Influence of Agronomic Factors on Mycotoxin Contamination in Maize and Changes during a 10-Day Harvest-Till-Drying Simulation Period: A Different Perspective
Abstract
:1. Introduction
2. Results and Discussion
2.1. Influence of Agronomic Factors on the Maize at Harvest Date
2.2. Correlations between the Studied Variables at the Harvest Date
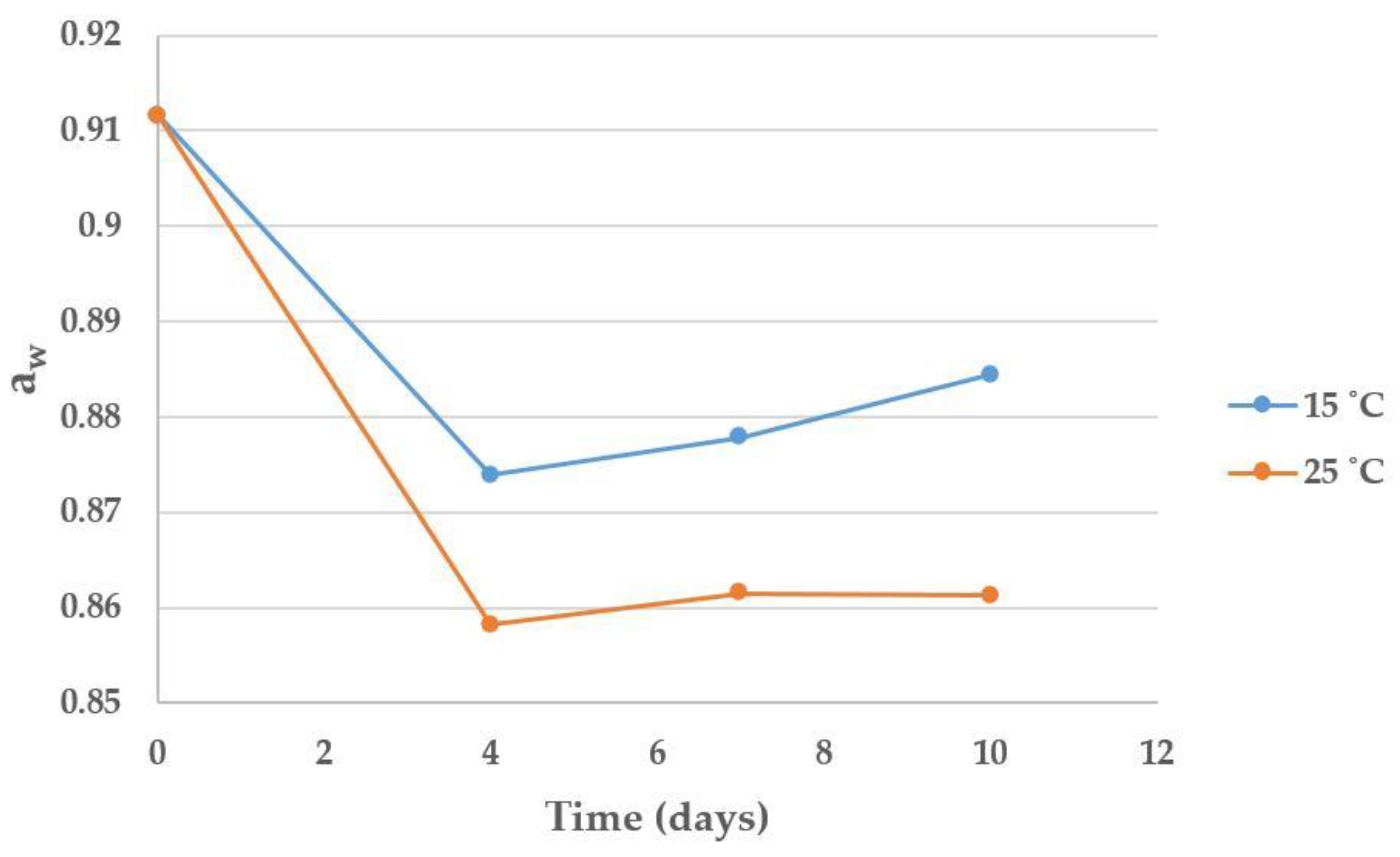
2.3. Effect of Time and Temperature on Maize Moisture, aw, Microbial Counts and Mycotoxin Contamination after Harvest
3. Conclusions
4. Materials and Methods
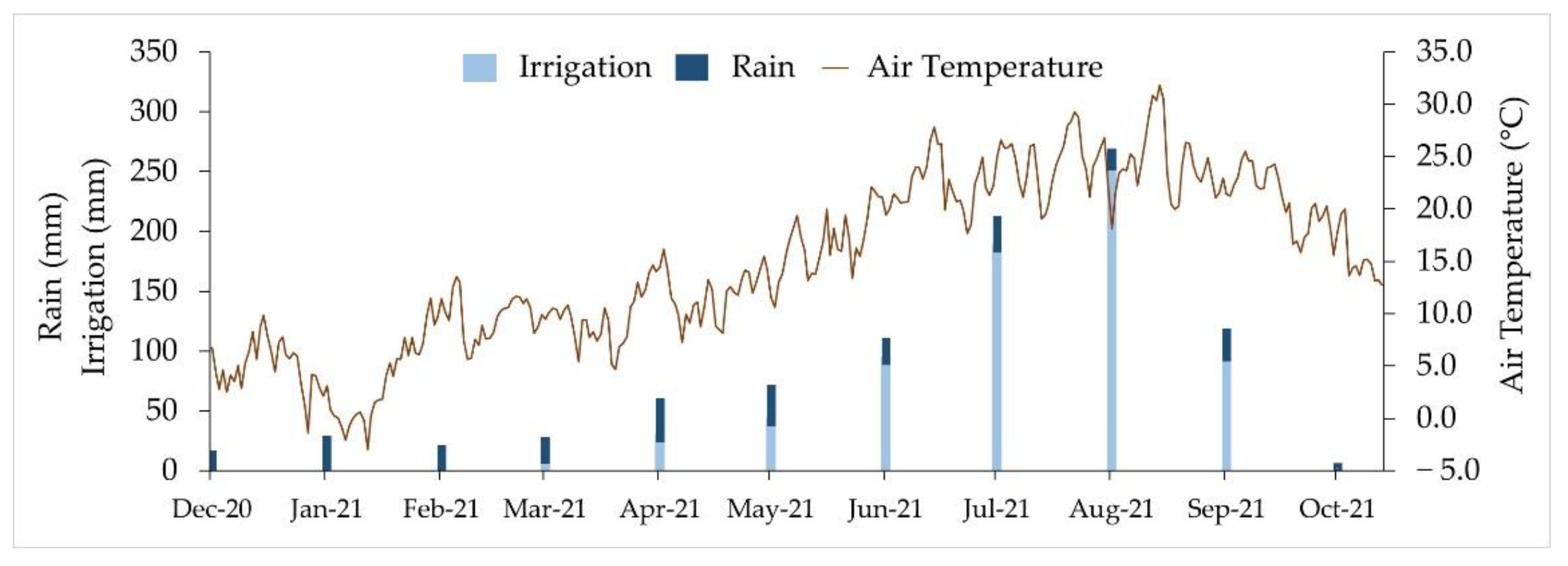
4.1. Climate and Soil Characteristics, Experimental Design and Crop Management
4.2. Maize Harvesting and Storage
4.3. Laboratory Determinations
4.3.1. Moisture
4.3.2. Water Activity (aw)
4.3.3. Total Fungal Contamination and Fusarium spp. Contamination
4.3.4. DON, FB1 and FB2 Contamination
Extraction of DON, FB1 and FB2
DON Extraction and Sample Preparation
DON HPLC-DAD Analysis
FB1 and FB2 Extraction and Sample Preparation
FB1 and FB2 HPLC-FLD Analysis
4.4. Reagents and Chemicals
4.5. Statistics
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO FAOSTAT. Production. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 March 2022).
