The Effects of Aflatoxin B1 on Liver Cholestasis and Its Nutritional Regulation in Ducks
Abstract
1. Introduction
2. Results
2.1. Production Performance and Organ Weight Changes in Ducks
2.2. Changes in Liver Antioxidant Indicators
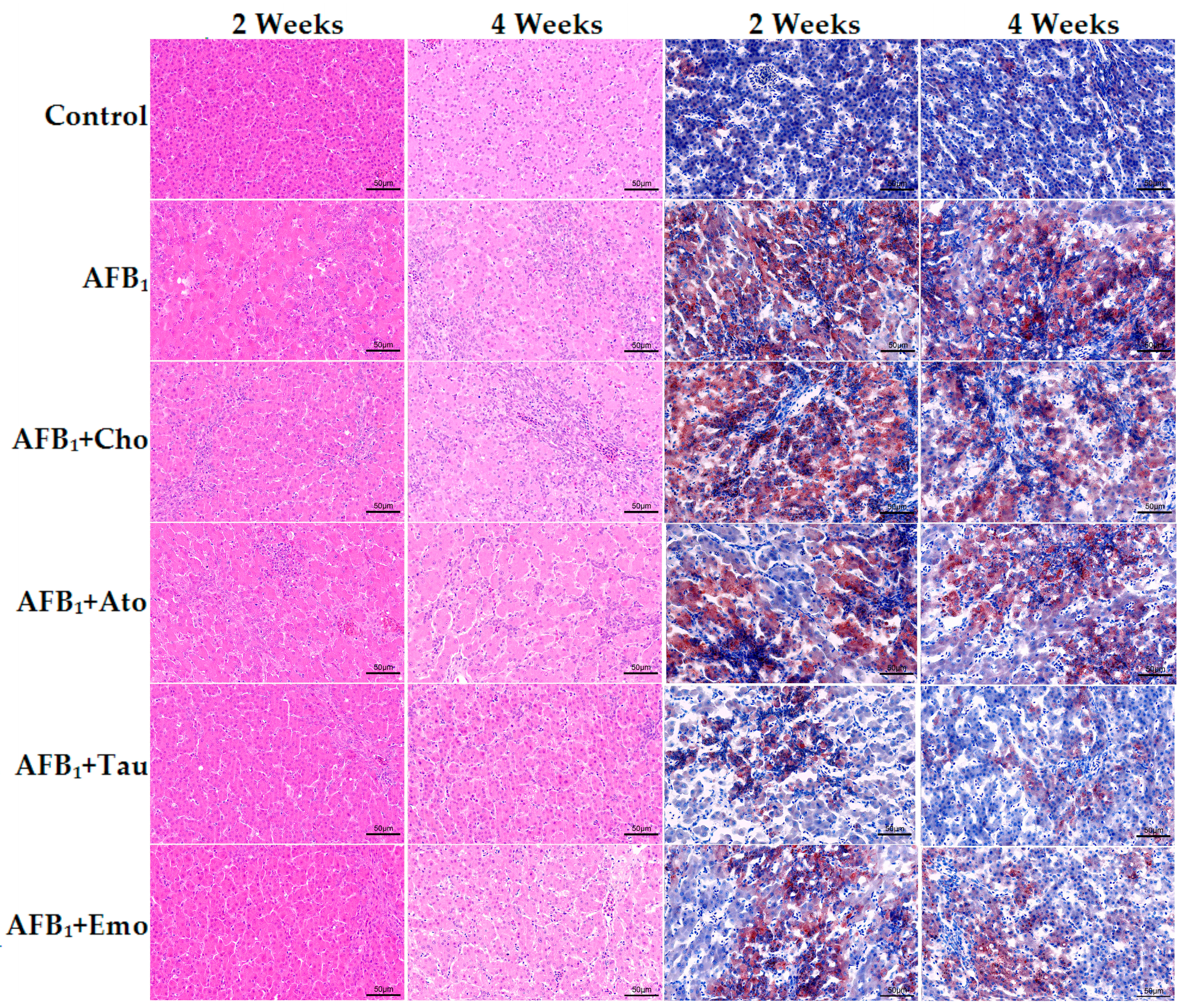
2.3. Histological Analysis of the Liver
2.4. Changes in Biochemical Indicators in Duck Serum
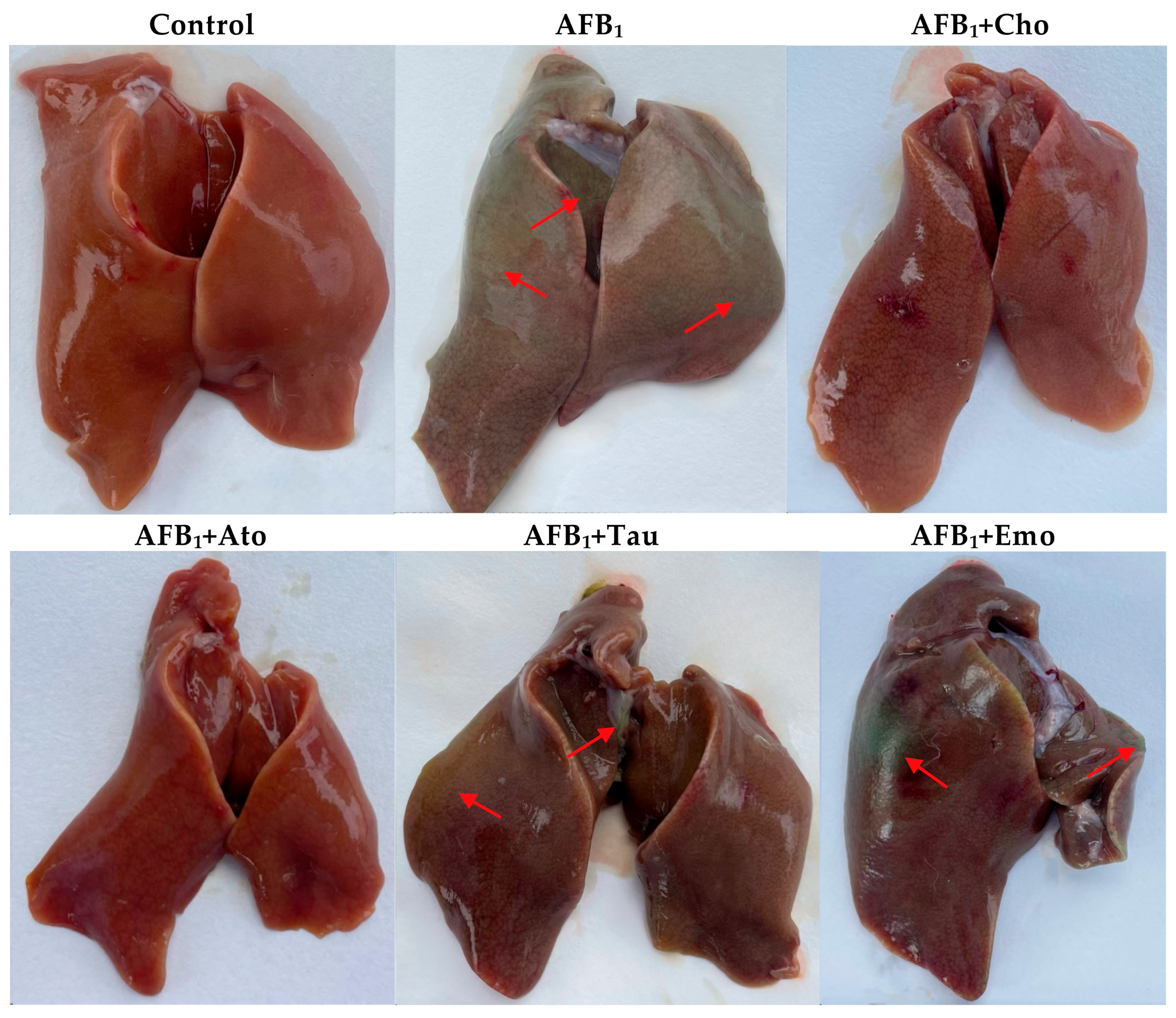
2.5. The Degree of Liver Cholestasis in the Treatment Groups
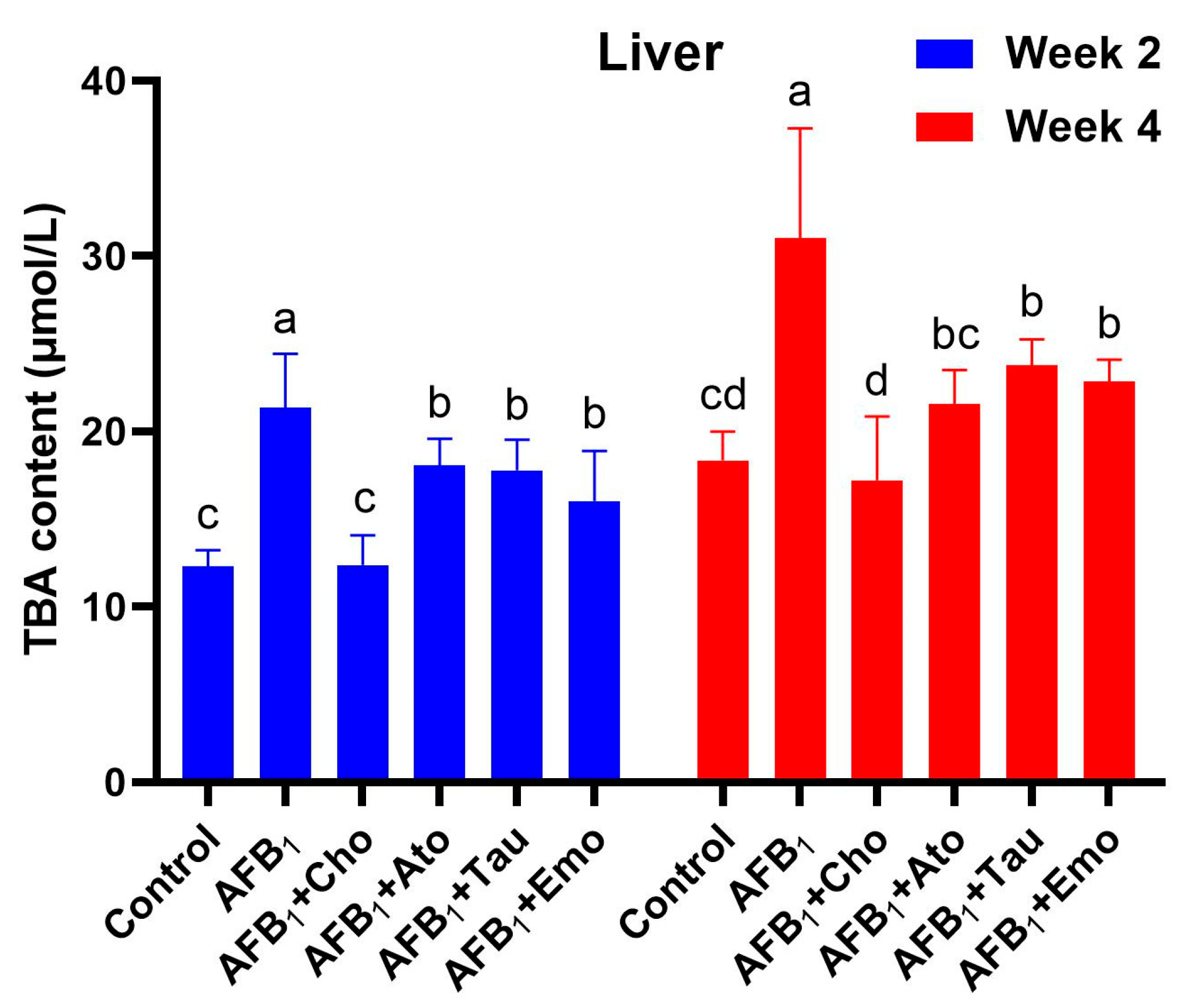
2.6. Changes in the Total Bile Acid Content in the Liver
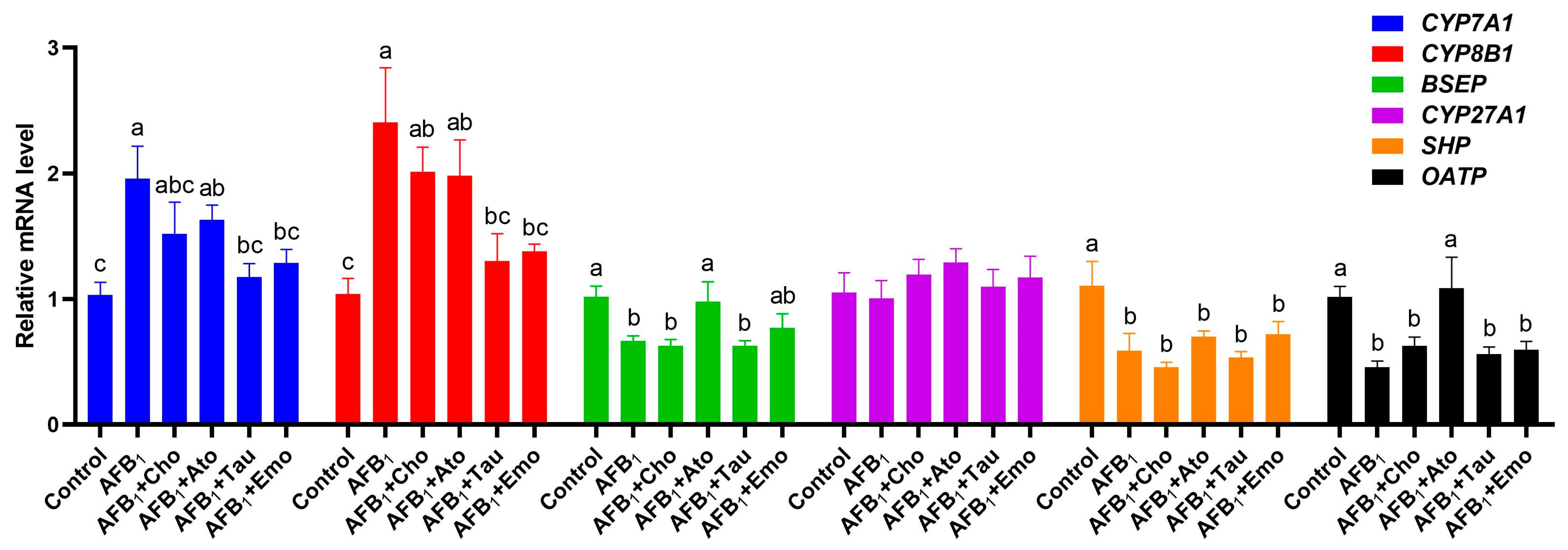
2.7. Changes in the Expression Levels of Liver-Bile-Acid-Metabolism-Related Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Duck, Treatments, and Sample Collection
5.2. Determination of Production Performance
5.3. Determination of Liver Antioxidant Indicators
5.4. H&E Staining
5.5. Oil Red O Staining
5.6. Determination of Serum Biochemical Indicators
5.7. Determination of the TBA Content in the Liver
5.8. RNA Extraction, cDNA Synthesis, and Real-Time qPCR
5.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimbalo, A.; Alonso-Garrido, M.; Font, G.; Manyes, L. Toxicity of mycotoxins in vivo on vertebrate organisms: A review. Food Chem. Toxicol. 2020, 137, 111161. [Google Scholar] [CrossRef] [PubMed]
- Shabeer, S.; Asad, S.; Jamal, A.; Ali, A. Aflatoxin Contamination, Its Impact and Management Strategies: An Updated Review. Toxins 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Beltran, A.; Bandyopadhyay, R. Contributions of integrated aflatoxin management strategies to achieve the sustainable development goals in various African countries. Glob. Food Secur. 2021, 30, 100559. [Google Scholar] [CrossRef]
