Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review
Abstract
1. Introduction
2. Results
2.1. In Vitro Studies
2.2. Animal Studies
2.3. Clinical Studies
2.3.1. Observational Studies
Chronic Kidney Disease
Dialysis
Kidney Transplant Recipients
Diabetes
Other
2.3.2. Interventional studies
Dialysis
Phosphate Binders
Magnesium
Other Treatments
3. Discussion
4. Methodology
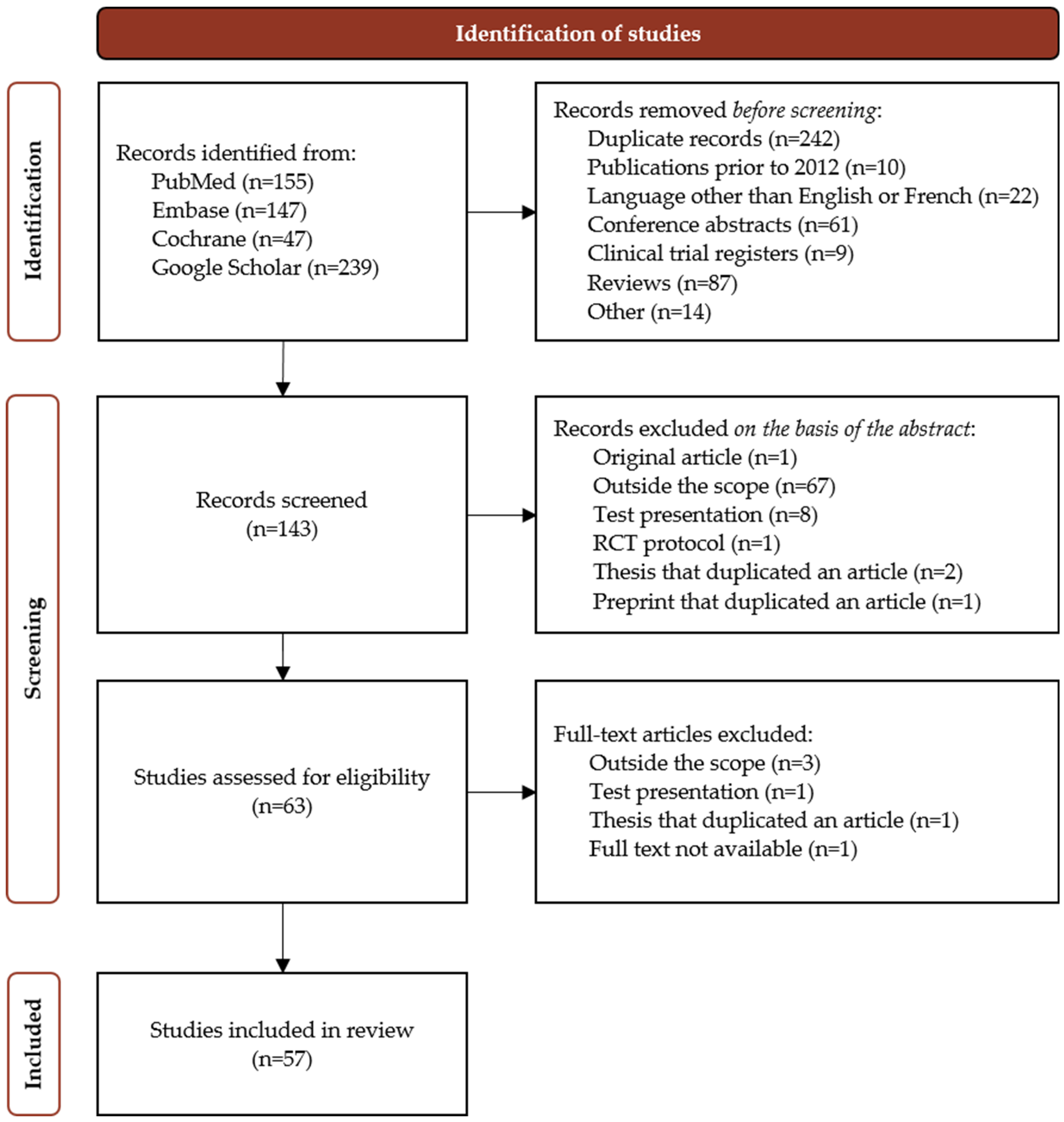
4.1. Search Strategy
4.2. Selection, Screening and Inclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, W.B.; Zhang, W.S.; Jiang, C.Q.; Liu, X.Y.; Jin, Y.L.; Lam, T.H.; Cheng, K.K.; Xu, L. Aortic Arch Calcification and Risk of All-Cause Mortality and Cardiovascular Disease: The Guangzhou Biobank Cohort Study. Lancet Reg. Health—West. Pac. 2022, 23, 100460. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, C.M. Mechanisms of Vascular Calcification in CKD—Evidence for Premature Ageing? Nat. Rev. Nephrol. 2013, 9, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Delanaye, P.; Cavalier, É.; Guérin, A.; Kamel, S.; Massy, Z.A.; Néphrologie, P. le groupe de travail « B. des calcifications vasculaires » de la S. et de la S. de Cardiovascular Calcification Inhibitors. Ann. Biol. Clin. 2015, 73, 315–322. [Google Scholar] [CrossRef]
- Pasch, A.; Farese, S.; Gräber, S.; Wald, J.; Richtering, W.; Floege, J.; Jahnen-Dechent, W. Nanoparticle-Based Test Measures Overall Propensity for Calcification in Serum. JASN 2012, 23, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- ter Braake, A.D.; Eelderink, C.; Zeper, L.W.; Pasch, A.; Bakker, S.J.L.; de Borst, M.H.; Hoenderop, J.G.J.; de Baaij, J.H.F. Calciprotein Particle Inhibition Explains Magnesium-Mediated Protection against Vascular Calcification. Nephrol. Dial. Transplant. 2020, 35, 765–773. [Google Scholar] [CrossRef]
- Nakatani, S.; Mori, K.; Sonoda, M.; Nishide, K.; Uedono, H.; Tsuda, A.; Emoto, M.; Shoji, T. Association between Serum Zinc and Calcification Propensity (T50) in Patients with Type 2 Diabetes Mellitus and In Vitro Effect of Exogenous Zinc on T50. Biomedicines 2020, 8, 337. [Google Scholar] [CrossRef]
- Mencke, R.; Sijbesma, J.W.A.; Doorduin, J.; Hoenderop, J.G.; Pasch, A.; Slart, R.H.J.A.; Hillebrands, J.L. Klotho in Vascular Biology; Rijksuniversiteit Groningen: Groningen, The Netherlands, 2018; ISBN 978-94-034-1025-8. [Google Scholar]
- Zarb, Y.; Weber-Stadlbauer, U.; Kirschenbaum, D.; Kindler, D.R.; Richetto, J.; Keller, D.; Rademakers, R.; Dickson, D.W.; Pasch, A.; Byzova, T.; et al. Ossified Blood Vessels in Primary Familial Brain Calcification Elicit a Neurotoxic Astrocyte Response. Brain 2019, 142, 885–902. [Google Scholar] [CrossRef]
- Schantl, A.E.; Verhulst, A.; Neven, E.; Behets, G.J.; D’Haese, P.C.; Maillard, M.; Mordasini, D.; Phan, O.; Burnier, M.; Spaggiari, D.; et al. Inhibition of Vascular Calcification by Inositol Phosphates Derivatized with Ethylene Glycol Oligomers. Nat. Commun. 2020, 11, 721. [Google Scholar] [CrossRef]
- Moor, M.B.; Ramakrishnan, S.K.; Legrand, F.; Bachtler, M.; Koesters, R.; Hynes, N.E.; Pasch, A.; Bonny, O. Elevated Serum Magnesium Lowers Calcification Propensity in Memo1-Deficient Mice. PLoS ONE 2020, 15, e0236361. [Google Scholar] [CrossRef]
- Ishida, K.; Ashizawa, N.; Morikane, S.; Kurita, N.; Kobashi, S.; Iwanaga, T. Assessment of Calciprotein Particle Formation by AUC of the Absorbance Change: Effect of FYB-931, a Novel Bisphosphonate Compound. J. Pharm. Pharmacol. 2021, 73, 947–955. [Google Scholar] [CrossRef]
- Smith, E.R.; Ford, M.L.; Tomlinson, L.A.; Bodenham, E.; McMahon, L.P.; Farese, S.; Rajkumar, C.; Holt, S.G.; Pasch, A. Serum Calcification Propensity Predicts All-Cause Mortality in Predialysis CKD. JASN 2014, 25, 339–348. [Google Scholar] [CrossRef] [PubMed]
- de Seigneux, S.; Ponte, B.; Berchtold, L.; Hadaya, K.; Martin, P.-Y.; Pasch, A. Living Kidney Donation Does Not Adversely Affect Serum Calcification Propensity and Markers of Vascular Stiffness. Transpl. Int. 2015, 28, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, C.A.; de Borst, M.H.; van den Berg, E.; Jahnen-Dechent, W.; Arampatzis, S.; Farese, S.; Bergmann, I.P.; Floege, J.; Navis, G.; Bakker, S.J.L.; et al. Calcification Propensity and Survival among Renal Transplant Recipients. JASN 2016, 27, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Dahle, D.O.; Åsberg, A.; Hartmann, A.; Holdaas, H.; Bachtler, M.; Jenssen, T.G.; Dionisi, M.; Pasch, A. Serum Calcification Propensity Is a Strong and Independent Determinant of Cardiac and All-Cause Mortality in Kidney Transplant Recipients. Am. J. Transplant. 2016, 16, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Dekker, M.; Pasch, A.; van der Sande, F.; Konings, C.; Bachtler, M.; Dionisi, M.; Meier, M.; Kooman, J.; Canaud, B. High-Flux Hemodialysis and High-Volume Hemodiafiltration Improve Serum Calcification Propensity. PLoS ONE 2016, 11, e0151508. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, L.; Ponte, B.; Moll, S.; Hadaya, K.; Seyde, O.; Bachtler, M.; Vallée, J.-P.; Martin, P.-Y.; Pasch, A.; Seigneux, S. de Phosphocalcic Markers and Calcification Propensity for Assessment of Interstitial Fibrosis and Vascular Lesions in Kidney Allograft Recipients. PLoS ONE 2016, 11, e0167929. [Google Scholar] [CrossRef] [PubMed]
- Pasch, A.; Block, G.A.; Bachtler, M.; Smith, E.R.; Jahnen-Dechent, W.; Arampatzis, S.; Chertow, G.M.; Parfrey, P.; Ma, X.; Floege, J. Blood Calcification Propensity, Cardiovascular Events, and Survival in Patients Receiving Hemodialysis in the EVOLVE Trial. CJASN 2017, 12, 315–322. [Google Scholar] [CrossRef]
