Development of a Novel Magnetic-Bead-Based Automated Strategy for Efficient and Low-Cost Sample Preparation for Ochratoxin A Detection Using Mycotoxin–Albumin Interaction
Abstract
1. Introduction
2. Results and Discussion
2.1. Adsorption Ability of the Three Types of Albumin–Magnetic Beads
2.2. Optimization of the Coupling Amount of HSA–Magnetic Beads
2.3. The Principle of This Automated System
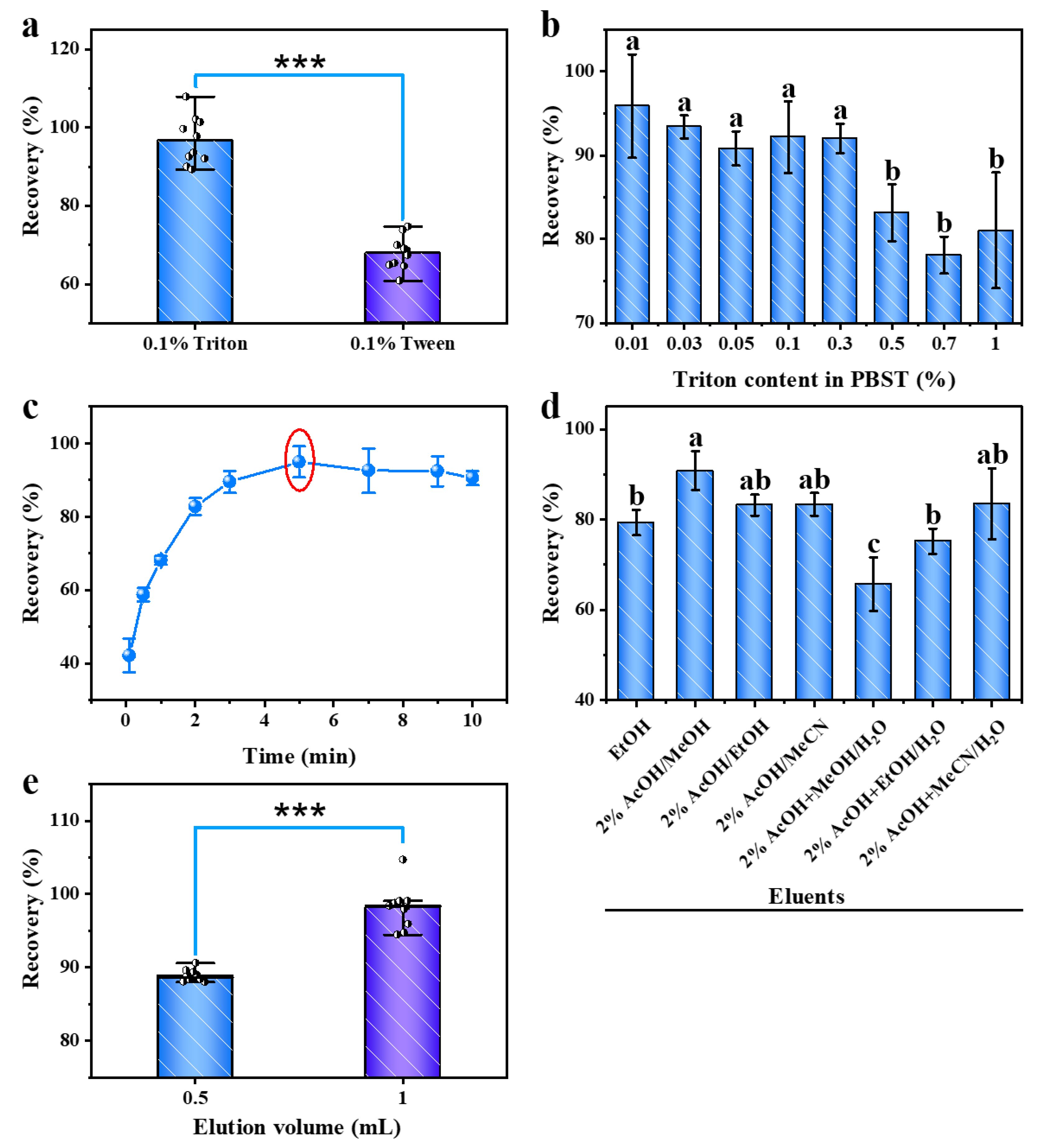
2.4. Optimization of the Sample Clean-Up Method
2.5. Analytical Performance
2.6. Detection of Actual Samples
3. Conclusions
4. Materials and Methods
4.1. Experimental Materials
4.2. Instrument and Analytical Conditions
4.3. Coupling of Magnetic Beads to Albumin
4.4. Identification Ability of Magnetic Beads
4.5. Optimization of Conditions
4.5.1. Optimization of the Protein/Magnetic Bead Coupling Ratio and Loading Volume
4.5.2. Optimization of the Elution Solvent and Volume
4.5.3. Optimization of the Sample Extract
4.5.4. Optimization of the Capture Time
4.5.5. Optimization of the Beer Loading Volume
4.5.6. Optimization of the Loading Volume of Red Wine Samples
4.6. Sample Treatment
4.7. Investigation of the Detection Performance
4.8. Manual IAC Clean-Up Procedure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duarte, S.; Pena, A.; Lino, C. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Z.; Jiang, X.; Fang, W.; Song, H.; Zhang, X. Rapid and sensitive detection of ochratoxin A by using a quantum dots-based immunochromatographic assay. Mycosystema 2019, 38, 1003–1013. [Google Scholar]
- Ringot, D.; Chango, A.; Schneider, Y.-J.; Larondelle, Y. Toxicokinetics and toxicodynamics of ochratoxin A, an update. Chem.-Biol. Interact. 2006, 159, 18–46. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Wei, M.; He, X.; Xie, Y. A novel signal-on fluorescent aptasensor for ochratoxin A detection based on RecJ f exonuclease-induced signal amplification. J. Chin. Chem. Soc. Taipei 2020, 67, 1247–1253. [Google Scholar] [CrossRef]
- Li, X.; Ma, W.; Ma, Z.; Zhang, Q.; Li, H. The occurrence and contamination level of ochratoxin A in plant and animal-derived food commodities. Molecules 2021, 26, 6928. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.B.; Fernandez-Franzon, M.; Ruiz, M.-J.; Juan-Garcia, A. Presence of ochratoxin A (OTA) mycotoxin in alcoholic drinks from southern European countries: Wine and beer. J. Agric. Food Chem. 2014, 62, 7643–7651. [Google Scholar] [CrossRef]
- Schabo, D.C.; Alvarenga, V.O.; Schaffner, D.W.; Magnani, M. A worldwide systematic review, meta-analysis, and health risk assessment study of mycotoxins in beers. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5742–5764. [Google Scholar] [CrossRef] [PubMed]
- Leal, T.; Abrunhosa, L.; Domingues, L.; Venâncio, A.; Oliveira, C. BSA-based sample clean-up columns for ochratoxin A determination in wine: Method development and validation. Food Chem. 2019, 300, 125204. [Google Scholar] [CrossRef] [PubMed]