- Cao, W.; Yu, P.; Yang, K.; Cao, D. Aflatoxin B1: Metabolism, toxicology, and its involvement in oxidative stress and cancer development. Toxicol. Mech. Methods 2022, 32, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Wang, L.; Yang, P.; Liu, Z.; Rajput, S.A.; Hassan, M.; Qi, D. Epigallocatechin Gallate and Glutathione Attenuate Aflatoxin B1-Induced Acute Liver Injury in Ducklings via Mitochondria-Mediated Apoptosis and the Nrf2 Signalling Pathway. Toxins 2022, 14, 876. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, Y.; Zhou, X.; Liu, M.; Jin, S.; Shan, A.; Feng, X. Resveratrol Relieved Acute Liver Damage in Ducks (Anas platyrhynchos) Induced by AFB1 via Modulation of Apoptosis and Nrf2 Signaling Pathways. Animals 2021, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Functional, Diagnostic and Therapeutic Aspects of Bile. Clin. Exp. Gastroenterol. 2022, 15, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yutuc, E.; Griffiths, W.J. Cholesterol metabolism pathways—Are the intermediates more important than the products? FEBS J. 2021, 288, 3727–3745. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Wei, M.; Rajani, C.; Zheng, X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell 2021, 12, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Garzel, B.; Hu, T.; Li, L.; Lu, Y.; Heyward, S.; Polli, J.; Zhang, L.; Huang, S.M.; Raufman, J.P.; Wang, H. Metformin Disrupts Bile Acid Efflux by Repressing Bile Salt Export Pump Expression. Pharm. Res. 2020, 37, 26. [Google Scholar] [CrossRef] [PubMed]
- Arab, J.P.; Cabrera, D.; Arrese, M. Bile Acids in Cholestasis and its Treatment. Ann. Hepatol. 2017, 16, s53–s57. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Horinouchi, A.; Higashimura, Y.; Akahori, R.; Matsumoto, K. Cholestyramine, a Bile Acid Sequestrant, Increases Cecal Short Chain Fatty Acids and Intestinal Immunoglobulin A in Mice. Biol. Pharm. Bull. 2020, 43, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Laatikainen, T. Effect of cholestyramine and phenobarbital on pruritus and serum bile acid levels in cholestasis of pregnancy. Am. J. Obstet. Gynecol. 