- Pruijm, M.; Lu, Y.; Megdiche, F.; Piskunowicz, M.; Milani, B.; Stuber, M.; Bachtler, M.; Vogt, B.; Burnier, M.; Pasch, A. Serum Calcification Propensity Is Associated with Renal Tissue Oxygenation and Resistive Index in Patients with Arterial Hypertension or Chronic Kidney Disease. J. Hypertens. 2017, 35, 2044–2052. [Google Scholar] [CrossRef]
- Lorenz, G.; Steubl, D.; Kemmner, S.; Pasch, A.; Koch-Sembdner, W.; Pham, D.; Haller, B.; Bachmann, Q.; Mayer, C.C.; Wassertheurer, S.; et al. Worsening Calcification Propensity Precedes All-Cause and Cardiovascular Mortality in Haemodialyzed Patients. Sci. Rep. 2017, 7, 13368. [Google Scholar] [CrossRef]
- Bielesz, B.; Reiter, T.; Marculescu, R.; Gleiss, A.; Bojic, M.; Kieweg, H.; Cejka, D. Calcification Propensity of Serum Is Independent of Excretory Renal Function. Sci. Rep. 2017, 7, 17941. [Google Scholar] [CrossRef]
- Dahdal, S.; Devetzis, V.; Chalikias, G.; Tziakas, D.; Chizzolini, C.; Ribi, C.; Trendelenburg, M.; Eisenberger, U.; Hauser, T.; Pasch, A.; et al. Serum Calcification Propensity Is Independently Associated with Disease Activity in Systemic Lupus Erythematosus. PLoS ONE 2018, 13, e0188695. [Google Scholar] [CrossRef]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.D.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro-o, M.; Tomaschitz, A.; et al. Zinc Inhibits Phosphate-Induced Vascular Calcification through TNFAIP3-Mediated Suppression of NF-ΚB. JASN 2018, 29, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Voelkl, J.; Luong, T.T.D.; Tuffaha, R.; Musculus, K.; Auer, T.; Lian, X.; Daniel, C.; Zickler, D.; Boehme, B.; Sacherer, M.; et al. SGK1 Induces Vascular Smooth Muscle Cell Calcification through NF-κB Signaling. J. Clin. Investig. 2018, 128, 3024–3040. [Google Scholar] [CrossRef]
- Bostom, A.; Pasch, A.; Madsen, T.; Roberts, M.B.; Franceschini, N.; Steubl, D.; Garimella, P.S.; Ix, J.H.; Tuttle, K.R.; Ivanova, A.; et al. Serum Calcification Propensity and Fetuin-A: Biomarkers of Cardiovascular Disease in Kidney Transplant Recipients. AJN 2018, 48, 21–31. [Google Scholar] [CrossRef]
- Chen, W.; Anokhina, V.; Dieudonne, G.; Abramowitz, M.K.; Kashyap, R.; Yan, C.; Wu, T.T.; de Mesy Bentley, K.L.; Miller, B.L.; Bushinsky, D.A. Patients with Advanced Chronic Kidney Disease and Vascular Calcification Have a Large Hydrodynamic Radius of Secondary Calciprotein Particles. Nephrol. Dial. Transplant. 2019, 34, 992–1000. [Google Scholar] [CrossRef] [PubMed]
- Bullen, A.L.; Anderson, C.A.M.; Hooker, E.R.; Kado, D.M.; Orwoll, E.; Pasch, A.; Ix, J.H. Correlates of T50 and Relationships with Bone Mineral Density in Community-Living Older Men: The Osteoporotic Fractures in Men (MrOS) Study. Osteoporos. Int. 2019, 30, 1529–1531. [Google Scholar] [CrossRef]
- Bundy, J.D.; Cai, X.; Scialla, J.J.; Dobre, M.A.; Chen, J.; Hsu, C.; Leonard, M.B.; Go, A.S.; Rao, P.S.; Lash, J.P.; et al. Serum Calcification Propensity and Coronary Artery Calcification Among Patients With CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2019, 73, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Henze, L.A.; Luong, T.T.D.; Boehme, B.; Masyout, J.; Schneider, M.P.; Brachs, S.; Lang, F.; Pieske, B.; Pasch, A.; Eckardt, K.-U.; et al. Impact of C-Reactive Protein on Osteo-/Chondrogenic Transdifferentiation and Calcification of Vascular Smooth Muscle Cells. Aging 2019, 11, 5445–5462. [Google Scholar] [CrossRef] [PubMed]
- Bundy, J.D.; Cai, X.; Mehta, R.C.; Scialla, J.J.; de Boer, I.H.; Hsu, C.; Go, A.S.; Dobre, M.A.; Chen, J.; Rao, P.S.; et al. Serum Calcification Propensity and Clinical Events in CKD. CJASN 2019, 14, 1562–1571. [Google Scholar] [CrossRef]
- van Dijk, P.R.; Hop, H.; Waanders, F.; Mulder, U.J.; Pasch, A.; Hillebrands, J.-L.; van Goor, H.; Bilo, H.J.G. Serum Calcification Propensity in Type 1 Diabetes Associates with Mineral Stress. Diabetes Res. Clin. Pract. 2019, 158, 107917. [Google Scholar] [CrossRef]
- Thorsen, I.S.; Bleskestad, I.H.; Åsberg, A.; Hartmann, A.; Skadberg, Ø.; Brede, C.; Ueland, T.; Pasch, A.; Reisæter, A.V.; Gøransson, L.G. Vitamin D as a Risk Factor for Patient Survival after Kidney Transplantation: A Prospective Observational Cohort Study. Clin. Transplant. 2019, 33, e13517. [Google Scholar] [CrossRef] [PubMed]
- Ponte, B.; Pruijm, M.; Pasch, A.; Dufey-Teso, A.; Martin, P.-Y.; de Seigneux, S. Dialysis Initiation Improves Calcification Propensity. Nephrol. Dial. Transplant. 2020, 35, 495–502. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, P.R.; Waanders, F.; Pasch, A.; Logtenberg, S.J.J.; Vriesendorp, T.; Groenier, K.H.; Hillebrands, J.-L.; Kleefstra, N.; Gans, R.O.B.; van Goor, H.; et al. Favourable Serum Calcification Propensity with Intraperitoneal as Compared with Subcutaneous Insulin Administration in Type 1 Diabetes. Ther. Adv. Endocrinol. 2020, 11, 2042018820908456. [Google Scholar] [CrossRef]
- Eelderink, C.; te Velde-Keyzer, C.A.; Frenay, A.-R.S.; Vermeulen, E.A.; Bachtler, M.; Aghagolzadeh, P.; van Dijk, P.R.; Gansevoort, R.T.; Vervloet, M.G.; Hillebrands, J.-L.; et al. Serum Calcification Propensity and the Risk of Cardiovascular and All-Cause Mortality in the General Population. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1942–1951. [Google Scholar] [CrossRef]
- Kakajiwala, A.; Pasch, A.; Rogers, R.; Hoofnagle, A.; Meloni, S.; Furth, S.L.; Leonard, M.B.; Copelovitch, L.; Denburg, M.R. Serum Calcification Propensity in Children on Chronic Hemodialysis. Kidney Int. Rep. 2020, 5, 1528–1531. [Google Scholar] [CrossRef]
- Alesutan, I.; Luong, T.T.D.; Schelski, N.; Masyout, J.; Hille, S.; Schneider, M.P.; Graham, D.; Zickler, D.; Verheyen, N.; Estepa, M.; et al. Circulating Uromodulin Inhibits Vascular Calcification by Interfering with Pro-Inflammatory Cytokine Signalling. Cardiovasc. Res. 2021, 117, 930–941. [Google Scholar] [CrossRef]
