Chimeric Peptides from Californiconus californicus and Heterodontus francisci with Antigen-Binding Capacity: A Conotoxin Scaffold to Create Non-Natural Antibodies (NoNaBodies)
Abstract
1. Introduction
2. Results
2.1. In-Silico Analysis (Homology Modeling, Molecular Dynamics, and Protein–Protein Docking)
2.1.1. NoNaBodies Models
2.1.2. Docking of VEGF165
2.1.3. Docking of TGF-β
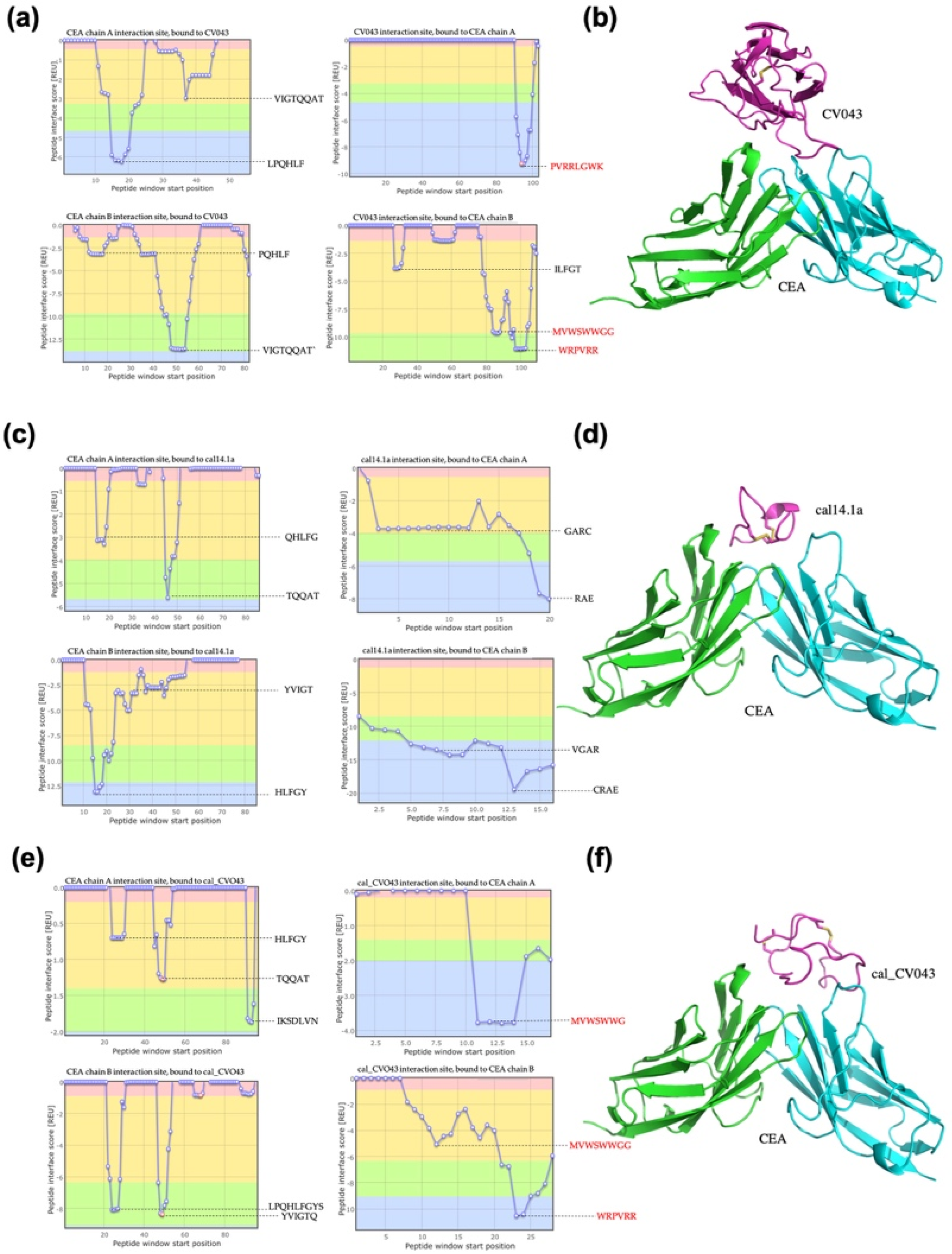
2.1.4. Docking of CEA
2.1.5. Docking of Other Pathological Targets
2.2. In-Vitro Analysis
2.2.1. Activity Evaluation of the Scaffold cal14.1a
2.2.2. VEGF165
VEGF165 Recognition Using ELISA
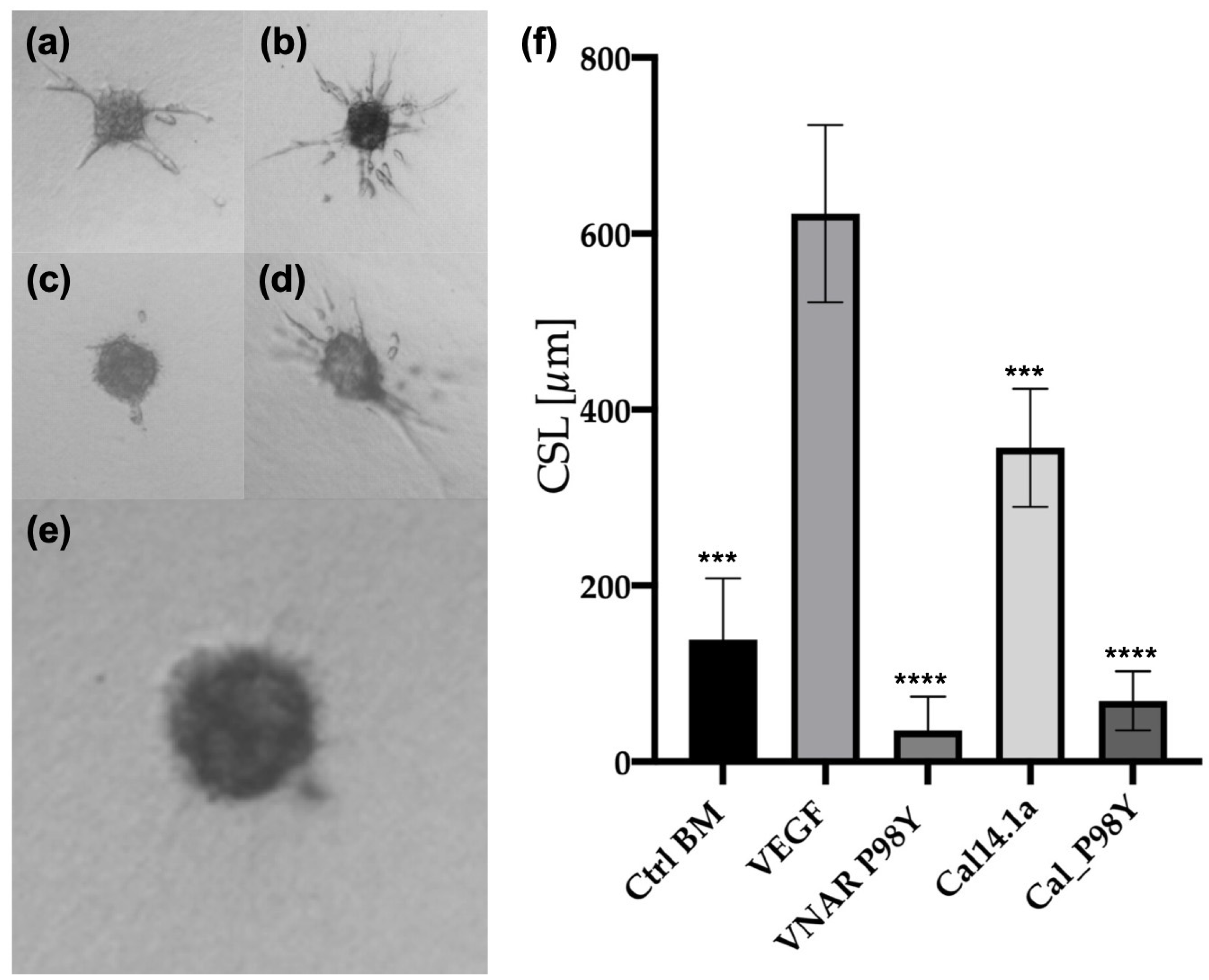
Three-Dimensional In Vitro Angiogenesis Assay Based on Endothelial Cell Spheroids
2.2.3. TGF-β
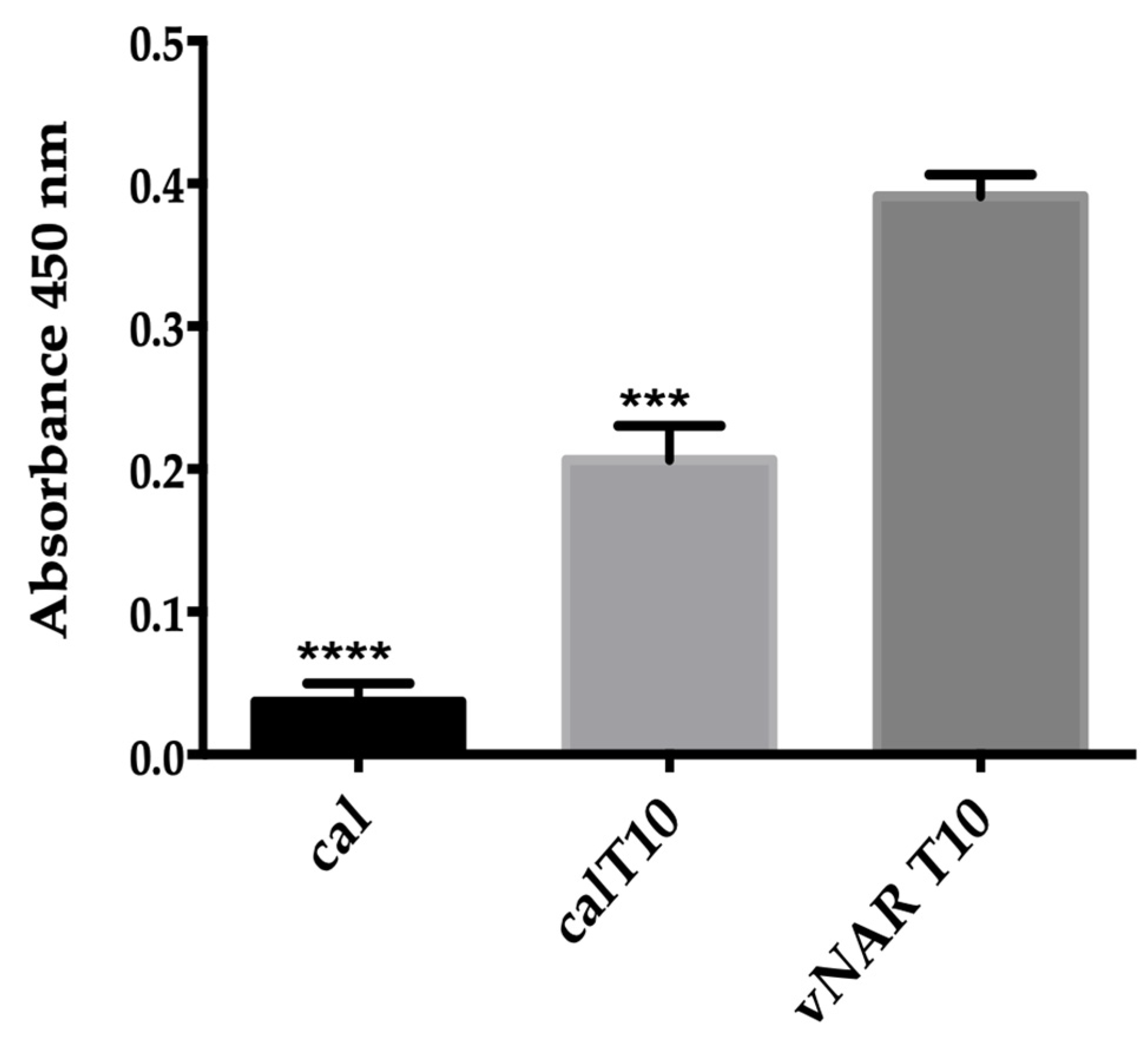
TGF-β Recognition Using ELISA
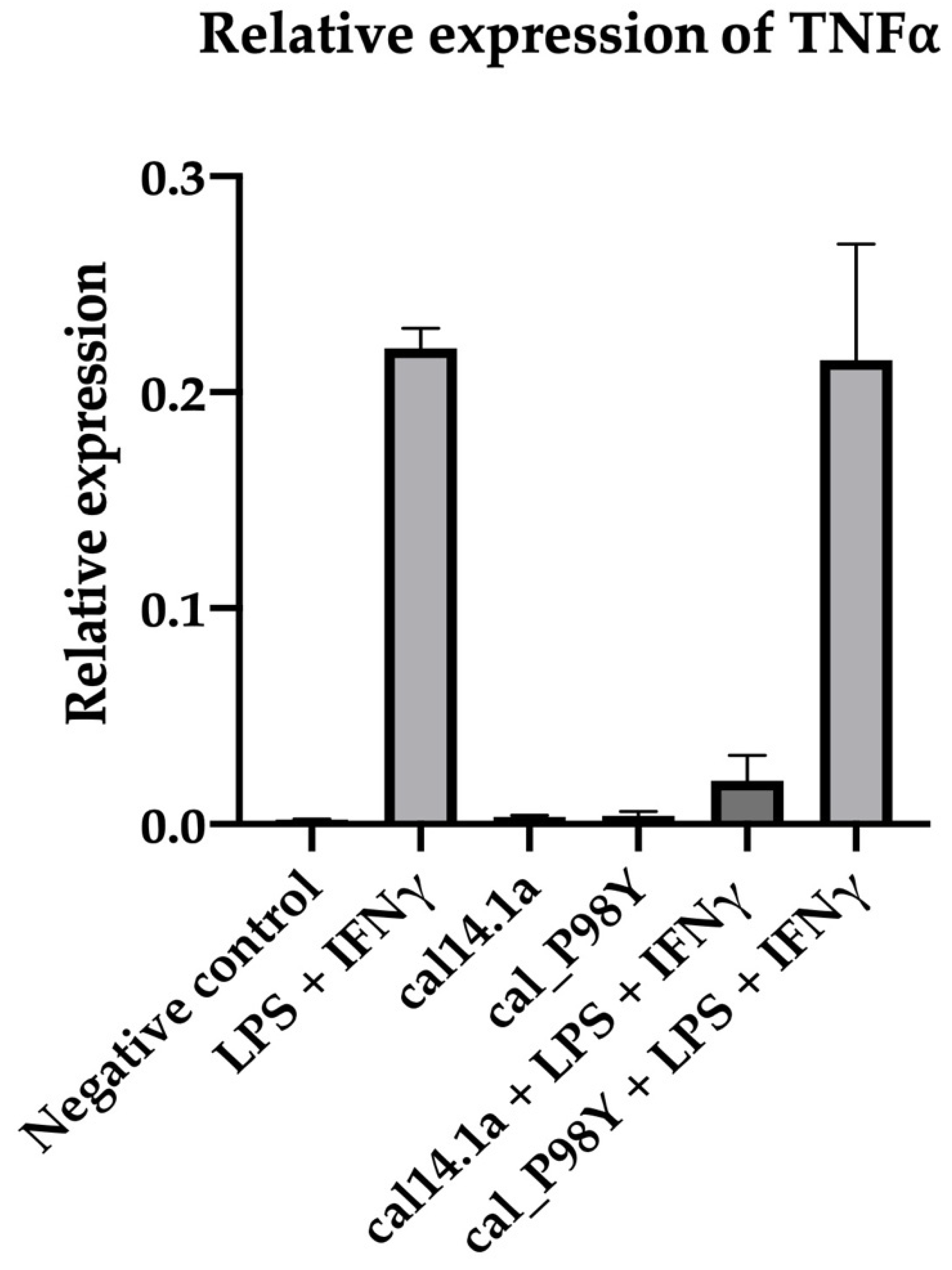
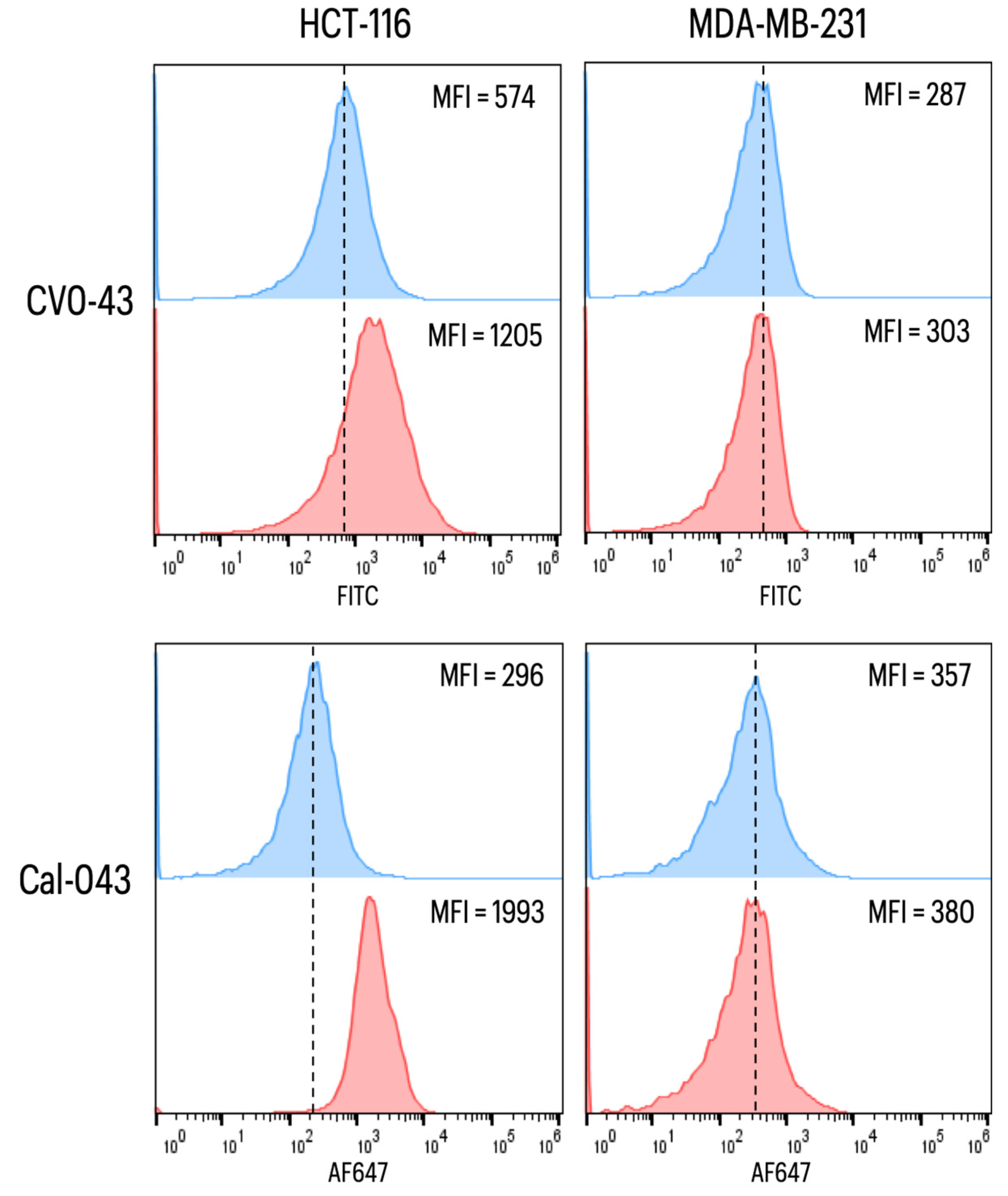
2.2.4. CEA
CEA Recognition Using ELISA
CEA Labeling on the Surface of Cancer Cells
3. Discussion
4. Materials and Methods
4.1. In Silico Analysis
4.1.1. NoNaBody Design
4.1.2. Homology Modeling
4.1.3. Molecular Dynamics and Simulated Annealing
4.1.4. Protein–Protein Docking
4.2. In Vitro Analysis
4.2.1. Protein Synthesis
4.2.2. Activity Evaluation of the Scaffold cal14.1a
4.2.3. VEGF165
VEGF165 Recognition Using ELISA Assay
Three-Dimensional In Vitro Angiogenesis Assay and Endothelial Cell Spheroid Model
Statistical Analysis
4.2.4. TGF-β
TGF-β Recognition Using ELISA Assay
4.2.5. CEA
CEA Recognition Using ELISA Assay
CEA Labeling on the Surface of Cancer Cells
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saerens, D.; Ghassabeh, G.H.; Muyldermans, S. Single-domain antibodies as building blocks for novel therapeutics. Curr. Opin. Pharmacol. 2008, 8, 600–608. [Google Scholar] [CrossRef]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef]
