Assessment of the Impact of Humic Acids on Intestinal Microbiota, Gut Integrity, Ileum Morphometry, and Cellular Immunity of Turkey Poults Fed an Aflatoxin B1-Contaminated Diet
Abstract
1. Introduction
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Animal Source, Diets, and Experimental Design
5.2. DNA Extraction and 16S rRNA Gene Sequencing
5.3. Bioinformatics and Statistical Analysis
5.4. Ileum Morphometry
5.5. Gut Integrity
5.6. Cellular Immunity
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghaemmaghami, S.S.; Rouhanipour, H.; Sharifi, S.D. Aflatoxin levels in poultry feed: A comparison of mash and pellet forms. Poult. Sci. 2024, 103, 103254. [Google Scholar] [CrossRef] [PubMed]
- Rauber, R.H.; Dilkin, P.; Giacomini, L.Z.; de Almeida, C.A.; Mallmann, C.A. Performance of turkey poults fed different doses of aflatoxins in the diet. Poult. Sci. 2007, 86, 1620–1624. [Google Scholar] [CrossRef]
- Diaz, G.J.; Cortés, A.; Botero, L. Evaluation of the ability of a feed additive to ameliorate the adverse effects of aflatoxins in turkey poults. Br. Poult. Sci. 2009, 50, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Maguey-Gonzalez, J.A.; Michel, M.A.; Baxter, M.F.; Solis-Cruz, B.; Hernandez-Patlan, D.; Merino-Guzman, R.; Hernandez-Velasco, X.; Latorre, J.D.; Hargis, B.M.; Tellez, G.; et al. Effects of humic acids on recovery of Salmonella enterica serovar Enteritidis. Ann. Anim. Sci. 2018, 18, 387–399. [Google Scholar] [CrossRef]
- Chen, X.; Naehrer, K.; Applegate, T.J. Interactive effects of dietary protein concentration and aflatoxin B1 on performance, nutrient digestibility, and gut health in broiler chicks. Poult. Sci. 2016, 95, 1312–1325. [Google Scholar] [CrossRef]
- Saleemi, M.K.; Raza, A.; Khatoon, A.; Zubair, M.; Gul, S.T.; Yongping, X.; Murtaza, B.; Muhammad, F.; Akhtar, B.; Jubeen, F.; et al. Pathological effects of feeding aflatoxin-contaminated feed on immune status and reproductive performance of juvenile white leghorn males and its mitigation with∝-tocopherol and Moringa oleifera. Environ. Sci. Pollut. Res. 2023, 31, 2156–2166. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, X.; Chai, X.; Jiao, Y.; Sun, J.; Wang, S.; Yu, H.; Feng, X. Curcumin mitigates oxidative damage in broiler liver and ileum caused by AFB1-contaminated feed through Nrf2 signaling pathway. Animals 2024, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Nava-Ramírez, M.d.J.; Vázquez-Durán, A.; Figueroa-Cárdenas, J.d.D.; Hernández-Patlán, D.; Solís-Cruz, B.; Téllez-Isaías, G.; López-Coello, C.; Méndez-Albores, A. Removal of Aflatoxin B1 Using Alfalfa Leaves as an Adsorbent Material: A Comparison between Two In Vitro Experimental Models. Toxins 2023, 15, 604. [Google Scholar] [CrossRef] [PubMed]