- Chen, W.; Fitzpatrick, J.; Monroy-Trujillo, J.M.; Sozio, S.M.; Jaar, B.G.; Estrella, M.M.; Serrano, J.; Anokhina, V.; Miller, B.L.; Melamed, M.L.; et al. Associations of Serum Calciprotein Particle Size and Transformation Time With Arterial Calcification, Arterial Stiffness, and Mortality in Incident Hemodialysis Patients. Am. J. Kidney Dis. 2021, 77, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Mencke, R.; van der Vaart, A.; Pasch, A.; Harms, G.; Waanders, F.; Bilo, H.J.G.; van Goor, H.; Hillebrands, J.-L.; Dijk, P.R. van Serum Calcification Propensity Is Associated with HbA1c in Type 2 Diabetes Mellitus. BMJ Open Diabetes Res. Care 2021, 9, e002016. [Google Scholar] [CrossRef] [PubMed]
- Bojic, M.; Koller, L.; Cejka, D.; Niessner, A.; Bielesz, B. Propensity for Calcification in Serum Associates With 2-Year Cardiovascular Mortality in Ischemic Heart Failure With Reduced Ejection Fraction. Front. Med. 2021, 8. [Google Scholar] [CrossRef]
- de Haan, A.; Ahmadizar, F.; van der Most, P.J.; Thio, C.H.L.; Kamali, Z.; Ani, A.; Ghanbari, M.; Chaker, L.; van Meurs, J.; Ikram, M.K.; et al. Genetic Determinants of Serum Calcification Propensity and Cardiovascular Outcomes in the General Population. Front. Cardiovasc. Med. 2022, 8. [Google Scholar] [CrossRef]
- Kantauskaite, M.; Bolten, K.; Boschheidgen, M.; Schmidt, C.; Kolb, T.; Eckardt, K.U.; Pasch, A.; Schimmöller, L.; Rump, L.C.; Voelkl, J.; et al. Serum Calcification Propensity and Calcification of the Abdominal Aorta in Patients With Primary Aldosteronism. Front. Cardiovasc. Med. 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Bristow, S.M.; Gamble, G.D.; Pasch, A.; O’Neill, W.C.; Stewart, A.; Horne, A.M.; Reid, I.R. Acute and 3-Month Effects of Calcium Carbonate on the Calcification Propensity of Serum and Regulators of Vascular Calcification: Secondary Analysis of a Randomized Controlled Trial. Osteoporos. Int. 2016, 27, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Silver, B.; Pasch, A.; Bouchelouche, P.; Pedersen, L.; Rasmussen, L.M.; Brandi, L. Oral Magnesium Supplementation in Chronic Kidney Disease Stages 3 and 4: Efficacy, Safety, and Effect on Serum Calcification Propensity—A Prospective Randomized Double-Blinded Placebo-Controlled Clinical Trial. Kidney Int. Rep. 2017, 2, 380–389. [Google Scholar] [CrossRef]
- Smerud, K.T.; Åsberg, A.; Kile, H.; Pasch, A.; Dahle, D.O.; Bollerslev, J.; Godang, K.; Hartmann, A. A Rapid and Sustained Improvement of Calcification Propensity Score (Serum T50) after Successful Kidney Transplantation: Reanalysis of a Randomized Controlled Trial of Ibandronate. Clin. Transplant. 2017, 31, e13131. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, G.; Mayer, C.C.; Bachmann, Q.; Stryeck, S.; Braunisch, M.C.; Haller, B.; Carbajo-Lozoya, J.; Schmidt, A.; Witthauer, S.; Abuzahu, J.; et al. Acetate-Free, Citrate-Acidified Bicarbonate Dialysis Improves Serum Calcification Propensity—A Preliminary Study. Nephrol. Dial. Transplant. 2018, 33, 2043–2051. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, J.; Scanni, R.; Bestmann, L.; Hulter, H.N.; Krapf, R. A Controlled Increase in Dietary Phosphate Elevates BP in Healthy Human Subjects. JASN 2018, 29, 2089–2098. [Google Scholar] [CrossRef] [PubMed]
- Bressendorff, I.; Hansen, D.; Schou, M.; Pasch, A.; Brandi, L. The Effect of Increasing Dialysate Magnesium on Serum Calcification Propensity in Subjects with End Stage Kidney Disease: A Randomized, Controlled Clinical Trial. CJASN 2018, 13, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Ussif, A.; Pihlstrøm, H.; Pasch, A.; Holdaas, H.; Hartmann, A.; Smerud, K.; Åsberg, A. Paricalcitol Supplementation during the First Year after Kidney Transplantation Does Not Affect Calcification Propensity Score. BMC Nephrol. 2018, 19, 212. [Google Scholar] [CrossRef]
- Kendrick, J.; Shah, P.; Andrews, E.; You, Z.; Nowak, K.; Pasch, A.; Chonchol, M. Effect of Treatment of Metabolic Acidosis on Vascular Endothelial Function in Patients with CKD: A Pilot Randomized Cross-Over Study. CJASN 2018, 13, 1463–1470. [Google Scholar] [CrossRef]
- Andrews, E.S.; Perrenoud, L.; Nowak, K.L.; You, Z.; Pasch, A.; Chonchol, M.; Kendrick, J.; Jalal, D. Examining the Effects of Uric Acid-Lowering on Markers Vascular of Calcification and CKD-MBD; A Post-Hoc Analysis of a Randomized Clinical Trial. PLoS ONE 2018, 13, e0205831. [Google Scholar] [CrossRef]
- Quiñones, H.; Hamdi, T.; Sakhaee, K.; Pasch, A.; Moe, O.W.; Pak, C.Y.C. Control of Metabolic Predisposition to Cardiovascular Complications of Chronic Kidney Disease by Effervescent Calcium Magnesium Citrate: A Feasibility Study. J. Nephrol. 2019, 32, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Aigner, C.; Cejka, D.; Sliber, C.; Fraunschiel, M.; Sunder-Plassmann, G.; Gaggl, M. Oral Sodium Bicarbonate Supplementation Does Not Affect Serum Calcification Propensity in Patients with Chronic Kidney Disease and Chronic Metabolic Acidosis. KBR 2019, 44, 188–199. [Google Scholar] [CrossRef] [PubMed]
- ter Meulen, K.J.; Dekker, M.J.E.; Pasch, A.; Broers, N.J.H.; van der Sande, F.M.; van der Net, J.B.; Konings, C.J.A.M.; Gsponer, I.M.; Bachtler, M.D.N.; Gauly, A.; et al. Citric-Acid Dialysate Improves the Calcification Propensity of Hemodialysis Patients: A Multicenter Prospective Randomized Cross-over Trial. PLoS ONE 2019, 14, e0225824. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Pan, F.F.M.; Hewitson, T.D.; Toussaint, N.D.; Holt, S.G. Effect of Sevelamer on Calciprotein Particles in Hemodialysis Patients: The Sevelamer Versus Calcium to Reduce Fetuin-A-Containing Calciprotein Particles in Dialysis (SCaRF) Randomized Controlled Trial. Kidney Int. Rep. 2020, 5, 1432–1447. [Google Scholar] [CrossRef] [PubMed]