- The Commission of the European Communities. Commission Regulation (EC) No.1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:364:0005:0024:EN:PDF (accessed on 21 March 2023).
- Dimzoska, B.S.; Musliu, Z.H.; Uzunov, R.; Angeleska, A.; Blagoevska, K.; Nikolovska, R.C.; Ilievska, G.; Stojkovic, E.D. Application of reliable cost-effective strategy for analysis of mycotoxins in corn-based foods with HPLC-FLD after multi-toxin immunoaffinity clean-up. Maced. J. Chem. Chem. Eng. 2022, 41, 77–88. [Google Scholar]
- Afsah-Hejri, L.; Jinap, S.; Mirhosseini, H. Ochratoxin A quantification: Newly developed HPLC conditions. Food Control. 2012, 23, 113–119. [Google Scholar] [CrossRef]
- Mikulíková, R.; Běláková, S.; Benešová, K.; Svoboda, Z. Study of ochratoxin A content in South Moravian and foreign wines by the UPLC method with fluorescence detection. Food Chem. 2012, 133, 55–59. [Google Scholar] [CrossRef]
- Gentile, F.; La Torre, G.L.; Potortì, A.G.; Saitta, M.; Alfa, M.; Dugo, G. Organic wine safety: UPLC-FLD determination of Ochratoxin A in Southern Italy wines from organic farming and winemaking. Food Control. 2016, 59, 20–26. [Google Scholar] [CrossRef]
- Ye, J.; Xuan, Z.; Zhang, B.; Wu, Y.; Li, L.; Wang, S.; Xie, G.; Wang, S. Automated analysis of ochratoxin A in cereals and oil by immunoaffinity magnetic beads coupled to UPLC-FLD. Food Control. 2019, 104, 57–62. [Google Scholar] [CrossRef]
- Rubert, J.; Soler, C.; Mañes, J. Evaluation of matrix solid-phase dispersion (MSPD) extraction for multi-mycotoxin determination in different flours using LC–MS/MS. Talanta 2011, 85, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.N.; Lai, E.P.; Miller, J.D. Analysis of wheat extracts for ochratoxin A by molecularly imprinted solid-phase extraction and pulsed elution. Anal. Bioanal. Chem. 2004, 378, 1903–1906. [Google Scholar] [CrossRef]
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; García-Campaña, A.M.; Gámiz-Gracia, L. Solid phase extraction as sample treatment for the determination of Ochratoxin A in foods: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3405–3420. [Google Scholar] [CrossRef]
- Belli, N.; Marin, S.; Sanchis, V.; Ramos, A. Ochratoxin A (OTA) in wines, musts and grape juices: Occurrence, regulations and methods of analysis. Food Sci. Technol. Int. 2002, 8, 325–335. [Google Scholar] [CrossRef]
- Garcia-Villanova, R.J.; Cordón, C.; Paramás, A.M.G.; Aparicio, P.; Rosales, M.E.G. Simultaneous immunoaffinity column cleanup and HPLC analysis of aflatoxins and ochratoxin A in Spanish bee pollen. J. Agric. Food Chem. 2004, 52, 7235–7239. [Google Scholar] [CrossRef]
- Trucksess, M.; Weaver, C.; Oles, C.; D’Ovidio, K.; Rader, J. Determination of aflatoxins and ochratoxin A in ginseng and other botanical roots by immunoaffinity column cleanup and liquid chromatography with fluorescence detection. J. AOAC Int. 2006, 89, 624–630. [Google Scholar] [CrossRef]