- Holliger, P.; Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 2005, 23, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Kolmar, H.; Christmann, A. The therapeutic potential of cystine-knot microprotein. Innov. Pharm. Technol. 2008, 26, 34–36. [Google Scholar]
- Moore, S.J.; Leung, C.L.; Cochran, J.R. Knottins: Disulfide-bonded therapeutic and diagnostic peptides. Drug Discov. Today Technol. 2012, 9, e3–e11. [Google Scholar] [CrossRef]
- Ackerman, S.E.; Currier, N.V.; Bergen, J.M.; Cochran, J.R. Cystine-knot peptides: Emerging tools for cancer imaging and therapy. Expert Rev. Proteom. 2014, 11, 561–572. [Google Scholar] [CrossRef]
- Gracy, J.; Chiche, L. Optimizing structural modeling for a specific protein scaffold: Knottins or inhibitor cystine knots. BMC BioInform. 2010, 11, 535. [Google Scholar] [CrossRef] [PubMed]
- Northfield, S.E.; Wang, C.K.; Schroeder, C.I.; Durek, T.; Kan, M.-W.; Swedberg, J.E.; Craik, D.J. Disulfide-rich macrocyclic peptides as templates in drug design. Eur. J. Med. Chem. 2014, 77, 248–257. [Google Scholar] [CrossRef]
- Daly, N.L.; Craik, D.J. Bioactive cystine knot proteins. Curr. Opin. Chem. Biol. 2011, 15, 362–368. [Google Scholar] [CrossRef]
- Karatt, E.; McCafferty, V.A.; Surade, J.; Luetkens, S.; Masters, T. Binding Members with Altered Diversity Scaffold Domains. EP3786292A1, 9 January 2017. [Google Scholar]
- Barbas, C.F.; Languino, L.R.; Smith, J.W. High-affinity self-reactive human antibodies by design and selection: Targeting the integrin ligand binding site. Proc. Natl. Acad. Sci. USA 1993, 90, 10003–10007. [Google Scholar] [CrossRef]
- Frederickson, S.; Renshaw, M.W.; Lin, B.; Smith, L.M.; Calveley, P.; Springhorn, J.P.; Johnson, K.; Wang, Y.; Su, X.; Shen, Y.; et al. A rationally designed agonist antibody fragment that functionally mimics thrombopoietin. Proc. Natl. Acad. Sci. USA 2006, 103, 14307–14312. [Google Scholar] [CrossRef]