- Solis-Cruz, B.; Hernandez-Patlan, D.; Petrone, V.M.; Pontin, K.P.; Latorre, J.D.; Beyssac, E.; Hernandez-Velasco, X.; Merino-Guzman, R.; Arreguin, M.A.; Hargis, B.M. Evaluation of a Bacillus-based direct-fed microbial on aflatoxin B1 toxic effects, performance, immunologic status, and serum biochemical parameters in broiler chickens. Avian Dis. 2019, 63, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Mucignat, G.; Bassan, I.; Giantin, M.; Pauletto, M.; Bardhi, A.; Iori, S.; Lopparelli, R.M.; Barbarossa, A.; Zaghini, A.; Novelli, E.; et al. Does Bentonite Cause Cytotoxic and Whole-Transcriptomic Adverse Effects in Enterocytes When Used to Reduce Aflatoxin B1 Exposure? Toxins 2022, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Maguey-Gonzalez, J.A.; Nava-Ramírez, M.D.J.; Gómez-Rosales, S.; Ángeles, M.D.L.; Solís-Cruz, B.; Hernández-Patlán, D.; Merino-Guzmán, R.; Hernández-Velasco, X.; Figueroa-Cárdenas, J.D.D.; Vázquez-Durán, A.; et al. Humic acids preparation, characterization, and their potential adsorption capacity for aflatoxin B1 in an in vitro poultry digestive model. Toxins 2023, 15, 83. [Google Scholar] [CrossRef] [PubMed]
- Maguey-Gonzalez, J.A.; de Jesús Nava-Ramírez, M.; Gómez-Rosales, S.; de Lourdes Ángeles, M.; Solís-Cruz, B.; Hernández-Patlán, D.; Merino-Guzmán, R.; Hernandez-Velasco, X.; Hernández-Ramírez, J.O.; Loeza, I.; et al. Evaluation of the efficacy of humic acids to counteract the toxic effects of aflatoxin B1 in turkey poults. Front. Vet. Sci. 2023, 10, 1276754. [Google Scholar] [CrossRef] [PubMed]
- Taklimi, S.M.S.M.; Ghahri, H.; Isakan, M.A. Influence of different levels of humic acid and esterified glucomannan on growth performance and intestinal morphology of broiler chickens. Agric. Sci. 2012, 3, 663–668. [Google Scholar] [CrossRef]
- López-García, Y.R.; Gómez-Rosales, S.; Angeles, M.D.L.; Jiménez-Severiano, H.; Merino-Guzman, R.; Téllez-Isaias, G. Effect of the addition of humic substances on morphometric analysis and number of goblet cells in the intestinal mucosa of broiler chickens. Animals 2023, 13, 212. [Google Scholar] [CrossRef]
- Maguey-Gonzalez, J.A.; Michel, M.A.; Baxter, M.F.; Tellez, G., Jr.; Moore, P.A., Jr.; Solis-Cruz, B.; Hernández-Patlan, D.; Merino-Guzman, R.; Hernandez-Velasco, X.; Latorre, J.D.; et al. Effect of humic acids on intestinal viscosity, leaky gut and ammonia excretion in a 24 hr feed restriction model to induce intestinal permeability in broiler chickens. Anim. Sci. J. 2018, 89, 1002–1010. [Google Scholar] [CrossRef]
- Li, Y.; Guo, B.; Wu, Z.; Wang, W.; Li, C.; Liu, G.; Cai, H. Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 2020, 10, 1098. [Google Scholar] [CrossRef]
- Aristimunha, P.C.; Mallheiros, R.D.; Ferket, P.R.; Cardinal, K.M.; Moreira Filho, A.L.D.B.; Santos, E.T.; Cavalcante, D.T.; Ribeiro, A.M.L. Effect of dietary organic acids and humic substance supplementation on performance, immune response and gut morphology of broiler chickens. J. Appl. Poult. Res. 2020, 29, 85–94. [Google Scholar] [CrossRef]
