasic propeptide processing site | |||
| 1d | 1 | Dibasic propeptide processing site, seventeen-residue N-terminal extension compared to typical 3FTxs | |||
| 1e | 2 | Monobasic propeptide processing site, six-residue N-terminal extension compared to typical 3FTxs | |||
| 1f | 1 | α-colubritoxin, unknown propeptide and processing site | |||
| 2 | 15 | N-terminal Pro, five-residue N-terminal extension, eight-residue propeptide, RRKKK-P processing site | |||
| 3 | 1 | N-terminal Pro, seven-residue N-terminal extension, eight-residue propeptide, KKK-P processing site | |||
| 4 | 9 | N-terminal Pro, six-residue N-terminal extension, seven-residue propeptide, RR/KR-P processing site | |||
| 5 | 56 | N-terminal Pro, seven-residue N-terminal extension, eight-residue propeptide, RK-P processing site | |||
| 6 | 2 | N-terminal Pro, six-residue N-terminal extension, seven-residue propeptide, monobasic processing site | |||
| 7 | 2 | one- or three-residue N-terminal extension, no propeptide | |||
| 8 | 8a | 43 | i–iv | Covalent heterodimeric 3FTxs (subunit A), additional Cys in loop II, varying proteolytic maturation characteristics | |
| 8b | 2 | ||||
| 8c | 2 | ||||
| 9 | 9a | 27 | Covalent heterodimeric 3FTxs (subunit B), additional Cys in loop I, varying proteolytic maturation characteristics | ||
| 9b | 3 | ||||
| 9c | 2 | ||||
| 9d | 2 | ||||
| 9e | 2 | ||||
| 10 | 10a | 2 | Similar to Classes 8 and 9, additional Cys in both loop I and II | ||
| 10b | 1 | ||||
| 11 | 2 | Two conserved Cys of typical nonconventional 3FTx missing, disulfide pairing pattern unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kini, R.M.; Koh, C.Y. Variations in “Functional Site” Residues and Classification of Three-Finger Neurotoxins in Snake Venoms. Toxins 2025, 17, 364. https://doi.org/10.3390/toxins17080364
Kini RM, Koh CY. Variations in “Functional Site” Residues and Classification of Three-Finger Neurotoxins in Snake Venoms. Toxins. 2025; 17(8):364. https://doi.org/10.3390/toxins17080364
Chicago/Turabian StyleKini, R. Manjunatha, and Cho Yeow Koh. 2025. "Variations in “Functional Site” Residues and Classification of Three-Finger Neurotoxins in Snake Venoms" Toxins 17, no. 8: 364. https://doi.org/10.3390/toxins17080364
APA StyleKini, R. M., & Koh, C. Y. (2025). Variations in “Functional Site” Residues and Classification of Three-Finger Neurotoxins in Snake Venoms. Toxins, 17(8), 364. https://doi.org/10.3390/toxins17080364