- Oroz-Parra, I.; Álvarez-Delgado, C.; Cervantes-Luevano, K.; Dueñas-Espinoza, S.; Licea-Navarro, A.F. Proapoptotic Index Evaluation of Two Synthetic Peptides Derived from the Coneshell Californiconus californicus in Lung Cancer Cell Line H1299. Mar. Drugs 2019, 18, 10. [Google Scholar] [CrossRef]
- Millán-Gómez, D.; Dueñas, S.; Muñoz, P.L.A.; Villegas, T.C.; Elosua, C.; Cabanillas-Bernal, O.; Escalante, T.; Perona, A.; Abia, D.; Drescher, F.; et al. In silico-designed mutations increase variable new-antigen receptor single-domain antibodies for VEGF165 neutralization. Oncotarget 2018, 9, 28016–28029. [Google Scholar] [CrossRef] [PubMed]
- Juma, S.; Gong, X.; Hu, S.; Lv, Z.; Shao, J.; Liu, L.; Chen, G. Shark New Antigen Receptor (IgNAR): Structure, Characteristics and Potential Biomedical Applications. Cells 2021, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Cheong, W.S.; Leow, C.Y.; Majeed, A.B.A. Diagnostic and therapeutic potential of shark variable new antigen receptor (VNAR) single domain antibody. Int. J. Biol. Macromol. 2020, 147, 369–375. [Google Scholar] [CrossRef]
- Zielonka, S.; Empting, M.; Grzeschik, J.; Könning, D.; Barelle, C.J.; Kolmar, H. Structural insights and biomedical potential of IgNAR scaffolds from sharks. mAbs 2015, 7, 15–25. [Google Scholar] [CrossRef]
- Juarez, K.; Dubberke, G.; Lugo, P.; Koch-Nolte, F.; Buck, F.; Haag, F.; Licea, A. Monoclonal Antibodies for the Identification and Purification of vNAR Domains and IgNAR Immunoglobulins from the Horn Shark Heterodontus francisci. Hybridoma 2011, 30, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Villegas, T.A.; Mata-González, M.T.; García-Ubbelohd, W.; Núñez-García, L.; Elosua, C.; Paniagua-Solis, J.F.; Licea-Navarro, A.F. Intraocular Penetration of a vNAR: In Vivo and In Vitro VEGF165 Neutralization. Mar. Drugs 2018, 16, 113. [Google Scholar] [CrossRef]
- Camacho-Villegas, T.; Mata-Gonzalez, T.; Paniagua-Solis, J.; Sanchez, E.; Licea, A. Human TNF cytokine neutralization with a vNAR from Heterodontus francisci shark: A potential therapeutic use. mAbs 2012, 5, 80–85. [Google Scholar] [CrossRef]