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in Maize. Annu. Rev. Phytopathol. 2003, 41, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Tan, Y.; Liu, N.; Liao, Y.; Sun, C.; Wang, S.; Wu, A. Functional agents to biologically control deoxynivalenol contamination in cereal grains. Front. Microbiol. 2016, 7, 00395. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A Review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Folcher, L.; Delos, M.; Marengue, E.; Jarry, M.; Weissenberger, A.; Eychenne, N.; Regnault-Roger, C. Lower mycotoxin levels in Bt Maize grain. Agron. Sustain. Dev. 2010, 30, 711–719. [Google Scholar] [CrossRef]
- Lanza, F.E.; Zambolim, L.; Veras da Costa, R.; Vieira Queiroz, V.A.; Cota, L.V.; Dionísia da Silva, D.; Coelho de Souza, A.G.; Fontes Figueiredo, J.E. Prevalence of fumonisin-producing Fusarium species in Brazilian corn grains. Crop Prot. 2014, 65, 232–237. [Google Scholar] [CrossRef]
- Logrieco, A.; Bottalico, A.; Mulé, G.; Moretti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur. J. Plant Pathol. 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Qu, L.; Wang, L.; Ji, H.; Fang, Y.; Lei, P.; Zhang, X.; Jin, L.; Sun, D.; Dong, H. Toxic mechanism and biological detoxification of fumonisins. Toxins 2022, 14, 182. [Google Scholar]
- Chu, F.S.; Li, G.Y. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 1994, 60, 847–852. [Google Scholar] [CrossRef]
- Thiel, P.G.; Marasas, W.F.O.; Sydenham, E.W.; Shephard, G.S.; Gelderblom, W.C.A. The implications of naturally occurring levels of fumonisins in corn for human and animal health. Mycopathologia 1992, 117, 3–9. [Google Scholar] [CrossRef]
- Ueno, Y. Mode of action of trichothecenes. Pure Appl. Chem. 1977, 49, 1737–1745. [Google Scholar]
- Pestka, J.J. Deoxynivalenol: Toxicity, Mechanisms and Animal Health Risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Commission of the European Communities. Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- Commission of the European Communities. Commission Recommention of 17 August 2006 on the prevention and reduction of Fusarium toxins in cereals and cereal products. Off. J. Eur. Union 2006, L234, 35–40. [Google Scholar]
- Commission of the European Communities. Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Liu, Z.; Zhang, G.; Zhang, Y.; Jin, Q.; Zhao, J.; Li, J. Factors controlling mycotoxin contamination in Maize and food in the Hebei province, China. Agron. Sustain. Dev. 2016, 36, 39. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Channaiah, L.H.; Maier, D.E. Best Stored Maize Management Practices for the Prevention of Mycotoxin Contamination. In Mycotoxin Reduction in Grain Chains; Wiley: Hoboken, NJ, USA, 2014; pp. 78–88. [Google Scholar]
- Cotten, T.K.; Munkvold, G.P. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in Maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [CrossRef]
- Council for Agricultural Science and Technology. Mycotoxins—Risks in Plant, Animal and Human Systems; CAST: Ames, IA, USA, 2003; ISBN 1-887383-22-0. [Google Scholar]
- Edwards, S.G. Influence of agricultural practices on Fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Maiorano, A.; Blandino, M.; Reyneri, A.; Vanara, F. Effects of Maize residues on the Fusarium spp. Infection and deoxynivalenol (DON) contamination of wheat grain. Crop Prot. 2008, 27, 182–188. [Google Scholar] [CrossRef]
- Rossi, V.; Scandolara, A.; Battilani, P. Effect of environmental conditions on spore production by Fusarium verticillioides, the causal agent of maize ear rot. Eur. J. Plant Pathol. 2008, 123, 159–169. [Google Scholar] [CrossRef]
- Mansfield, M.A.; De Wolf, E.D.; Kuldau, G.A. Relationships between weather conditions, agronomic practices, and fermentation characteristics with deoxynivalenol content in fresh and ensiled Maize. Plant Dis. 2005, 89, 1151–1157. [Google Scholar] [CrossRef]
- Dill-Macky, R.; Jones, R.K. The effect of previous crop residues and tillage on fusarium head blight of wheat. Plant Dis. 2000, 84, 71–76. [Google Scholar] [CrossRef]