1978, 132, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, K.; Mi, X.; Rajput, S.A.; Qi, D. Effects of 3-Hydroxy-3-methylglutaryl-CoA Reductase Inhibitors on Cholesterol Metabolism in Laying Hens. Animals 2023, 13, 1868. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, H.; Liang, J.; Wang, T.; Liu, J.; Pi, X.; Zou, W.; Qu, L. Effects of Atorvastatin on Bile Acid Metabolism in High-fat Diet-fed ApoE -/- Mice. J. Cardiovasc. Pharmacol. 2023, 81, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More Than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, L.J.; Huang, T.; Ying, J.Q.; Li, J. The pharmacology, toxicology and therapeutic potential of anthraquinone derivative emodin. Chin. J. Nat. Med. 2020, 18, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Guertin, F.; Roy, C.C.; Lepage, G.; Perea, A.; Giguere, R.; Yousef, I.; Tuchweber, B. Effect of taurine on total parenteral nutrition-associated cholestasis. JPEN J. Parenter. Enter. Nutr. 1991, 15, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Liu, J.; Xue, X.; Li, Y. The effect of emodin on liver disease—Comprehensive advances in molecular mechanisms. Eur. J. Pharmacol. 2020, 882, 173269. [Google Scholar] [CrossRef]
- Miyazaki, T.; Sasaki, S.I.; Toyoda, A.; Shirai, M.; Ikegami, T.; Matsuzaki, Y.; Honda, A. Influences of Taurine Deficiency on Bile Acids of the Bile in the Cat Model. Adv. Exp. Med. Biol. 2019, 1155, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.L.; Ding, Y.; Chen, Z.L.; Wang, Y.; Liu, P.; Qin, H.; Zhou, L.S.; Zhang, L.L.; Huang, J.; Zhao, L. Emodin Rescues Intrahepatic Cholestasis via Stimulating FXR/BSEP Pathway in Promoting the Canalicular Export of Accumulated Bile. Front. Pharmacol. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Jiang, L.; Xu, J.; Liu, W.; Li, S.; Huang, W.; Zhao, H.; Yang, Z.; Yu, X.; Wei, Z. Aflatoxin B1-activated heterophil extracellular traps result in the immunotoxicity to liver and kidney in chickens. Dev. Comp. Immunol. 2022, 128, 104325. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, F.; Qin, S.; Ma, J.; Li, L.; Jin, T.; Zhao, R. Effects of Selenium-Enriched Yeast Improved Aflatoxin B1-Induced Changes in Growth Performance, Antioxidation Capacity, IL-2 and IFN-gamma Contents, and Gene Expression in Mice. Biol. Trace Elem. Res. 2019, 191, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Khan, M.Z.; Khan, A.; Javed, I.; Saleemi, M.K.; Mahmood, S.; Asi, M.R. Residues of aflatoxin B1 in broiler meat: Effect of age and dietary aflatoxin B1 levels. Food Chem. Toxicol. 2010, 48, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.L.; Stine, J.G.; Bisanz, J.E.; Okafor, C.D.; Patterson, A.D. Bile acids and the gut microbiota: Metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 2023, 21, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J.; Kang, W.; Liu, S.; Liu, J.; Shi, M.; Wang, Y.; Liu, X.; Chen, X.; Huang, K. Aflatoxin B1 induces liver injury by disturbing gut microbiota-bile acid-FXR axis in mice. Food Chem. Toxicol. 2023, 176, 113751. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, M.C.; Rodriguez, J.V.; Monti, J.A.; Pellegrino, J.M.; Rodriguez, G.E. Impairment of bile secretion induced by aflatoxin B1 in the rat. Res. Commun. Chem. Pathol. Pharmacol. 1982, 38, 521–524. [Google Scholar] [PubMed]