- Leow, C.H.; Fischer, K.; Leow, C.Y.; Braet, K.; Cheng, Q.; McCarthy, J. Isolation and characterization of malaria PfHRP2 specific VNAR antibody fragments from immunized shark phage display library. Malar. J. 2018, 17, 383. [Google Scholar] [CrossRef]
- Gauhar, A.; Privezentzev, C.V.; Demydchuk, M.; Gerlza, T.; Rieger, J.; Kungl, A.J.; Stocki, P. Single domain shark VNAR antibodies neutralize SARS-CoV-2 infection in vitro. FASEB J. 2021, 35, e21970. [Google Scholar] [CrossRef] [PubMed]
- Könning, D.; Rhiel, L.; Empting, M.; Grzeschik, J.; Sellmann, C.; Schröter, C.; Zielonka, S.; Dickgießer, S.; Pirzer, T.; Yanakieva, D.; et al. Semi-synthetic vNAR libraries screened against therapeutic antibodies primarily deliver anti-idiotypic binders. Sci. Rep. 2017, 7, 9676. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas-Bernal, O.; Dueñas, S.; Ayala-Avila, M.; Rucavado, A.; Escalante, T.; Licea-Navarro, A.F. Synthetic libraries of shark vNAR domains with different cysteine numbers within the CDR3. PLoS ONE 2019, 14, e0213394. [Google Scholar] [CrossRef]
- Oroz-Parra, I.; Navarro, M.; Cervantes-Luevano, K.E.; Álvarez-Delgado, C.; Salvesen, G.; Sanchez-Campos, L.N.; Licea-Navarro, A.F. Apoptosis Activation in Human Lung Cancer Cell Lines by a Novel Synthetic Peptide Derived from Conus californicus Venom. Toxins 2016, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Moertel, C.G.; Fleming, T.R.; Macdonald, J.S.; Haller, D.G.; Laurie, J.A.; Tangen, C. An Evaluation of the Carcinoembryonic Antigen (CEA) Test for Monitoring Patients with Resected Colon Cancer. JAMA J. Am. Med. Assoc. 1993, 270, 943–947. [Google Scholar] [CrossRef]
- Grunnet, M.; Sorensen, J. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012, 76, 138–143. [Google Scholar] [CrossRef]