- Karami-Osboo, R.; Miri, R.; Javidnia, K.; Kobarfard, F.; AliAbadi, M.H.S.; Maham, M. A validated dispersive liquid-liquid microextraction method for extraction of ochratoxin A from raisin samples. J. Food Sci. Technol. 2015, 52, 2440–2445. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Gámiz-Gracia, L.; García-Campaña, A.M. Determination of ochratoxin A in wines by capillary liquid chromatography with laser induced fluorescence detection using dispersive liquid–liquid microextraction. Food Chem. 2012, 135, 368–372. [Google Scholar] [CrossRef]
- Paíga, P.; Morais, S.; Oliva-Teles, T.; Correia, M.; Delerue-Matos, C.; Duarte, S.C.; Pena, A.; Lino, C.M. Extraction of ochratoxin A in bread samples by the QuEChERS methodology. Food Chem. 2012, 135, 2522–2528. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Mañes, J.; Font, G.; Juan-García, A. Determination of mycotoxins in fruit berry by-products using QuEChERS extraction method. Lwt 2017, 86, 344–351. [Google Scholar] [CrossRef]
- Rubert, J.; Soler, C.; Mañes, J. Optimization of Matrix Solid-Phase Dispersion method for simultaneous extraction of aflatoxins and OTA in cereals and its application to commercial samples. Talanta 2010, 82, 567–574. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.-H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Guo, J.-X.; Pan, L.-M.; Wang, M.-C.; Chen, L.-J.; Zhao, X. Exogenous Interference and Autofluorescence-free Ratiometric Aptasensor for Detection of OTA Based on Dual-Colored Persistent Luminescence Nanoparticles. Food Chem. 2023, 413, 135611. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Huang, T.; Zhou, Q.; Yang, Z.; Liu, B.; Li, M.; Li, C.; Chen, J.-X.; Dai, Z.; Chen, J. A label-free fluorescent aptasensor based on a novel exponential rolling circle amplification for highly sensitive Ochratoxin A detection. Food Chem. 2023, 410, 135427. [Google Scholar] [CrossRef]
- Zheng, M.; Liu, H.; Ye, J.; Ni, B.; Xie, Y.; Wang, S. Target-responsive aptamer-cross-linked hydrogel sensors for the visual quantitative detection of aflatoxin B1 using exonuclease I-Triggered target cyclic amplification. Food Chem. X 2022, 15, 100395. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Liu, Z. A boronate affinity sandwich assay: An appealing alternative to immunoassays for the determination of glycoproteins. Angew. Chem. Int. Ed. 2014, 53, 10386–10389. [Google Scholar] [CrossRef]
- Hu, M.; Huang, P.; Suo, L.; Wu, F. Polydopamine-based molecularly imprinting polymers on magnetic nanoparticles for recognition and enrichment of ochratoxins prior to their determination by HPLC. Microchim. Acta 2018, 185, 300. [Google Scholar] [CrossRef]
- Pichon, V.; Combès, A. Selective tools for the solid-phase extraction of Ochratoxin A from various complex samples: Immunosorbents, oligosorbents, and molecularly imprinted polymers. Anal. Bioanal. Chem. 2016, 408, 6983–6999. [Google Scholar] [CrossRef]