- de Lourdes Angeles, M.; Gómez-Rosales, S.; Téllez-Isaias, G. Mechanisms of action of humic substances as growth promoters in animals. In Humus and Humic Substances-Recent Advances; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar] [CrossRef]
- Vigneault, B.; Percot, A.; Lafleur, M.; Campbell, P.G.C. Permeability changes in model and phytoplankton membranes in the presence of aquatic humic substances. Environ. Sci. Technol. 2000, 34, 3907–3913. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Yadav, P.; Ruknuddin, G.; Prajapati, P.K. Research developments in immunomodulatory and antioxidant activities of shilajatu. Indian Drugs 2021, 58, 7–20. [Google Scholar] [CrossRef]
- Huang, J.; Xu, P.; Shao, M.; Wei, B.; Zhang, C.; Zhang, J. Humic acids alleviate dextran sulfate sodium-induced colitis by positively modulating gut microbiota. Front. Microbiol. 2023, 14, 1147110. [Google Scholar] [CrossRef] [PubMed]
- Mudroňová, D.; Karaffová, V.; Pešulová, T.; Koščová, J.; Maruščáková, I.C.; Bartkovský, M.; Marcinčaková, D. The effect of humic substances on gut microbiota and immune response of broilers. Food Agric. Immunol. 2020, 31, 137–149. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Thippareddi, H.; Kim, W.K. Effect of dietary fructooligosaccharide (FOS) supplementation on ileal microbiota in broiler chickens. Poult. Sci. 2018, 97, 3622–3634. [Google Scholar] [CrossRef]
- Wang, D.; Du, Y.; Wang, S.; You, Z.; Liu, Y. Effects of sodium humate and glutamine combined supplementation on growth performance, diarrhea incidence, blood parameters, and intestinal microflora of weaned calves. Anim. Sci. J. 2021, 92, e13584. [Google Scholar] [CrossRef] [PubMed]
- Voth-Gaeddert, L.E.; Torres, O.; Maldonado, J.; Krajmalnik-Brown, R.; Rittmann, B.E.; Oerther, D.B. Aflatoxin exposure, child stunting, and dysbiosis in the intestinal microbiome among children in Guatemala. Environ. Eng. Sci. 2019, 36, 958–968. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Tsim, K.W.K.; Shen, X.; Li, X.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 induces inflammatory liver injury via gut microbiota in mice. J. Agric. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, J.; Cao, Q.; Zhang, C.; Dong, Z.; Feng, D.; Ye, H.; Zuo, J. Dietary catalase supplementation alleviates deoxynivalenol-induced oxidative stress and gut microbiota dysbiosis in broiler chickens. Toxins 2022, 14, 830. [Google Scholar] [CrossRef]
- Liew, W.P.; Sabran, M.R.; Than, L.T.; Abd-Ghani, F.; Sabran, M.R.; Abd-Ghani, F. Metagenomic and proteomic approaches in elucidating aflatoxin B1 detoxification mechanisms of probiotic Lactobacillus casei Shirota towards intestine. Food Chem. Toxicol. 2022, 160, 112808. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Beekmann, K.; Ringø, E.; Rietjens, I.M.; Xing, F. Interaction between food-borne mycotoxins and gut microbiota: A review. Food Control 2021, 126, 107998. [Google Scholar] [CrossRef]