- Henderson, K.A.; Streltsov, V.A.; Coley, A.M.; Dolezal, O.; Hudson, P.J.; Batchelor, A.H.; Nuttall, S.D. Structure of an IgNAR-AMA1 complex: Targeting a conserved hydrophobic cleft broadens malarial strain recognition. Structure 2007, 15, 1452–1466. [Google Scholar] [CrossRef]
- Burgess, S.G.; Oleksy, A.; Cavazza, T.; Richards, M.W.; Vernos, I.; Matthews, D.; Bayliss, R. Allosteric inhibition of Aurora-A kinase by a synthetic vNAR domain. Open Biol. 2016, 6, 160089. [Google Scholar] [CrossRef]
- Webb, B.; Sali, A. Comparative Protein Structure Modeling Using MODELLER. Curr. Protoc. Bioinform. 2016, 54, 5.6.1–5.6.37. [Google Scholar] [CrossRef]
- Kohno, T.; Kim, J.I.; Kobayashi, K.; Kodera, Y.; Maeda, T.; Sato, K. Three-Dimensional Structure in Solution of the Calcium Channel Blocker.omega.-Conotoxin MVIIA. Biochemistry 1995, 34, 10256–10265. [Google Scholar] [CrossRef]
- Goldenberg, D.P.; Koehn, R.E.; Gilbert, D.E.; Wagner, G. Solution structure and backbone dynamics of an omega-conotoxin precursor. Protein Sci. 2001, 10, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, K.J.; Thomas, L.; Lewis, R.J.; Alewood, P.F.; Craik, D.J. A Consensus Structure for ω-Conotoxins with Different Selectivities for Voltage-sensitive Calcium Channel Subtypes: Comparison of MVIIA, SVIB and SNX-202. J. Mol. Biol. 1996, 263, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model 1996, 14, 27–28,33–38. [Google Scholar] [CrossRef]
- Huang, J.; MacKerell, A.D., Jr. CHARMM36 all-atom additive protein force field: Validation based on comparison to NMR data. J. Comput. Chem. 2013, 34, 2135–2145. [Google Scholar] [CrossRef] [PubMed]
- Wallner, B.; Elofsson, A. Can correct protein models be identified? Protein Sci. 2003, 12, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Desta, I.T.; Porter, K.A.; Xia, B.; Kozakov, D.; Vajda, S. Performance and Its Limits in Rigid Body Protein-Protein Docking. Structure 2020, 28, 1071–1081.e3. [Google Scholar] [CrossRef]