- Thiem, U.; Soellradl, I.; Robl, B.; Watorek, E.; Blum, S.; Dumfarth, A.; Marculescu, R.; Pasch, A.; Haller, M.C.; Cejka, D. The Effect of Phosphate Binder Therapy with Sucroferric Oxyhydroxide on Calcification Propensity in Chronic Haemodialysis Patients: A Randomized, Controlled, Crossover Trial. Clin. Kidney J. 2021, 14, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Ketteler, M.; Wiecek, A.; Rosenkranz, A.R.; Pasch, A.; Rekowski, J.; Hellmann, B.; Karus, M.; Ammer, R. Efficacy and Safety of a Novel Nicotinamide Modified-Release Formulation in the Treatment of Refractory Hyperphosphatemia in Patients Receiving Hemodialysis—A Randomized Clinical Trial. Kidney Int. Rep. 2021, 6, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Shoji, T.; Nakatani, S.; Kabata, D.; Mori, K.; Shintani, A.; Yoshida, H.; Takahashi, K.; Ota, K.; Fujii, H.; Ueda, S.; et al. Comparative Effects of Etelcalcetide and Maxacalcitol on Serum Calcification Propensity in Secondary Hyperparathyroidism: A Randomized Clinical Trial. CJASN 2021, 16, 599–612. [Google Scholar] [CrossRef]
- Hammer, F.; Buehling, S.S.; Masyout, J.; Malzahn, U.; Hauser, T.; Auer, T.; Grebe, S.; Feger, M.; Tuffaha, R.; Degenhart, G.; et al. Protective Effects of Spironolactone on Vascular Calcification in Chronic Kidney Disease. Biochem. Biophys. Res. Commun. 2021, 582, 28–34. [Google Scholar] [CrossRef]
- Wang, A.Y.-M.; Pasch, A.; Wong, C.-K.; Chu, I.M.-T.; Tang, T.-K.; Chu, J.; Cheuk-Ying Fong, C.; Yau, Y.-Y.; Lo, W.-K. Long-Term Effects of Sevelamer on Vascular Calcification, Arterial Stiffness, and Calcification Propensity in Patients Receiving Peritoneal Dialysis: The Randomized Pilot SERENE (Sevelamer on Vascular Calcification, Arterial Stiffness) Trial. Kidney Med. 2022, 4, 100384. [Google Scholar] [CrossRef]
- Tiong, M.K.; Cai, M.M.X.; Toussaint, N.D.; Tan, S.-J.; Pasch, A.; Smith, E.R. Effect of Nutritional Calcium and Phosphate Loading on Calciprotein Particle Kinetics in Adults with Normal and Impaired Kidney Function. Sci. Rep. 2022, 12, 7358. [Google Scholar] [CrossRef]
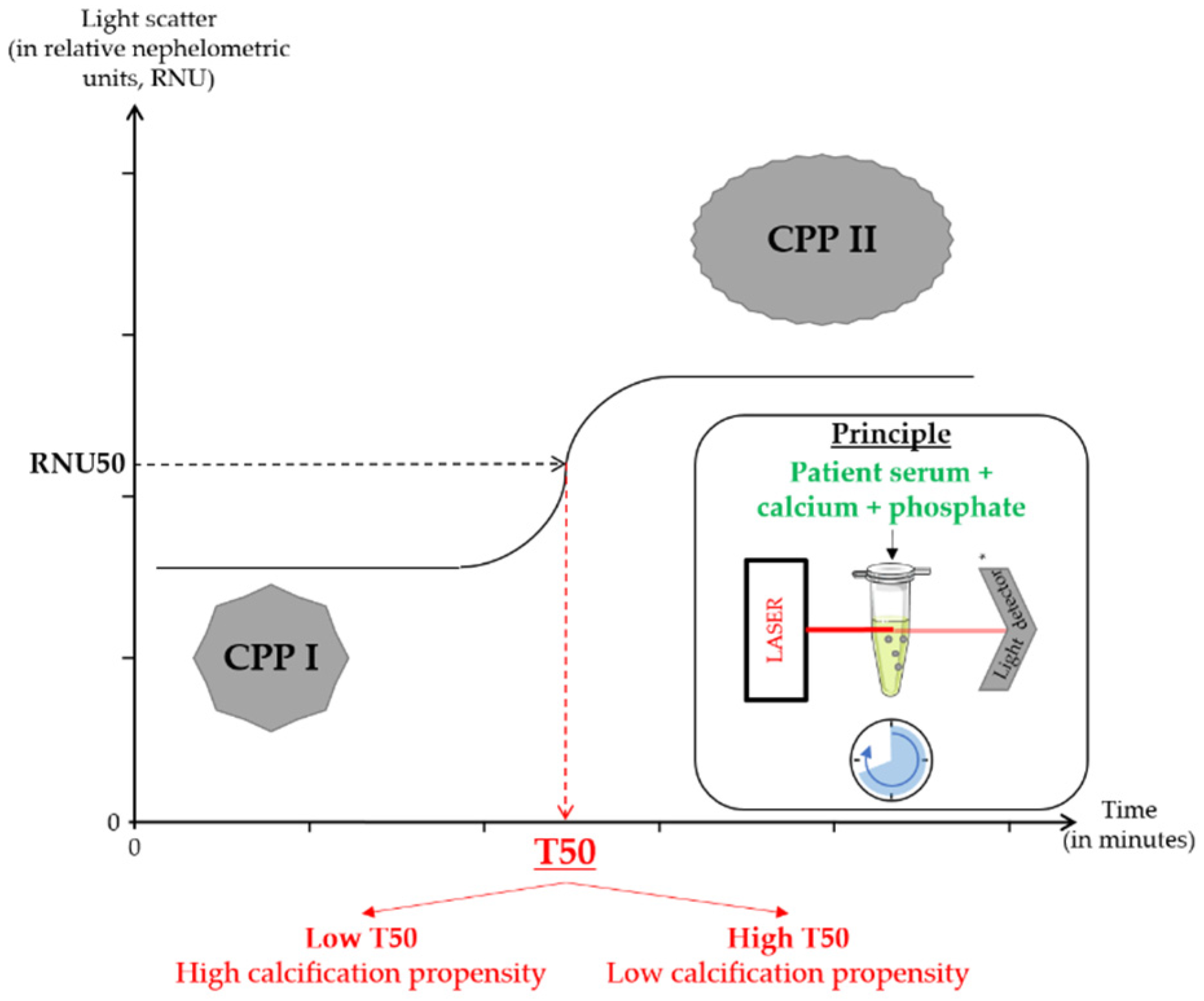
| Study | Experimental Models | Main Results |
|---|---|---|
| Ter Braake et al., 2020 [5] Calciprotein particle inhibition explains magnesium-mediated protection against vascular calcification | Human serum with or without the addition of MgCl2-containing solutions, resulting in elevations Mg2+ concentrations (0.2, 0.4, 0.6, 0.8 and 1.0 mmol/L):
| Mg2+ dose-dependently delayed the maturation of CPP1 to CPP2 in vitro. In human serum, addition of 0.2 mmol/L Mg2+ significantly lengthened T50 in healthy controls and CKD patients. Each 0.2 mmol/L increment in Mg2+ led to a similar increase in T50 in CKD patients and in healthy controls. |
| Nakatani et al., 2020 [6] Association between Serum zinc and calcification propensity (T(50)) in patients with type 2 diabetes mellitus and in vitro effect of exogenous zinc on T(50) | Pooled serum samples:
| Addition of a physiological concentration of exogenous zinc chloride (ZnCl2) was shown to significantly increase T50 (and thus decrease the calcification propensity) in serum from healthy volunteers and from patients on hemodialysis. |
| Mencke et al., 2018 [7] Imaging of incipient vascular calcification in Klotho deficiency | Mice:
| T50 was significantly shorter in serum from Klotho−/− mice than in serum from Klotho+/− mice or wild-type mice. |
| Zarb et al., 2019 [8] Ossified blood vessels in primary familial brain calcification elicit a neurotoxic astrocyte response | Mice:
| There was no significant difference in T50 values between Pdgfbret/ret mice and control animals. |
| Schantl et al., 2020 [9] Inhibition of vascular calcification by inositol phosphates derivatized with ethylene glycol oligomers | Rats (adenine and high-phosphate-diet):
| T50 did not differ when comparing the treatment groups. |
| Moor et al., 2020 [10] Elevated serum magnesium lowers calcification propensity in Memo1-deficient mice | Mice:
| Memo1 cKO mice had no soft tissue calcifications and had a lower serum calcification propensity (i.e., a longer T50) and a higher serum magnesium concentration, relative to controls. |
| Ishida et al., 2021 [11] Assessment of calciprotein particle formation by AUC of the absorbance change: effect of FYB-931, a novel bisphosphonate compound | Rats (treated or not with vitamin D3 (Subcutaneous)):
| In rats not treated with vitamin D3, FYB-931 and etidronate inhibited the increase in absorbance in a dose-dependent manner, but T50 was prolonged in a non-dose-dependent manner by FYB-931 and not prolonged by etidronate (up to 30 µmol/L). FYB-931 showed the most potent inhibitory activity against CPP formation. In vitamin D3-treated rats, T50 was prolonged in a dose-dependent manner by FYB-931 for a concentration of 0.3 or 0.6 mg/kg, but not when it was less concentrated. In contrast, etidronate did not show a significant change in T50 values. T50 was not prolonged by etidronate. |
| Clinical Studies | |||
|---|---|---|---|
| Observational Studies | |||
| Smith et al., 2014 [12] Serum calcification propensity predicts all-cause mortality in predialysis CKD | Median follow-up = 5.3 years Prospective cohort | N = 184 CKD stage 3–4 patients | Greater serum calcification propensity was independently associated with progressive aortic stiffening and an increased risk of all-cause mortality. |
| De Seigneux et al., 2015 [13] Living kidney donation does not adversely affect serum calcification propensity and markers of vascular stiffness | 1-year follow-up Prospective cohort | N = 21 Living kidney donors | Kidney donation did not worsen calcification propensity or markers of the progression of vascular stiffness measured 1 year after donation. |