- Chang, F.; He, S.; Dang, C. Assisted selection of biomarkers by linear discriminant analysis effect size (LEfSe) in microbiome data. J. Vis. Exp. 2022, 16, e61715. [Google Scholar] [CrossRef]
- Klaus, B. Effect size estimation and misclassification rate based variable selection in linear discriminant analysis. J. Data Sci. 2013, 11, 537–558. [Google Scholar] [CrossRef]
- Meehan, C.J.; Beiko, R.G. A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 2014, 6, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Zhao, X.; Liu, C.; Wang, B.; Zhou, J. Mechanistic basis and preliminary practice of butyric acid and butyrate sodium to mitigate gut inflammatory diseases: A comprehensive review. Nutr. Res. 2021, 95, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Huertas-Díaz, L.; Kyhnau, R.; Ingribelli, E.; Neuzil-Bunesova, V.; Li, Q.; Sasaki, M.; Lauener, R.P.; Roduit, C.; Frei, R.; Study Group, C.C.; et al. Breastfeeding and the major fermentation metabolite lactate determine occurrence of Peptostreptococcaceae in infant feces. Gut Microbes 2023, 15, 2241209. [Google Scholar] [CrossRef] [PubMed]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Ratajczak, W.; Rył, A.; Mizerski, A.; Walczakiewicz, K.; Sipak, O.; Laszczyńska, M. Immunomodulatory potential of gut microbiome-derived short-chain fatty acids (SCFAs). Acta Biochim. Pol. 2019, 66, 1–12. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.; Fei, W.; Ye, Y.; Zhao, M.; Zheng, C. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Younes, H.; Demigné, C.; Rémésy, C. Acidic fermentation in the caecum increases absorption of calcium and magnesium in the large intestine of the rat. Br. Nutr. 1996, 75, 301–314. [Google Scholar] [CrossRef]
- Bourquin, L.D.; Titgemeyer, E.C.; Garleb, K.A.; Fahey, G.C., Jr. Short-chain fatty acid production and fiber degradation by human colonic bacteria: Effects of substrate and cell wall fractionation procedures. J. Nutr. 1992, 122, 1508–1520. [Google Scholar] [CrossRef]
- Langfeld, L.Q.; Du, K.; Bereswill, S.; Heimesaat, M.M. A review of the antimicrobial and immune-modulatory properties of the gut microbiota-derived short chain fatty acid propionate–What is new? Eur. J. Microbiol. Immunol. 2021, 11, 50–56. [Google Scholar] [CrossRef]
- Hassan, A.A.; Salem, A.Z.M.; Elghandour, M.M.Y.; Hafsa, S.H.A.; Reddy, P.R.K.; Atia, S.E.S.; Vidu, L. Humic substances isolated from clay soil may improve the ruminal fermentation, milk yield, and fatty acid profile: A novel approach in dairy cows. Anim. Feed Sci. Technol. 2020, 268, 114601. [Google Scholar] [CrossRef]