- Vajda, S.; Yueh, C.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Kozakov, D. New additions to the ClusPro server motivated by CAPRI. Proteins Struct. Funct. Bioinform. 2017, 85, 435–444. [Google Scholar] [CrossRef]
- Kozakov, D.; Beglov, D.; Bohnuud, T.; Mottarella, S.E.; Xia, B.; Hall, D.R.; Vajda, S. How good is automated protein docking? Proteins Struct. Funct. Bioinform. 2013, 81, 2159–2166. [Google Scholar] [CrossRef]
- Kozakov, D.; Hall, D.R.; Xia, B.; Porter, K.A.; Padhorny, D.; Yueh, C.; Beglov, D.; Vajda, S. The ClusPro web server for protein–protein docking. Nat. Protoc. 2017, 12, 255–278. [Google Scholar] [CrossRef]
- Sedan, Y.; Marcu, O.; Lyskov, S.; Schueler-Furman, O. Peptiderive server: Derive peptide inhibitors from protein–protein interactions. Nucleic Acids Res. 2016, 44, W536–W541. [Google Scholar] [CrossRef] [PubMed]
- London, N.; Raveh, B.; Movshovitz-Attias, D.; Schueler-Furman, O. Can self-inhibitory peptides be derived from the interfaces of globular protein-protein interactions? Proteins Struct. Funct. Bioinforma. 2010, 78, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Lyskov, S.; Chou, F.-C.; Conchúir, S.Ó.; Der, B.S.; Drew, K.; Kuroda, D.; Xu, J.; Weitzner, B.D.; Renfrew, P.D.; Sripakdeevong, P.; et al. Serverification of molecular modeling applications: The Rosetta Online Server that Includes Everyone (ROSIE). PLoS ONE 2013, 8, e63906. [Google Scholar] [CrossRef] [PubMed]
- Korff, T. Three-Dimensional in Vitro Angiogenesis Assays. In Methods in Endothelial Cell Biology; Augustin, H., Ed.; Springer: Freiburg, Germany, 2004; pp. 115–124. [Google Scholar]




| NoNaBody | Sequence |
|---|---|
| cal_P98Y | GDCPPWCGARCKNLLPRYLVNGIAAMGYSSSC |
| cal_T10 | GDCPPWCGARCHTKWGFFPLSWKLVGAALINRSC |
| cal_CV043 | GDCPPWCGARCDMVWSWWGGWRPVRRLGWKGWSC |
| cal_Tn16 | GDCPPWCGARCKAQGLIDTSVRGLAVPGNCERCSSYHC |
| cal_PK13 | GDCPPWCGARCARVWVSWVARAFFRGINFLPVFSC |
| cal_SP240 | GDCPPWCGARCRAFGARARHEEGLEYYC |
| cal_lis | GDCPPWCGARCESRYGSYDAECAALNDC |
| cal_AMA1 | GDCPPWCGARCFYSLPLRDYNYSLLC |