- Poór, M.; Kunsági-Máté, S.; Bencsik, T.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Flavonoid aglycones can compete with Ochratoxin A for human serum albumin: A new possible mode of action. Int. J. Biol. Macromol. 2012, 51, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Poór, M.; Kunsági-Máté, S.; Czibulya, Z.; Li, Y.; Peles-Lemli, B.; Petrik, J.; Vladimir-Knežević, S.; Kőszegi, T. Fluorescence spectroscopic investigation of competitive interactions between ochratoxin A and 13 drug molecules for binding to human serum albumin. Luminescence 2013, 28, 726–733. [Google Scholar] [CrossRef]
- Poór, M.; Li, Y.; Matisz, G.; Kiss, L.; Kunsági-Máté, S.; Kőszegi, T. Quantitation of species differences in albumin–ligand interactions for bovine, human and rat serum albumins using fluorescence spectroscopy: A test case with some Sudlow’s site I ligands. J. Lumin. 2014, 145, 767–773. [Google Scholar] [CrossRef]
- Il’ichev, Y.V.; Perry, J.L.; Rüker, F.; Dockal, M.; Simon, J.D. Interaction of ochratoxin A with human serum albumin. Binding sites localized by competitive interactions with the native protein and its recombinant fragments. Chem.-Biol. Interact. 2002, 141, 275–293. [Google Scholar] [CrossRef]
- Chu, F.S. A comparative study of the interaction of ochratoxins with bovine serum albumin. Biochem. Pharmacol. 1974, 23, 1105–1113. [Google Scholar] [PubMed]
- Xuan, Z.; Liu, H.; Ye, J.; Li, L.; Tian, W.; Wang, S. Reliable and disposable quantum dot–based electrochemical immunosensor for aflatoxin B 1 simplified analysis with automated magneto-controlled pretreatment system. Anal. Bioanal. Chem. 2020, 412, 7615–7625. [Google Scholar] [CrossRef]
- Chen, J.; Ye, J.; Li, L.; Wu, Y.; Liu, H.; Xuan, Z.; Chen, M.; Wang, S. One-step automatic sample pretreatment for rapid, simple, sensitive, and efficient determination of aflatoxin M1 in milk by immunomagnetic beads coupled to liquid chromatography-tandem mass spectrometry. Food Control 2022, 137, 108927. [Google Scholar] [CrossRef]
- Sarwat, A.; Rauf, W.; Majeed, S.; De Boevre, M.; De Saeger, S.; Iqbal, M. LC-MS/MS based appraisal of multi-mycotoxin co-occurrence in poultry feeds from different regions of Punjab, Pakistan. Food Addit. Contam. Part B 2022, 15, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Ye, J.; Zhang, B.; Li, L.; Wu, Y.; Wang, S. An automated and high-throughput immunoaffinity magnetic bead-based sample clean-up platform for the determination of aflatoxins in grains and oils using UPLC-FLD. Toxins 2019, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- ISO 15141, I.; Cereals and Cereal Products-Determination of Ochratoxin A-High Performance Liquid Chromatographic Method with Immunoaffinity Column Cleanup and Fluorescence Detection. ISO International Standard: Geneva, Switzerland, 2018; Volume 5.