- El-Zaiat, H.M.; Morsy, A.S.; El-Wakeel, E.A.; Answer, M.M.; Sallam, S.M. Impact of humic acid as an organic additive on ruminal fermentation constituents, blood parameters and milk production in goats and their kids growth rate. J. Anim. Feed Sci. 2018, 27, 105–113. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Hassan, A.A.; Sabek, A. Extracted and characterized humic substances as feed supplement in rabbit feeding: Effects on performance, blood metabolites and caecal fermentation activity. Waste Biomass Valorization 2021, 12, 5471–5479. [Google Scholar] [CrossRef]
- Melaku, M.; Zhong, R.; Han, H.; Wan, F.; Yi, B.; Zhang, H. Butyric and citric acids and their salts in poultry nutrition: Effects on gut health and intestinal microbiota. Int. J. Mol. Sci. 2021, 22, 10392. [Google Scholar] [CrossRef]
- Dillon, K.M.; Morrison, H.A.; Powell, C.R.; Carrazzone, R.J.; Ringel-Scaia, V.M.; Winckler, E.W.; Council-Troche, R.M.; Allen, I.C.; Matson, J.B. Targeted delivery of persulfides to the gut: Effects on the microbiome. Angew. Chem. Int. Ed. Engl. 2021, 60, 6061–6067. [Google Scholar] [CrossRef] [PubMed]
- Nair, D.V.T.; Johnson, T.J.; Noll, S.L.; Johny, A.K. Effect of supplementation of a dairy-originated probiotic bacterium, Propionibacterium freudenreichii subsp. freudenreichii, on the cecal microbiome of turkeys challenged with multidrug-resistant Salmonella Heidelberg. Poult. Sci. 2021, 100, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Ricaboni, D.; Mailhe, M.; Khelaifia, S.; Raoult, D.; Million, M. Romboutsia timonensis, a new species isolated from human gut. New Microbes New Infect. 2016, 12, 6–7. [Google Scholar] [CrossRef] [PubMed]
- Wiersema, M.L.; Koester, L.R.; Schmitz-Esser, S.; Koltes, D.A. Comparison of intestinal permeability, morphology, and ileal microbial communities of commercial hens housed in conventional cages and cage-free housing systems. Poult. Sci. 2021, 100, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.F.; Merino-Guzman, R.; Latorre, J.D.; Mahaffey, B.D.; Yang, Y.; Teague, K.D.; Graham, L.E.; Wolfenden, A.D.; Hernandez-Velasco, X.; Bielke, L.R.; et al. Optimizing fluorescein isothiocyanate dextran measurement as a biomarker in a 24-h feed restriction model to induce gut permeability in broiler chickens. Front. Vet. Sci. 2017, 4, 56. [Google Scholar] [CrossRef] [PubMed]
- Zykova, M.V.; Schepetkin, I.A.; Belousov, M.V.; Krivoshchekov, S.V.; Logvinova, L.A.; Bratishko, K.A.; Yusubov, M.S.; Romanenko, S.V.; Quinn, M.T. Physicochemical characterization and antioxidant activity of humic acids isolated from peat of various origins. Molecules 2018, 23, 753. [Google Scholar] [CrossRef]
- Maguey-Gonzalez, J.A.; Gómez-Rosales, S.; de Lourdes Angeles, M.; López-Hernández, L.H.; Rodríguez-Hernández, E.; Solís-Cruz, B.; Hernández-Patlán, D.; Merino-Gúzman, R.; Téllez-Isaías, G. Effects of humic acids on the recovery of different bacterial strains in an in vitro chicken digestive model. Res. Vet. Sci. 2020, 145, 21–28. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Corrêa, R.O.; Vieira, A.; Sernaglia, E.M.; Lancellotti, M.; Vieira, A.T.; Avila-Campos, M.J.; Rodrigues, H.G.; Vinolo, M.A.R. Bacterial short-chain fatty acid metabolites modulate the inflammatory response against infectious bacteria. Cell. Microbiol. 2017, 19, e12720. [Google Scholar] [CrossRef]