| Molecule | Interaction Site Bound to VEGF165 Chain A | VEGF165 Chain A Interaction Site | Interaction Site Bound to VEGF165 Chain B | VEGF165 Chain B Interaction Site | Total Score (REU) |
|---|---|---|---|---|---|
| P98Y | RRKNLLPRYLV | RKHLFVQDPQT | IGRRKNLLPRYL | IETLVDIFQ | −45.98 |
| cal14.1a | VGARCR | SYCHPI | ARC | PIETL | −14.88 |
| cal_P98Y | RCKNLLPRYLVN | ERRKHLFVQ | KNLLPRYLVN | VDIFQEYPDE | −41.54 |
| Molecule | Interaction Site Bound to TGF-β Chain A | TGF-β Chain A Interaction Site | Interaction Site Bound to TGF-β Chain B | TGF-β Chain B Interaction Site | Total Score (REU) |
|---|---|---|---|---|---|
| T10 | KWGFFPLSWKLV | QHNPGASAAP | KWGFFPLSWKLV | QHNPGASAAP | −27.24 |
| cal14.1a | GDCPPW | ANFCL | GDCPPW | NFCLGP | −12.83 |
| cal_T10 | PCHTKWGFFPL | QHNPGASAA | KLVGAAL | YNQHNPGASA | −34.55 |
| Molecule | Interaction Site Bound to CEA Chain A | CEA Chain A Interaction Site | Interaction Site Bound to CEA Chain B | CEA Chain B Interaction Site | Total Score (REU) |
|---|---|---|---|---|---|
| CV0-43 | PVRRLGWK | LPQHLF | WRPVRR | VIGTQQAT | −18.76 |
| Cal14.1a | RAE | TQQAT | CRAE | HLFGY | −8.53 |
| Cal_CV043 | MVWSWWG | IKSDLVN | WRPVRR | YVIGTQ | −22.16 |
| Molecules | Binding Strength | Figure | |
|---|---|---|---|
| TNF-⍺ | NoNaBody cal_T16 | −27.05 REU | Supplementary Figure S1 |
| Conotoxin cal14.1a | −14.04 REU | ||
| VNAR Tn16 | −33.01 REU | ||
| PCSK9 | NoNaBody cal_pk13 | −28.20 REU | Supplementary Figure S2 |
| Conotoxin cal14.1a | −8.98 REU | ||
| VNAR PK13 | −20.39 REU | ||
| SARS-CoV-2 Delta SPIKE | NoNaBody cal_SP240 | −41.06 REU | Supplementary Figure S3 |
| Conotoxin cal14.1a | −12.60 REU | ||
| VNAR SP240 | −30.29 REU | ||
| Lysozyme (G. cirratum) | NoNaBody cal_lis | −29.78 REU | Supplementary Figure S4 |
| Conotoxin cal14.1a | −13.10 REU | ||
| VNAR A07 | −32.29 REU | ||
| AMA1 (O.maculatus) | NoNaBody cal_AMA1 | −37.18 REU | Supplementary Figure S5 |
| Conotoxin cal14.1a | −13.08 REU | ||