| Toxins | Spiking Concentration (µg/L) | Recovery (%) | RSD (%) |
|---|---|---|---|
| DON | 30.00 | ND | ND |
| 15-AcDON | 4.00 | ND | ND |
| 3-AcDON | 8.00 | ND | ND |
| 3G-DON | 5.00 | ND | ND |
| AFB1 | 0.20 | ND | ND |
| AFB2 | 0.20 | ND | ND |
| AFG1 | 0.20 | ND | ND |
| AFG2 | 0.20 | ND | ND |
| HT-2 | 2.00 | ND | ND |
| NIV | 40.00 | ND | ND |
| ST | 0.20 | 1.1 | 0.2 |
| T-2 | 0.16 | ND | ND |
| ZEN | 4.00 | ND | ND |
| OTA | 1.00 | 111.8 | 0.04 |
| Spiked Level (µg/L) | Beer | Wine | ||
|---|---|---|---|---|
| Average Recovery (%) | RSD (%) | Average Recovery (%) | RSD (%) | |
| 1 | 101.4 | 8.2 | 102.1 | 2.9 |
| 2 | 91.2 | 2.9 | 96.4 | 2.2 |
| 4 | 97.0 | 1.2 | 100.4 | 2.7 |
| Brand | Concentrations (µg/L) | |
|---|---|---|
| This Method | Reference IAC Method | |
| RIO 3° Cocktail White Peach Brandy Beer | ND | ND |
| Qingyi Pineapple Beer | ND | ND |
| Corona Beer | ND | ND |
| Brave Rudolf Rock Beer | 0.16 | 0.16 |
| Kaiser Yellow Beer | ND | ND |
| Tsingtao Augerta Beer | ND | ND |
| Kingway King Of Golden Wheat Beer | ND | ND |
| Qingdao Bingchun Beer | ND | ND |
| Tsingtao Laoshan Beer | ND | ND |
| Barreker Wheat Beer | ND | ND |
| Qingyi Wheat King Beer | ND | ND |
| Tsingtao Whole Wheat Whitebeer | ND | ND |
| Hoegaarden Whitebeer | ND | ND |
| Hoegaarden Amber Orange Beer | ND | ND |
| Budweiser Blackgold Beer | ND | ND |
| Budweiser Supreme Beer | ND | ND |
| Yanjing U8 Beer | ND | ND |
| Beijing Yanjing Brewery | ND | ND |
| Yanjing Boutique Beer | 0.26 | 0.20 |
| Weijixiong Craft Beer | ND | ND |
| Harbin Beer Chunshuang Pale Lager | 0.17 | 0.26 |
| Hoegaarden Rosee Beer | ND | ND |
| Fort Shengfei Reserve Dry Red Wine | 1.25 | 1.35 |
| Changbai Mountain Ice-Red Wine | ND | ND |
| Home-made Red wine (1) | 7.89 | 7.56 |
| Home-made Red wine (2) | 3.26 | 3.17 |
| Step | Well | Mixing Time/min | Mixing Frequency/Hz | Volume/mL |
|---|---|---|---|---|
| Transfer | 2 | 1.0 | 6.5 | 0.8 |
| Reaction | 1 | 5.0 | 1.5 | 10.0 |
| Wash 1 | 3 | 1.0 | 6.5 | 1.0 |
| Wash 2 | 4 | 1.0 | 6.5 | 1.0 |
| Elution/Collection | 5 | 1.0 | 6.5 | 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Bao, H.; Zheng, M.; Liu, H.; Chen, J.; Wang, S.; Ma, H.; Zhang, Y. Development of a Novel Magnetic-Bead-Based Automated Strategy for Efficient and Low-Cost Sample Preparation for Ochratoxin A Detection Using Mycotoxin–Albumin Interaction. Toxins 2023, 15, 270. https://doi.org/10.3390/toxins15040270
Ye J, Bao H, Zheng M, Liu H, Chen J, Wang S, Ma H, Zhang Y. Development of a Novel Magnetic-Bead-Based Automated Strategy for Efficient and Low-Cost Sample Preparation for Ochratoxin A Detection Using Mycotoxin–Albumin Interaction. Toxins. 2023; 15(4):270. https://doi.org/10.3390/toxins15040270
Chicago/Turabian StyleYe, Jin, Hui Bao, Mengyao Zheng, Hongmei Liu, Jinnan Chen, Songxue Wang, Haihua Ma, and Yuan Zhang. 2023. "Development of a Novel Magnetic-Bead-Based Automated Strategy for Efficient and Low-Cost Sample Preparation for Ochratoxin A Detection Using Mycotoxin–Albumin Interaction" Toxins 15, no. 4: 270. https://doi.org/10.3390/toxins15040270
APA StyleYe, J., Bao, H., Zheng, M., Liu, H., Chen, J., Wang, S., Ma, H., & Zhang, Y. (2023). Development of a Novel Magnetic-Bead-Based Automated Strategy for Efficient and Low-Cost Sample Preparation for Ochratoxin A Detection Using Mycotoxin–Albumin Interaction. Toxins, 15(4), 270. https://doi.org/10.3390/toxins15040270