- Gilani, S.; Chrystal, P.V.; Barekatain, R. Current experimental models, assessment and dietary modulations of intestinal permeability in broiler chickens. Anim. Nutr. 2021, 7, 801–811. [Google Scholar] [CrossRef]
- Dvorak, H.F. Cutaneous basophil hypersensitivity. J. Allergy Clin. Immunol. 1976, 58, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Amir, A.; McDonald, D.; Navas-Molina, J.A.; Kopylova, E.; Morton, J.T.; Zech Xu, Z.; Kightley, E.P.; Thompson, L.R.; Hyde, E.R.; Gonzalez, A.; et al. Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2017, 2, e00191–e00216. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.N.; Stine, O.C.; Bravo, H.C.; Pop, M. Differential abundance analysis for microbial marker-gene surveys. Nat. Methods 2013, 10, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package, Package version 2.5-5. 2019. Available online: https://github.com/vegandevs/vegan (accessed on 28 November 2023).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/Share: 9.4 User’s Guide, 2nd ed.; Cary, SAS Documentation: Cary, NC, USA, 2002; 288p. [Google Scholar]
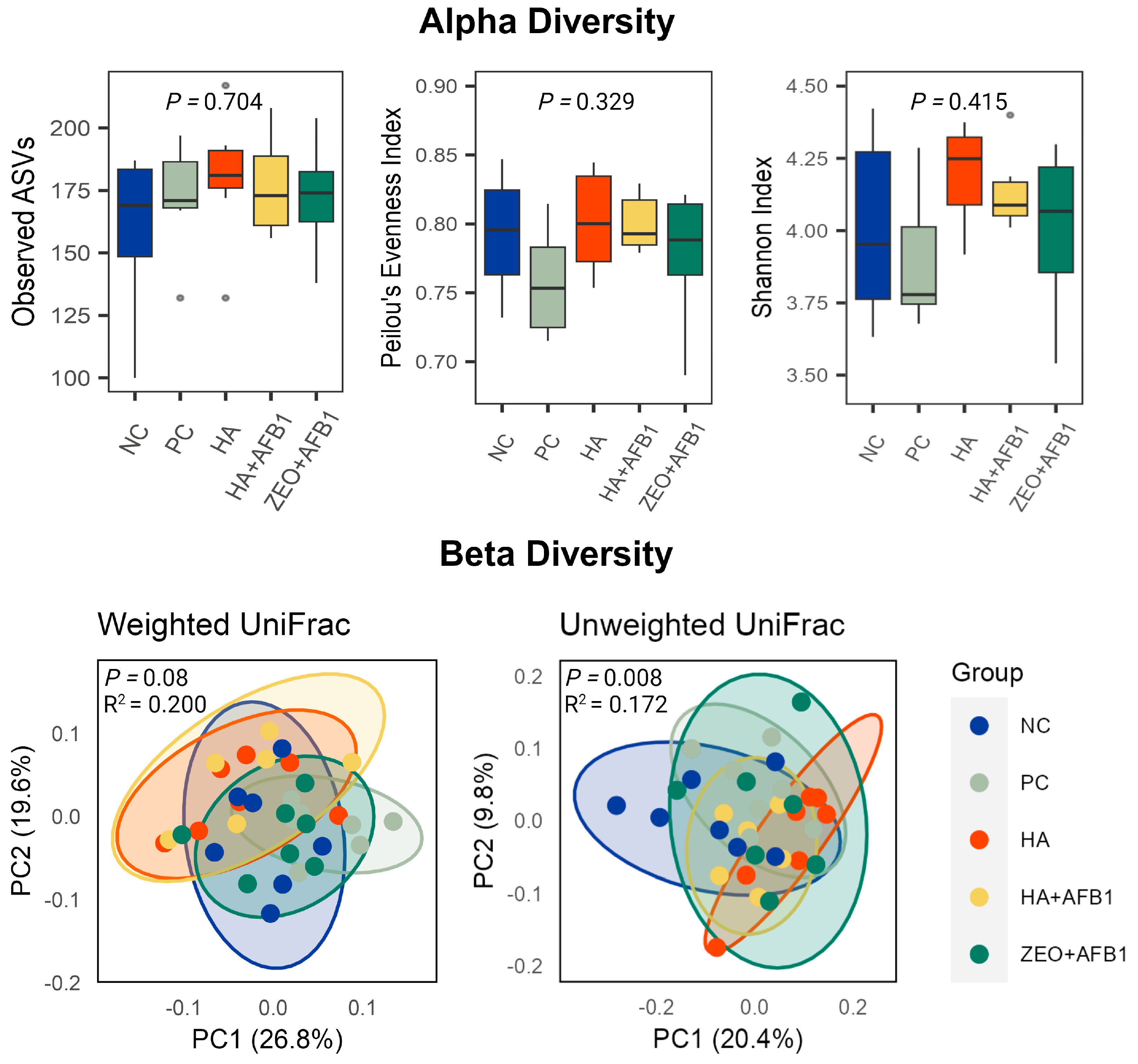
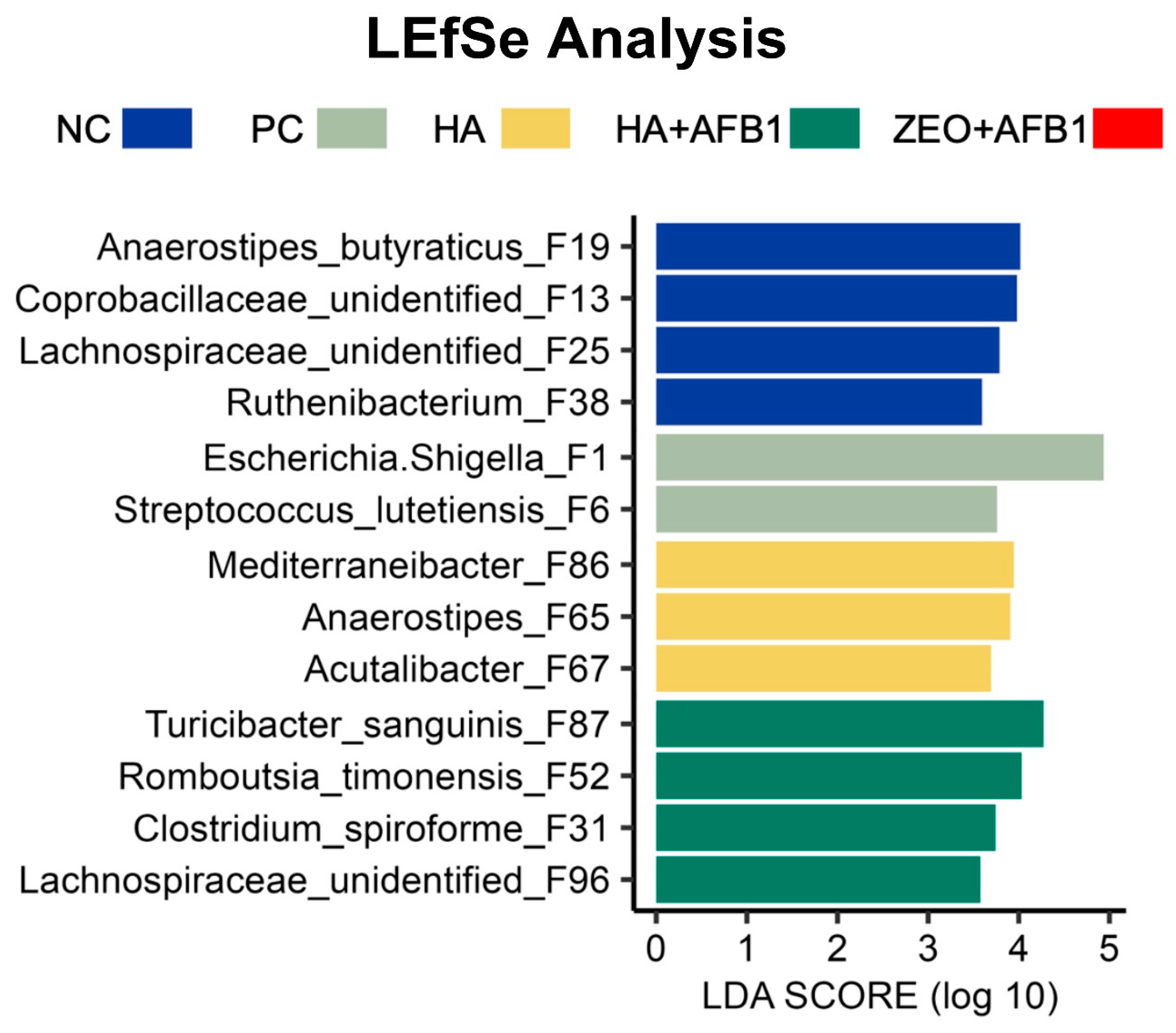
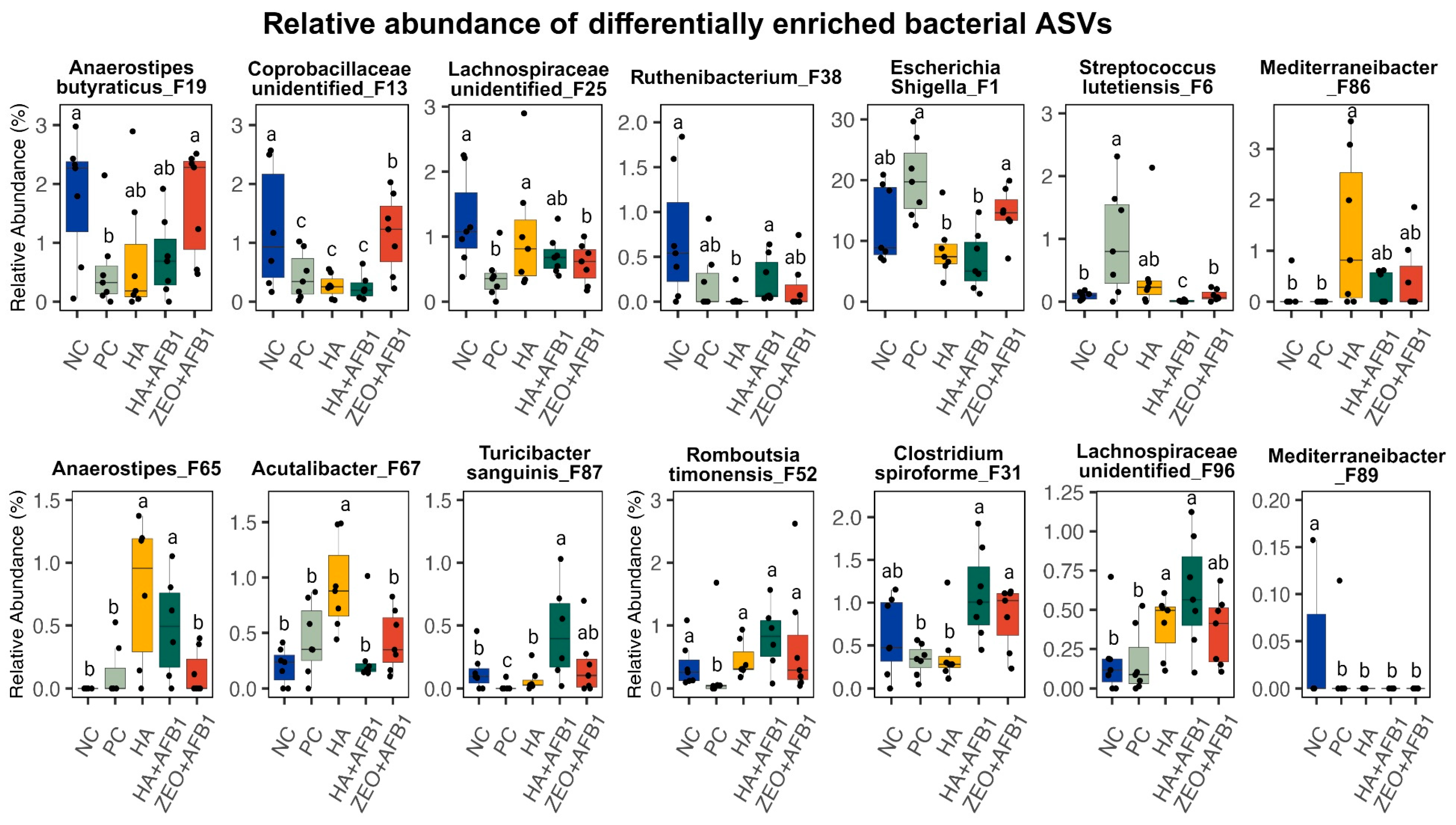
| Taxon | NC | PC | HA | HA + AFB1 | ZEO + AFB1 | SEM * | p-Value |
|---|---|---|---|---|---|---|---|
| Phyla | |||||||
| Firmicutes | 79.40 b | 74.08 b | 86.28 a | 88.5 a | 80.46 b | 2.57 | 0.007 |
| Proteobacteria | 14.11 a | 20.71 a | 8.79 b | 6.92 b | 15.02 a | 2.44 | 0.002 |
| Tenericutes | 3.85 | 2.84 | 1.97 | 2.71 | 2.35 | 0.32 | 0.696 |
| Cyanobacteria | 0.78 | 1.45 | 1.38 | 0.42 | 0.14 | 0.26 | 0.305 |
| Actinobacteria | 0.45 | 0.08 | 0.23 | 0.45 | 0.96 | 0.15 | 0.464 |
| Families | |||||||
| Oscillospiraceae | 32.75 | 32.43 | 32.83 | 35.87 | 35.37 | 0.82 | 0.836 |
| Lachnospiraceae | 32.22 ab | 25.76 b | 40.28 a | 27.42 ab | 32.16 ab | 2.82 | 0.049 |
| Enterobacteriaceae | 14.11a | 20.71 a | 8.79 b | 6.92 b | 15.02 a | 2.73 | 0.002 |
| Clostridiales_unidentified | 4.90 | 6.01 | 4.04 | 5.05 | 4.00 | 0.41 | 0.261 |
| Erysipelotrichaceae | 3.77 | 1.74 | 2.06 | 6.86 | 3.31 | 1.02 | 0.106 |
| Lactobacillaceae | 1.66 | 1.83 | 2.25 | 4.44 | 1.30 | 0.62 | 0.283 |
| Mollicutes_unidentified | 3.04 | 2.56 | 1.63 | 2.49 | 1.43 | 0.34 | 0.572 |
| Bacillaceae | 1.55 | 1.47 | 0.16 | 2.19 | 1.25 | 0.37 | 0.209 |
| Christensenellaceae | 0.76 | 0.80 | 1.35 | 2.65 | 0.83 | 0.40 | 0.492 |
| Peptostreptococcaceae | 0.41 a | 0.29 b | 0.61 a | 2.32 a | 0.71 a | 0.41 | 0.020 |