| VNAR 14I-1 | −36.53 REU | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dueñas, S.; Escalante, T.; Gasperin-Bulbarela, J.; Bernáldez-Sarabia, J.; Cervantes-Luévano, K.; Jiménez, S.; Sánchez-Campos, N.; Cabanillas-Bernal, O.; Valdovinos-Navarro, B.J.; Álvarez-Lee, A.; et al. Chimeric Peptides from Californiconus californicus and Heterodontus francisci with Antigen-Binding Capacity: A Conotoxin Scaffold to Create Non-Natural Antibodies (NoNaBodies). Toxins 2023, 15, 269. https://doi.org/10.3390/toxins15040269
Dueñas S, Escalante T, Gasperin-Bulbarela J, Bernáldez-Sarabia J, Cervantes-Luévano K, Jiménez S, Sánchez-Campos N, Cabanillas-Bernal O, Valdovinos-Navarro BJ, Álvarez-Lee A, et al. Chimeric Peptides from Californiconus californicus and Heterodontus francisci with Antigen-Binding Capacity: A Conotoxin Scaffold to Create Non-Natural Antibodies (NoNaBodies). Toxins. 2023; 15(4):269. https://doi.org/10.3390/toxins15040269
Chicago/Turabian StyleDueñas, Salvador, Teresa Escalante, Jahaziel Gasperin-Bulbarela, Johanna Bernáldez-Sarabia, Karla Cervantes-Luévano, Samanta Jiménez, Noemí Sánchez-Campos, Olivia Cabanillas-Bernal, Blanca J. Valdovinos-Navarro, Angélica Álvarez-Lee, and et al. 2023. "Chimeric Peptides from Californiconus californicus and Heterodontus francisci with Antigen-Binding Capacity: A Conotoxin Scaffold to Create Non-Natural Antibodies (NoNaBodies)" Toxins 15, no. 4: 269. https://doi.org/10.3390/toxins15040269
APA StyleDueñas, S., Escalante, T., Gasperin-Bulbarela, J., Bernáldez-Sarabia, J., Cervantes-Luévano, K., Jiménez, S., Sánchez-Campos, N., Cabanillas-Bernal, O., Valdovinos-Navarro, B. J., Álvarez-Lee, A., De León-Nava, M. A., & Licea-Navarro, A. F. (2023). Chimeric Peptides from Californiconus californicus and Heterodontus francisci with Antigen-Binding Capacity: A Conotoxin Scaffold to Create Non-Natural Antibodies (NoNaBodies). Toxins, 15(4), 269. https://doi.org/10.3390/toxins15040269