| Keyzer et al., 2016 [14] Calcification propensity and survival among renal transplant recipients | Median follow-up = 3.1 years Longitudinal cohort | N = 699 Kidney transplant recipients | A short serum T50 was associated with an increased risk of all-cause mortality, cardiovascular mortality, and graft failure. |
| Dahle et al., 2016 [15] Serum calcification propensity is a strong and independent determinant of cardiac and all-cause mortality in kidney transplant recipients | Median follow-up = 5.1 years Prospective cohort | N = 1435 Kidney transplant recipients | Serum T50 was strongly associated with all-cause and cardiac mortality. |
| Dekker et al., 2016 [16] High-flux hemodialysis and high-volume hemodiafiltration improve serum calcification propensity | Cross-sectional study | N = 64
| T50 increased to a similar extent in both the hemodialysis and hemodiafiltration groups. |
| Berchtold et al., 2016 [17] Phosphocalcic markers and calcification propensity for assessment of interstitial fibrosis and vascular lesions in kidney allograft recipients | Retrospective study | N = 129 Kidney transplant recipients | T50 and vitamin D were inversely associated with greater interstitial fibrosis severity, while PTH elevation was positively associated with greater interstitial fibrosis severity. T50 decreased as the extent of arterial lesions increased. |
| Pasch et al., 2017 [18] Blood calcification propensity, cardiovascular events, and survival in patients receiving hemodialysis in the EVOLVE Trial | 64 months Post-hoc analysis of an interventional trial | N = 2785 Patients on hemodialysis with secondary hyperparathyroidism | A lower T50 was associated with all-cause mortality, myocardial infarction, and peripheral vascular events. |
| Pruijm et al., 2017 [19] Serum calcification propensity is associated with renal tissue oxygenation and resistive index in patients with arterial hypertension or chronic kidney disease | Cross-sectional study | N = 145
| Calcification propensity was higher in CKD patients and in hypertensive patients with preserved kidney function. Higher calcification propensity was associated with lower renal tissue oxygenation and perfusion, and higher renal vascular resistance and stiffness in both hypertensive patients with preserved kidney function and in CKD patients. |
| Lorenz et al., 2017 [20] Worsening calcification propensity precedes all-cause and cardiovascular mortality in hemodialyzed patients | 24 months Prospective cohort | N = 188 Hemodialysis | T50′s rate of decline was a significant predictor of all-cause and cardiovascular mortality, while cross-sectional T50 at inclusion and 24 months were not. |
| Bielesz et al., 2017 [21] Calcification propensity of serum is independent of excretory renal function | Cross-sectional study | N = 118
| T50 was associated with serum phosphate, magnesium, fetuin-A, albumin, bicarbonate and cross-lap levels (but not with eGFR) in multivariate adjusted models. Alterations in mineral homeostasis and serum protein composition (i.e., consequences of CKD) but not excretory kidney function per se influenced the serum calcification propensity. |
| Dahdal et al., 2018 [22] Serum calcification propensity is independently associated with disease activity in systemic lupus erythematosus | Cross-sectional study | N = 168 Systemic lupus erythematosus | T50 was negatively associated with systemic lupus erythematosus disease activity. |
| Voelkl et al., 2018 [23] Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB | Cross-sectional study | N = 57
| The serum zinc level and T50 tended to be lower in CKD patients than in controls. In patients on dialysis, serum zinc and T50 were lower still. Serum zinc levels were significantly correlated with T50 in controls and in patients with CKD. |
| Voelkl et al., 2018 [24] SGK1 induces vascular smooth muscle cell calcification through NF-κB signaling | Cross-sectional study | N = 14
| Serum calcification propensity was significantly higher in uremic serum samples than in normal serum samples. |
| Bostom et al., 2018 [25] Serum calcification propensity and fetuin-a: biomarkers of cardiovascular disease in kidney transplant recipients | Median follow-up = 2.18 years A longitudinal case-cohort analysis | N = 433 Kidney transplant recipients | A lower T50 and low fetuin-A levels were associated with a greater risk of cardiovascular disease outcomes. |
| Chen et al., 2019 [26] Patients with advanced chronic kidney disease and vascular calcification have a large hydrodynamic radius of secondary calciprotein particles | Cross-sectional study | N = 62
| Compared with healthy volunteers, CKD patients with or without vascular calcification had a lower T50. Among the CKD patients, there was no difference in T50 according to the presence or absence of vascular calcification. |
| Bullen et al., 2019 [27] Correlates of T(50) and relationships with bone mineral density in community-living older men: the osteoporotic fractures in men (MrOS) study | Cross-sectional study | N = 149 Men, aged 65 or over | No significant associations between T50 and total hip or total spine bone mineral density. |
| Bundy et al., 2019 [28] Serum calcification propensity and coronary artery calcification among patients with CKD: The CRIC (Chronic Renal Insufficiency Cohort) Study | Mean follow-up = 3.2 years Prospective cohort | N = 1274 (baseline) N = 780 (follow-up) CKD stage 2–4 patients | At baseline, T50 was not associated with CAC prevalence but was significantly associated with greater CAC severity among participants with prevalent CAC. Among participants with follow-up data, T50 was not associated with incident CAC but was significantly associated with CAC progression (a one-standard-deviation decrement in T50 was associated with a 28% [95% confidence interval 7–53%] greater risk of CAC progression). |