| Vampirovibrio_unidentified | 0.78 | 1.45 | 1.38 | 0.42 | 0.14 | 0.28 | 0.305 |
| Streptococcaceae | 0.09 | 0.97 | 0.46 | 0.39 | 0.11 | 0.18 | 0.181 |
| Clostridia_unidentified | 0.14 | 0.85 | 0.50 | 0.08 | 0.15 | 0.16 | 0.929 |
| Clostridiaceae 1 | 0.25 | 0.29 | 0.29 | 0.39 | 0.42 | 0.04 | 0.737 |
| Enterococcaceae | 0.67 | 0.14 | 0.09 | 0.16 | 0.44 | 0.12 | 0.072 |
| Taxon | NC | PC | HA | HA + AFB1 | ZEO + AFB1 | SEM * | p-Value |
|---|---|---|---|---|---|---|---|
| Genera | |||||||
| Escherichia/Shigella | 13.16 a | 20.68 a | 8.70 b | 6.92 b | 15.01 a | 2.43 | 0.004 |
| Mediterraneibacter | 10.54 | 7.49 | 19.07 | 10.4 | 13.27 | 1.96 | 0.296 |
| Oscillospiraceae_unidentified | 10.23 | 11.05 | 11.44 | 10.69 | 10.83 | 0.20 | 0.943 |
| Lachnospiraceae_unidentified | 6.24 | 6.18 | 7.47 | 5.44 | 6.90 | 0.35 | 0.285 |
| Subdoligranulum | 8.69 | 4.92 | 2.44 | 7.32 | 4.34 | 1.11 | 0.205 |
| Pseudoflavonifractor | 5.04 | 5.91 | 4.39 | 4.99 | 4.44 | 0.27 | 0.413 |
| Clostridiales_unidentified | 4.90 | 6.01 | 4.04 | 5.05 | 4.00 | 0.37 | 0.261 |
| Enterocloster | 4.64 | 3.53 | 5.37 | 4.16 | 4.31 | 0.30 | 0.693 |
| Faecalibacterium | 0.17 | 0.26 | 4.71 | 3.74 | 5.04 | 1.07 | 0.848 |
| Blautia | 4.25 | 2.67 | 1.80 | 1.65 | 1.83 | 0.49 | 0.276 |
| Anaerostipes | 2.51 a | 0.75 b | 2.01 a | 1.71 a | 1.94 a | 0.29 | 0.023 |
| Eisenbergiella | 1.50 | 1.55 | 1.93 | 1.39 | 1.33 | 0.11 | 0.874 |
| Acutalibacter | 1.12 | 1.33 | 2.11 | 1.17 | 1.42 | 0.18 | 0.087 |
| Lactobacillus | 0.86 | 0.94 | 1.90 | 2.41 | 1.04 | 0.31 | 0.148 |
| Mollicutes_unidentified | 3.04 | 2.56 | 1.63 | 2.49 | 1.43 | 0.30 | 0.572 |
| ASVs | |||||||
| Escherichia/Shigella_F1 | 12.8 a | 20.21 a | 8.55 b | 6.77 b | 14.56 a | 2.37 | 0.004 |
| Mediterraneibacter_F2 | 2.65 | 2.02 | 9.08 | 2.36 | 2.03 | 1.37 | 0.851 |
| Mediterraneibacter_F3 | 2.14 | 3.31 | 1.35 | 2.43 | 4.21 | 0.49 | 0.559 |
| Subdoligranulum_variabile_F4 | 5.10 | 1.88 | 0.83 | 2.18 | 1.34 | 0.75 | 0.815 |
| Enterocloster_F5 | 2.65 | 1.25 | 3.04 | 1.94 | 2.39 | 0.31 | 0.165 |
| Mediterraneibacter_F10 | 0.79 | 0.37 | 4.94 | 2.05 | 0.99 | 0.83 | 0.758 |
| Faecalibacterium_F7 | 0.13 | 0.24 | 3.02 | 2.39 | 3.18 | 0.67 | 0.678 |
| Lactobacillus_crispatus_F12 | 0.86 | 0.93 | 1.90 | 2.37 | 1.03 | 0.30 | 0.148 |
| Mollicutes_unidentified_F9 | 2.77 | 1.19 | 1.33 | 0.30 | 1.14 | 0.40 | 0.167 |
| Bacillus_F8 | 1.55 | 1.47 | 0.16 | 2.19 | 1.25 | 0.33 | 0.209 |
| Enterocloster_F11 | 1.24 | 1.36 | 1.13 | 1.43 | 1.36 | 0.05 | 0.762 |
| Oscillibacter_F15 | 0.92 | 1.24 | 1.96 | 0.43 | 1.55 | 0.26 | 0.077 |
| Pseudoflavonifractor_F14 | 1.10 | 1.57 | 1.23 | 0.76 | 1.26 | 0.13 | 0.376 |
| Blautia_obeum_F18 | 2.93 | 1.68 | 0.40 | 0.33 | 0.53 | 0.50 | 0.240 |
| Anaerostipes_butyraticus_F19 | 1.77 a | 0.56 b | 0.74 ab | 0.75 ab | 1.68 a | 0.26 | 0.044 |
| Pseudoflavonifractor_F20 | 1.35 | 1.14 | 0.64 | 1.27 | 0.95 | 0.13 | 0.666 |