- Zhou, J.; Tang, L.; Wang, J.; Wang, J.S. Aflatoxin B1 Disrupts Gut-Microbial Metabolisms of Short-Chain Fatty Acids, Long-Chain Fatty Acids, and Bile Acids in Male F344 Rats. Toxicol. Sci. 2018, 164, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Stieger, B.; Beuers, U. The canalicular bile salt export pump BSEP (ABCB11) as a potential therapeutic target. Curr. Drug Targets 2011, 12, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.I.; Donmez-Cakil, Y.; Szollosi, D.; Stockner, T.; Chiba, P. The Bile Salt Export Pump: Molecular Structure, Study Models and Small-Molecule Drugs for the Treatment of Inherited BSEP Deficiencies. Int. J. Mol. Sci. 2021, 22, 784. [Google Scholar] [CrossRef] [PubMed]
- Kis, E.; Ioja, E.; Rajnai, Z.; Jani, M.; Mehn, D.; Heredi-Szabo, K.; Krajcsi, P. BSEP inhibition: In vitro screens to assess cholestatic potential of drugs. Toxicol. In Vitro 2012, 26, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, M.C.; Nichifor, M.; Teaca, C.A. Bile Acid Sequestrants Based on Natural and Synthetic Gels. Gels 2023, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Lastuvkova, H.; Faradonbeh, F.A.; Schreiberova, J.; Hroch, M.; Mokry, J.; Faistova, H.; Nova, Z.; Hyspler, R.; Igreja Sa, I.C.; Nachtigal, P.; et al. Atorvastatin Modulates Bile Acid Homeostasis in Mice with Diet-Induced Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2021, 22, 6468. [Google Scholar] [CrossRef]
- Schade, D.S.; Shey, L.; Eaton, R.P. Cholesterol Review: A Metabolically Important Molecule. Endocr. Pract. 2020, 26, 1514–1523. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Horisaka, K. Effect of taurine and homotaurine on bile acid metabolism in dietary hyperlipidemic rats. J. Pharmacobiodyn. 1986, 9, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; San, J.; Pang, H.; Du, Y.; Li, W.; Zhou, X.; Yang, X.; Hu, J.; Yang, J. Taurine attenuates AFB1-induced liver injury by alleviating oxidative stress and regulating mitochondria-mediated apoptosis. Toxicon 2022, 215, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Han, L.; Bi, Y.; Li, C.; Gao, X.; Fan, G.; Zhang, Y. Paradoxical Effects of Emodin on ANIT-Induced Intrahepatic Cholestasis and Herb-Induced Hepatotoxicity in Mice. Toxicol. Sci. 2019, 168, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Ni, Y.; Zhou, Q.; Liu, H.; Xiang, H.; Sui, H.; Shang, D. Emodin Attenuates Severe Acute Pancreatitis via Antioxidant and Anti-inflammatory Activity. Inflammation 2019, 42, 2129–2138. [Google Scholar] [CrossRef] [PubMed]
| Items 2 | Control | AFB1 | AFB1 + Cho | AFB1 + Ato | AFB1 + Tau | AFB1 + Emo |
|---|---|---|---|---|---|---|
| IW (g) | 54.26 ± 0.69 | 54.27 ± 0.60 | 54.18 ± 0.75 | 54.15 ± 0.57 | 54.24 ± 0.67 | 54.14 ± 0.60 |
| 1–2 weeks | ||||||
| BW (g) | 438.86 ± 17.74 a | 351.30 ± 8.70 c | 392.52 ± 17.87 b | 363.97 ± 8.86 c | 404.31 ± 23.59 b | 388.92 ± 10.86 b |
| ADWG (g) | 27.47 ± 1.31 a | 21.22 ± 0.63 c | 24.17 ± 1.28 b | 22.13 ± 0.62 c | 25.01 ± 1.66 b | 23.91 ± 0.78 b |
| ADFI (g) | 50.71 ± 3.63 a | 42.50 ± 1.43 b | 46.67 ± 5.13 ab | 41.53 ± 5.42 b | 46.81 ± 5.71 ab | 46.42 ± 5.25 ab |
| FCR | 1.85 ± 0.12 | 2.00 ± 0.07 | 1.93 ± 0.18 | 1.88 ± 0.24 | 1.87 ± 0.17 | 1.94 ± 0.21 |
| 3–4 weeks | ||||||
| BW(g) | 1365.58 ± 56.40 a | 1082.15 ± 76.64 d | 1220.16 ± 65.88 b | 1113.13 ± 88.91 cd | 1249.74 ± 89.56 b | 1191.24 ± 65.51 bc |
| ADWG (g) | 66.19 ± 4.59 a | 52.20 ± 5.36 d | 59.12 ± 5.18 bc | 53.51 ± 6.54 cd | 60.39 ± 5.33 ab | 57.31 ± 4.64 bcd |
| ADFI (g) | 145.47 ± 9.15 a | 121.16 ± 21.29 bc | 127.98 ± 8.87 b | 112.00 ± 7.37 c | 131.15 ± 11.21 ab | 132.38 ± 11.80 ab |
| FCR | 2.20 ± 0.06 | 2.33 ± 0.40 | 2.17 ± 0.06 | 2.12 ± 0.29 | 2.17 ± 0.10 | 2.18 ± 0.27 |
| Week 4 | ||||||
| Liver (g) | 32.42 ± 4.79 b | 43.53 ± 10.18 a | 34.46 ± 5.46 b | 31.41 ± 3.73 b | 32.08 ± 4.45 b | 27.93 ± 7.31 b |
| Spleen (g) | 1.32 ± 0.35 b | 2.09 ± 0.84 a | 1.66 ± 0.26 ab | 2.09 ± 0.78 a | 1.36 ± 0.25 b | 1.29 ± 0.39 b |