| Henze et al., 2019 [29] Impact of C-reactive protein on osteo-/chondrogenic transdifferentiation and calcification of vascular smooth muscle cells | Cross-sectional study | N = 309 CKD patients | The serum CRP concentration was inversely correlated with T50 in patients with moderately severe CKD. |
| Bundy et al., 2019 [30] Serum calcification propensity and clinical events in CKD | Mean follow-up = 7.1 years Prospective cohort | N = 3404 CKD stage 2–4 patients | After adjustment for conventional cardiovascular risk factors, higher serum calcification propensity was associated with cardiovascular events, end-stage kidney disease and all-cause mortality. This association was not independent of kidney function. After adjustment for eGFR and 24-h urinary protein, these associations were no longer significant. |
| Van Dijk et al., 2019 [31] Serum calcification propensity in type 1 diabetes associates with mineral stress | Median follow-up = 15.3 years Prospective cohort | N = 216 Patients with T1DM | T50 was associated with serum markers of elevated mineral stress but not with the development of long-term macrovascular complications. |
| Thorsen et al., 2019 [32] Vitamin D as a risk factor for patient survival after kidney transplantation: a prospective observational cohort study | Median follow-up = 82 months Prospective cohort | N = 762 Kidney transplant recipients | T50 was significantly higher in the vitamin D sufficient group (25(OH)D > 50 nmol/L) than in the group of patients with vitamin D deficiency (30–50 nmol/L) or insufficiency (<30 nmol/L). A lower T50 at 10 weeks post-transplant was associated with death and graft failure. |
| Ponte et al., 2020 [33] Dialysis initiation improves calcification propensity | 3 months Prospective cohort | N = 58
| Dialysis initiation significantly decreased calcification propensity. |
| Van Dijk et al., 2020 [34] Favorable serum calcification propensity with intraperitoneal as compared with subcutaneous insulin administration in type 1 diabetes | 26 weeks Prospective, observational matched case-control study | N = 181 Patients with T1DM
| Intraperitoneal insulin administration resulted in a higher T50 than subcutaneous administration. |
| Eelderink et al., 2020 [35] Serum calcification propensity and the risk of cardiovascular and all-cause mortality in the general population: The PREVEND Study | Median follow-up = 8.3 years Prospective cohort | N = 6231 General population | T50 was inversely associated with circulating phosphate, age, eGFR, alcohol consumption, and an elevated risk of cardiovascular mortality in the general population. T50 was positively associated with the plasma magnesium level. |
| Nakatani et al., 2020 [6] Association between Serum Zinc and Calcification Propensity (T(50)) in Patients with Type 2 Diabetes Mellitus and In Vitro Effect of Exogenous Zinc on T(50) | Cross-sectional | N = 132 Patients with T2DM | The serum zinc level was found to be an independent factor associated positively with the serum T50 in patients with T2DM. |
| Kakajiwala et al., 2020 [36] Serum calcification propensity in children on chronic hemodialysis | 12 weeks A prospective, single-center study | N = 9 Children on hemodialysis | Participants who had greater serum calcification propensity (i.e., a lower median T50 value) had a higher median calcium x phosphate product level, driven by higher phosphate concentrations. In a multivariate analysis, higher serum magnesium, calcium and fetuin-A concentrations were independently associated with a longer T50, and a higher serum phosphate was independently associated with a shorter T50. |
| Alesutan et al., 2021 [37] Circulating uromodulin inhibits vascular calcification by interfering with pro-inflammatory cytokine signaling | Cross-sectional study | N = 311 CKD patients N = 138
| In three independent cohorts of CKD patients, serum uromodulin concentrations were correlated with T50. |
| Chen et al., 2021 [38] Associations of Serum Calciprotein Particle Size and Transformation Time With Arterial Calcification, Arterial Stiffness, and Mortality in Incident Hemodialysis Patients | Median follow-up = 3.5 years Prospective cohort | N = 402 Hemodialysis patients | Baseline T50 and CPP2 size were not associated with the presence and severity of coronary arterial calcification and thoracic aortic calcification, or repeated measures of pulse wave velocity. There was no association between baseline T50 and the risk of death. The size of the CPP aggregates was negatively correlated with T50. |
| Mencke et al., 2021 [39] Serum calcification propensity is associated with HbA1c in type 2 diabetes mellitus | Cross-sectional study | N = 932 Patients with T2DM | Serum calcification propensity was negatively and independently associated with the HbA1c level. T50 was not associated with previous macrovascular events or the presence of microvascular disease. |
| Bojic et al., 2021 [40] Propensity for calcification in serum associates with 2-year cardiovascular mortality in ischemic heart failure with reduced ejection fraction | Median follow-up = 3.2 years Prospective cohort | N = 306 Patients with chronic heart failure and reduced ejection fraction (HfrEF) | T50 was associated with 2-year cardiovascular mortality in patients with ischemic HfrEF but not in patients with non-ischemic HfrEF. There were no significant differences in the severity and the underlying form of HfrEF (ischemic vs. non-ischemic) between T50 tertiles. There were significant differences in serum phosphate, albumin and intact FGF-23 levels between T50 tertiles. |