| Subdoligranulum_F26 | 0.50 | 0.07 | 0.23 | 3.37 | 0.97 | 0.61 | 0.161 |
| Acutalibacter_F22 | 0.92 | 0.89 | 1.18 | 0.87 | 0.99 | 0.06 | 0.372 |
| Pseudoflavonifractor_capillosus_F16 | 0.93 | 1.22 | 0.63 | 1.24 | 0.82 | 0.11 | 0.227 |
| Pseudoflavonifractor_F21 | 0.82 | 1.07 | 0.88 | 0.94 | 1.03 | 0.05 | 0.925 |
| Parameter | NC | PC | HA | HA + AFB1 | ZEO + AFB1 | SEM * | p-Value |
|---|---|---|---|---|---|---|---|
| Villi height (μm) | 862.01 a | 389.65 c | 778.48 a | 766.14 a | 510.06 b | 227.71 | <0.0001 |
| Villi width (μm) | 118.94 b | 146.80 ab | 116.85 b | 157.43 a | 125.88 b | 60.85 | 0.02 |
| Total area (μm2) | 103.24 a | 44.16 c | 74.07 b | 101.39 a | 57.12 bc | 36.69 | <0.0001 |
| FITC-d (ng/mL) | 263.3 b | 858.2 a | 182.7 b | 272.1 b | 410 b | 669.37 | 0.01 |
| CBH (mm) | 0.37 c | 0.50 bc | 0.68 ab | 0.76 a | 0.7 a | 0.23 | 0.0003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maguey-González, J.A.; Liu, J.; Zhang, G.; Latorre, J.D.; Hernández-Ramírez, J.O.; de Jesús Nava-Ramírez, M.; Senas-Cuesta, R.; Gómez-Rosales, S.; de Lourdes Ángeles, M.; Stein, A.; et al. Assessment of the Impact of Humic Acids on Intestinal Microbiota, Gut Integrity, Ileum Morphometry, and Cellular Immunity of Turkey Poults Fed an Aflatoxin B1-Contaminated Diet. Toxins 2024, 16, 122. https://doi.org/10.3390/toxins16030122
Maguey-González JA, Liu J, Zhang G, Latorre JD, Hernández-Ramírez JO, de Jesús Nava-Ramírez M, Senas-Cuesta R, Gómez-Rosales S, de Lourdes Ángeles M, Stein A, et al. Assessment of the Impact of Humic Acids on Intestinal Microbiota, Gut Integrity, Ileum Morphometry, and Cellular Immunity of Turkey Poults Fed an Aflatoxin B1-Contaminated Diet. Toxins. 2024; 16(3):122. https://doi.org/10.3390/toxins16030122
Chicago/Turabian StyleMaguey-González, Jesús A., Jing Liu, Guolong Zhang, Juan D. Latorre, Juan O. Hernández-Ramírez, María de Jesús Nava-Ramírez, Roberto Senas-Cuesta, Sergio Gómez-Rosales, María de Lourdes Ángeles, Andressa Stein, and et al. 2024. "Assessment of the Impact of Humic Acids on Intestinal Microbiota, Gut Integrity, Ileum Morphometry, and Cellular Immunity of Turkey Poults Fed an Aflatoxin B1-Contaminated Diet" Toxins 16, no. 3: 122. https://doi.org/10.3390/toxins16030122
APA StyleMaguey-González, J. A., Liu, J., Zhang, G., Latorre, J. D., Hernández-Ramírez, J. O., de Jesús Nava-Ramírez, M., Senas-Cuesta, R., Gómez-Rosales, S., de Lourdes Ángeles, M., Stein, A., Solís-Cruz, B., Hernández-Patlán, D., Merino-Guzmán, R., Hernandez-Velasco, X., Castellanos-Huerta, I., Uribe-Diaz, S., Vázquez-Durán, A., Méndez-Albores, A., Petrone-Garcia, V. M., ... Téllez-Isaías, G. (2024). Assessment of the Impact of Humic Acids on Intestinal Microbiota, Gut Integrity, Ileum Morphometry, and Cellular Immunity of Turkey Poults Fed an Aflatoxin B1-Contaminated Diet. Toxins, 16(3), 122. https://doi.org/10.3390/toxins16030122