| Items | Control | AFB1 | AFB1 + Cho | AFB1 + Ato | AFB1 + Tau | AFB1 + Emo |
|---|---|---|---|---|---|---|
| Week 2 | ||||||
| T-SOD (U/mg protein) | 100.41 ± 16.65 a | 77.94 ± 5.65 b | 74.36 ± 6.28 b | 53.85 ± 7.39 c | 84.78 ± 11.26 b | 88.88 ± 19.70 ab |
| MDA (nmol/ mg protein) | 1.90 ± 0.22 b | 2.77 ± 0.60 a | 2.41 ± 0.48 ab | 2.41 ± 0.35 ab | 2.26 ± 0.47 ab | 1.94 ± 0.38 b |
| GSH-Px (U/mg protein) | 57.34 ± 7.14 a | 42.51 ± 7.65 b | 46.00 ± 5.77 b | 42.17 ± 6.08 b | 50.69 ± 7.13 ab | 51.08 ± 9.18 ab |
| GSH (U/mg protein) | 37.33 ± 2.85 a | 24.84 ± 4.09 c | 30.12 ± 4.47 bc | 33.06 ± 9.83 ab | 26.95 ± 3.29 bc | 28.13 ± 4.00 bc |
| Week 4 | ||||||
| T-SOD (U/mg protein) | 119.56 ± 6.06 a | 90.70 ± 12.30 b | 102.51 ± 13.70 ab | 105.33 ± 17.25 ab | 119.47 ± 12.21 a | 98.88 ± 16.05 b |
| MDA (nmol/ mg protein) | 1.70 ± 0.10 b | 2.46 ± 0.48 a | 1.96 ± 0.47 b | 1.73 ± 0.46 b | 1.70 ± 0.21 b | 1.73 ± 0.58 b |
| GSH-Px (U/mg protein) | 69.42 ± 10.79 a | 44.80 ± 5.52 c | 51.30 ± 17.29 bc | 51.06 ± 5.60 bc | 63.10 ± 13.37 ab | 57.34 ± 7.63 abc |
| GSH (U/mg protein) | 39.46 ± 2.03 a | 29.68 ± 5.19 b | 34.76 ± 4.28 ab | 36.52 ± 6.21 a | 36.97 ± 1.98 a | 39.87 ± 5.96 a |
| Items | Control | AFB1 | AFB1 + Cho | AFB1 + Ato | AFB1 + Tau | AFB1 + Emo |
|---|---|---|---|---|---|---|
| Week 2 | ||||||
| AST (U/L) | 33.25 ± 3.41 d | 59.17 ± 7.01 a | 53.58 ± 11.11 ab | 46.22 ± 12.31 bc | 43.37 ± 7.59 c | 32.95 ± 2.52 d |
| ALT (U/L) | 46.05 ± 8.83 | 53.77 ± 5.34 | 46.77 ± 9.49 | 49.95 ± 4.85 | 52.18 ± 9.88 | 43.82 ± 6.41 |
| TP (g/L) | 31.32 ± 2.80 a | 20.37 ± 1.58 c | 23.77 ± 4.86 bc | 21.38 ± 1.73 c | 23.18 ± 2.78 bc | 25.13 ± 1.91 b |
| ALB (g/L) | 9.88 ± 1.30 a | 5.77 ± 1.05 c | 6.27 ± 0.86 bc | 6.13 ± 0.98 bc | 7.35 ± 0.94 b | 6.93 ± 1.17 bc |