| De Haan et al., 2022 [41] Genetic determinants of serum calcification propensity and cardiovascular outcomes in the general population | Genome-wide association study + a two-sample Mendelian randomization study | N = 2739 members of the general population + N = 8566 members of the general population | Three independent genome-wide-significant single nucleotide polymorphisms in the AHSG gene (encoding fetuin-A) were identified: rs4917, rs2077119 and rs9870756 together explained 18.3% of the variance in T50. Mendelian randomization did not evidence a causal effect of T50 on cardiovascular outcomes in the general population. In patient-level analyses, rs9870756 was associated with a primary composite endpoint of all-cause mortality or cardiovascular disease and all-cause mortality alone. In patients with T2DM or CKD, the association between rs9870756 and the primary composite endpoint was stronger. |
| Kantauskaite et al., 2022 [42] Serum calcification propensity and calcification of the abdominal aorta in patients with primary aldosteronism | Median follow-up:
| N = 94
| In patients with PA, a higher aldosterone-to-renin ratio was associated with a lower T50. The decline in T50 over the follow-up period was associated with higher calcium levels, an increase in phosphate levels, and a decrease in magnesium levels. In both the PA and RH groups, a higher atherosclerotic cardiovascular disease score was associated with a lower T50. Eighteen patients with PA underwent a CT scan of the abdomen: T50 was negatively associated with the extent of vascular calcification in the abdominal aorta. |
| Interventional studies | |||
| Bristow et al., 2016 [43] Acute and 3-month effects of calcium carbonate on the calcification propensity of serum and regulators of vascular calcification: secondary analysis of a randomized controlled trial | 3 months A randomized controlled trial | N = 41 Postmenopausal women
| T50 declined in both groups. The changes were slightly but not significantly greater in the calcium carbonate group. |
| Bressendorff et al., 2016 [44] Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial | 8 weeks Prospective, double-blind, placebo-controlled, randomized clinical trial | N = 36 CKD stage 3–4 patients:
| Oral Mg supplementation did not increase intracellular Mg levels. T50 increased significantly over baseline at weeks 4 and 8 in the Mg 30 mmol/d group. T50 increased significantly over baseline only at week 4 in the Mg 15 mmol/d group. There were no significant changes in the placebo group. |
| Smerud et al., 2017 [45] A rapid and sustained improvement of calcification propensity score (serum T(50)) after successful kidney transplantation: Reanalysis of a randomized controlled trial of ibandronate | 1 year Post-hoc analysis of a prospective, double-blind, placebo-controlled, randomized controlled trial | N = 123 Kidney transplant recipients:
| T50 increased from baseline to 10 weeks after transplantation, with no further change after 1 year. Ibandronate had no effect on T50, relative to placebo. |
| Lorenz et al., 2018 [46] Acetate-free, citrate-acidified bicarbonate dialysis improves serum calcification propensity-a preliminary study | 3 months Pre-post-quasi-interventional study | N = 78 Hemodialysis patients:
| Three months of dialysis with acetate-free, citrate-acidified, bicarbonate dialysis solution was associated with a longer T50, compared with acetate-acidified bicarbonate dialysis solution. |
| Mohammad et al., 2018 [47] A controlled increase in dietary phosphate elevates BP in healthy human subjects | 11 weeks Prospective, randomized, single-blind study | N = 20 Healthy young adults:
| Modulation of dietary phosphate loading did not significantly affect T50. |
| Bressendorff et al., 2018 [48] The effect of increasing dialysate magnesium on serum calcification propensity in subjects with end stage kidney disease: a randomized, controlled clinical trial | 28 days Single-center, double blind, parallel group, controlled randomized clinical trial | N = 57 Hemodialysis patients:
| Increasing the dialysate Mg level increased T50 in patients on maintenance hemodialysis, relative to standard-dialysate Mg. T50 returned to baseline levels when the high-dialysate Mg group was switched back to dialysate with 1.0 mEq/L Mg. |
| Ussif et al., 2018 [49] Paricalcitol supplementation during the first year after kidney transplantation does not affect calcification propensity score | 1 year Open-label, randomized, controlled trial | N = 76 Kidney transplant recipients:
| Paricalcitol had no effect on T50 during the first year following transplantation. |
| Kendrick et al., 2018 [50] Effect of treatment of metabolic acidosis on vascular endothelial function in patients with CKD: a pilot randomized cross-over study | 14 weeks Prospective, open-label, randomized crossover study | N = 18 Patients with CKD stage 3b-4 + metabolic acidosis:
| Oral sodium bicarbonate supplementation had no effect on T50. |
| Andrews et al., 2018 [51] Examining the effects of uric acid-lowering on markers vascular of calcification and CKD-MBD; A post-hoc analysis of a randomized clinical trial | 12 weeks Post-hoc analysis of a double-blind, placebo-controlled, randomized clinical trial | N = 63 Patients with stage 3 CKD + hyperuricemia:
| Allopurinol lowered uric acid levels but had no effect on T50 and CKD-mineral and bone disorder parameters. |
| Quiñones et al., 2019 [52] Control of metabolic predisposition to cardiovascular complications of chronic kidney disease by effervescent calcium magnesium citrate: a feasibility study | 3 weeks Randomized crossover study | N = 18 Patients with stage 3 (n = 9) or 5D (n = 9) CKD:
| In stage 3 CKD, neither calcium magnesium citrate nor calcium acetate altered T50. In stage 5D CKD, calcium magnesium citrate was associated with a significantly longer T50 and calcium acetate was not associated with any change. |
| Aigner et al., 2019 [53] Oral Sodium Bicarbonate Supplementation Does Not Affect Serum Calcification Propensity in Patients with Chronic Kidney Disease and Chronic Metabolic Acidosis | 4 weeks Randomized controlled trial | N = 35 Patients with stage 3–4 CKD + chronic metabolic acidosis:
| Oral sodium bicarbonate supplementation had no effect on T50 in CKD patients with acidosis. |
| Ter Meulen et al., 2019 [54] Citric-acid dialysate improves the calcification propensity of hemodialysis patients: A multicenter prospective randomized cross-over trial | 4 weeks Prospective multicenter randomized crossover study | N = 18 Hemodialysis patients:
| Citric acid-buffered dialysis solution was associated with a longer T50, relative to acetate-buffered solution. |
| Smith et al., 2020 [55] Effect of sevelamer on calciprotein particles in hemodialysis patients: the sevelamer versus calcium to reduce fetuin-a-containing calciprotein particles in dialysis (ScaRF) randomized controlled trial | 24 weeks Multicenter, 3-arm, parallel group, open-label randomized controlled trial | N = 31 Hemodialysis patients:
| At 24 weeks, the serum CPP-1 level (but not the CPP-2 level), aortic pulse wave velocity and interleukin-8 levels were lower in the SH and SC groups than in the CC group. T50 increased from baseline over 24 weeks to the same extent in all three groups. |
| Thiem et al., 2020 [56] The effect of phosphate binder therapy with sucroferric oxyhydroxide on calcification propensity in chronic hemodialysis patients: a randomized, controlled, crossover trial | 6 weeks Open-label, single-center, crossover, randomized controlled trial | N = 39 Hemodialysis + hyperphosphatemia
| Treatment with the phosphate binder sucroferric oxyhydroxide significantly increased T50 and decreased serum phosphate levels. |
| Ketteler et al., 2020 [57] Efficacy and safety of a novel nicotinamide modified-release formulation in the treatment of refractory hyperphosphatemia in patients receiving hemodialysis-a randomized clinical trial | 12 weeks Multicenter, double-blind, placebo-controlled, prospective randomized clinical trial | N = 722 Patient with hemodialysis + refractory hyperphosphatemia (i.e., despite treatment with phosphate binder):
| A combination of modified-release nicotinamide and an oral phosphate binder was associated with significantly lower serum phosphate and intact PTH levels and a longer T50, compared with a combination of placebo and phosphate binder. |
| Shoji et al., 2021 [58] Comparative effects of etelcalcetide and maxacalcitol on serum calcification propensity in secondary hyperparathyroidism: a randomized clinical trial | 12 months Multicenter, open-label, blind end-point, randomized controlled trial | N = 321 Patients with secondary hyperparathyroidism on hemodialysis
| The increase in T50 was significantly greater in the etelcalcetide group than in the maxacalcitol group. |
| Hammer et al., 2021 [59] Protective effects of spironolactone on vascular calcification in chronic kidney disease | 40 weeks Multicenter, double-blind, placebo-controlled randomized clinical trial | N = 85 Hemodialysis patients:
| Serum calcification propensity was lower in hemodialysis patients treated with spironolactone, relative to placebo. |
| Wang et al., 2022 [60] Long-term effects of sevelamer on vascular calcification, arterial stiffness, and calcification propensity in patients receiving peritoneal dialysis: the randomized pilot SERENE (Sevelamer on Vascular Calcification, Arterial Stiffness) Trial | 2 years Prospective, multicenter, open-label, randomized pilot study | N = 60 Peritoneal dialysis patients:
| A combination of sevelamer (used as a second-line, low-dose therapy) and calcium carbonate had much the same effects on coronary artery calcium score, aortic valve calcium score, mitral annulus calcium score, pulse wave velocity, and T50 as first-line (high-dose) sevelamer therapy. After 2 years of treatment, there were no significant within-group and between-group differences in T50 or serum calcium and phosphate. |
| Tiong et al., 2022 [61] Effect of nutritional calcium and phosphate loading on calciprotein particle kinetics in adults with normal and impaired kidney function | 240 min Controlled study | N = 30 CKD patients (n = 14) and healthy controls (n = 16):
| In both groups, there was an early but transient within-group increase from fasting levels in T50. There were no pairwise between-group differences. There was a strong correlation between deviations from baseline in T50 and in fetuin-A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluquet, M.; Kamel, S.; Choukroun, G.; Liabeuf, S.; Laville, S.M. Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review. Toxins 2022, 14, 637. https://doi.org/10.3390/toxins14090637
Pluquet M, Kamel S, Choukroun G, Liabeuf S, Laville SM. Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review. Toxins. 2022; 14(9):637. https://doi.org/10.3390/toxins14090637
Chicago/Turabian StylePluquet, Maxime, Said Kamel, Gabriel Choukroun, Sophie Liabeuf, and Solène M. Laville. 2022. "Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review" Toxins 14, no. 9: 637. https://doi.org/10.3390/toxins14090637
APA StylePluquet, M., Kamel, S., Choukroun, G., Liabeuf, S., & Laville, S. M. (2022). Serum Calcification Propensity Represents a Good Biomarker of Vascular Calcification: A Systematic Review. Toxins, 14(9), 637. https://doi.org/10.3390/toxins14090637