| TBA (μmol/L) | 12.65 ± 1.27 c | 20.96 ± 3.64 a | 12.66 ± 1.96 c | 18.64 ± 1.22 ab | 17.77 ± 1.75 b | 16.78 ± 2.95 b |
| TbiL (μmol/L) | 5.83 ± 1.15 b | 7.43 ± 1.09 a | 6.29 ± 1.01 ab | 6.65 ± 0.87 ab | 6.98 ± 1.58 ab | 6.75 ± 1.27 ab |
| Week 4 | ||||||
| AST (U/L) | 33.12 ± 7.31 b | 43.78 ± 8.73 a | 38.48 ± 12.36 ab | 38.90 ± 3.61 ab | 37.85 ± 6.81 ab | 39.07 ± 4.51 ab |
| ALT (U/L) | 35.38 ± 7.74 b | 45.58 ± 8.58 a | 44.23 ± 6.16 ab | 43.35 ± 6.59 ab | 39.45 ± 4.56 ab | 37.10 ± 7.19 ab |
| TP (g/L) | 28.62 ± 1.58 a | 22.98 ± 2.81 c | 22.82 ± 1.61 c | 25.40 ± 2.25 bc | 27.67 ± 2.67 ab | 24.83 ± 2.1 bc |
| ALB (g/L) | 12.70 ± 0.89 a | 9.70 ± 0.98 b | 9.65 ± 0.94 b | 9.82 ± 1.07 b | 11.90 ± 1.68 a | 11.10 ± 2.90 ab |
| TBA (μmol/L) | 18.58 ± 2.04 c | 29.03 ± 6.78 a | 18.22 ± 5.81 c | 21.63 ± 1.99 bc | 24.92 ± 1.60 ab | 22.83 ± 1.27 bc |
| TbiL (μmol/L) | 5.88 ± 1.43 bc | 9.99 ± 0.90 a | 4.64 ± 1.43 c | 6.78 ± 1.61 b | 8.68 ± 0.99 a | 8.59 ± 2.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, A.; Wang, H.; Cheng, Q.; Rajput, S.A.; Qi, D. The Effects of Aflatoxin B1 on Liver Cholestasis and Its Nutritional Regulation in Ducks. Toxins 2024, 16, 239. https://doi.org/10.3390/toxins16060239
Yu A, Wang H, Cheng Q, Rajput SA, Qi D. The Effects of Aflatoxin B1 on Liver Cholestasis and Its Nutritional Regulation in Ducks. Toxins. 2024; 16(6):239. https://doi.org/10.3390/toxins16060239
Chicago/Turabian StyleYu, Aimei, Huanbin Wang, Qianhui Cheng, Shahid Ali Rajput, and Desheng Qi. 2024. "The Effects of Aflatoxin B1 on Liver Cholestasis and Its Nutritional Regulation in Ducks" Toxins 16, no. 6: 239. https://doi.org/10.3390/toxins16060239
APA StyleYu, A., Wang, H., Cheng, Q., Rajput, S. A., & Qi, D. (2024). The Effects of Aflatoxin B1 on Liver Cholestasis and Its Nutritional Regulation in Ducks. Toxins, 16(6), 239. https://doi.org/10.3390/toxins16060239