- Obst, A.; Lepschy-Von Gleissenthall, J.; Beck, R. On The etiology of Fusarium head blight of wheat in South Germany—Preceding crops, weather conditions for inoculum production and head infection, proneness of the crop to infection and mycotoxin production. Cereal Res. Commun. 1997, 25, 699–703. [Google Scholar] [CrossRef]
- Schöneberg, T.; Martin, C.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Keller, B.; Vogelgsang, S. Fusarium and mycotoxin spectra in Swiss barley are affected by various cropping techniques. Food Addit. Contam. Part A 2016, 33, 1608–1619. [Google Scholar] [CrossRef]
- Roucou, A.; Bergez, C.; Méléard, B.; Orlando, B. An agro-climatic approach to developing a national prevention tool for deoxynivalenol in French Maize-growing areas. Toxins 2022, 14, 74. [Google Scholar] [CrossRef]
- Supronienė, S.; Mankevičienė, A.; Kadžienė, G.; Kačergius, A.; Feiza, V.; Feizienė, D.; Semaškienė, R.; Dabkevičius, Z.; Tamošiūnas, K. The impact of tillage and fertilization on Fusarium infection and mycotoxin production in wheat grains. Žemdirbystė 2012, 99, 265–272. [Google Scholar]
- Kaukoranta, T.; Hietaniemi, V.; Rämö, S.; Koivisto, T.; Parikka, P. Contrasting responses of T-2, HT-2 and DON mycotoxins and Fusarium species in oat to climate, weather, tillage and cereal intensity. Eur. J. Plant Pathol. 2019, 155, 93–110. [Google Scholar] [CrossRef]
- Ramos, M.C.; Pareja-Sánchez, E.; Plaza-Bonilla, D.; Cantero-Martínez, C.; Lampurlanés, J. Soil sealing and soil water content under no-tillage and conventional tillage in irrigated corn: Effects on grain yield. Hydrol. Process. 2019, 33, 2095–2109. [Google Scholar] [CrossRef]
- Pareja-Sánchez, E.; Plaza-Bonilla, D.; Ramos, M.C.; Lampurlanés, J.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Long-term no-till as a means to maintain soil surface structure in an agroecosystem transformed into irrigation. Soil Tillage Res. 2017, 174, 221–230. [Google Scholar] [CrossRef]
- Arjmand Sajjadi, S.; Mahmoodabadi, M. Aggregate breakdown and surface seal development influenced by rain intensity, slope gradient and soil particle size. Solid Earth 2015, 6, 311–321. [Google Scholar] [CrossRef]
- Awadhwal, N.K.; Thierstein, G.E. Soil crust and its impact on crop establishment: A review. Soil Tillage Res. 1985, 5, 289–302. [Google Scholar] [CrossRef]
- Ramos, M.C.; Nacci, S.; Pla, I. Soil sealing and its influence on erosion rates for some soils in the Mediterranean area. Soil Sci. 2000, 165, 398–403. [Google Scholar] [CrossRef]
- Manstretta, V.; Rossi, V. Effects of weather variables on ascospore discharge from Fusarium graminearum perithecia. PLoS ONE 2015, 10, e0138860. [Google Scholar] [CrossRef]
- Sutton, J.C. Epidemiology of wheat head blight and Maize ear rot Caused by Fusarium graminearum. Can. J. Plant Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (Scab) in Small Grain Cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Nieminen, M.; Ketoja, E.; Mikola, J.; Terhivuo, J.; Sirén, T.; Nuutinen, V. Local land use effects and regional environmental limits on earthworm communities in Finnish arable landscapes. Ecol. Appl. 2011, 21, 3162–3177. [Google Scholar] [CrossRef]
- Briones, M.J.I.; Schmidt, O. Conventional tillage decreases the abundance and biomass of earthworms and alters their community structure in a global meta-analysis. Glob. Chang. Biol. 2017, 23, 4396–4419. [Google Scholar] [CrossRef]
- Ojha, R.B.; Devkota, D. Earthworms: “Soil and ecosystem engineers”—A review. World J. Agric. Res. 2014, 2, 257–260. [Google Scholar] [CrossRef]
- Imaz, M.J.; Virto, I.; Bescansa, P.; Enrique, A.; Fernandez-Ugalde, O.; Karlen, D.L. Soil quality indicator response to tillage and residue management on semi-arid Mediterranean cropland. Soil Tillage Res. 2010, 107, 17–25. [Google Scholar] [CrossRef]
- Chan, K.Y. An overview of some tillage impacts on earthworm population abundance and diversity—Implications for functioning in soils. Soil Tillage Res. 2001, 57, 179–191. [Google Scholar] [CrossRef]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Tillage Res. 2001, 61, 61–76. [Google Scholar] [CrossRef]
- Oldenburg, E.; Kramer, S.; Schrader, S.; Weinert, J. Impact of the Earthworm Lumbricus terrestris on the degradation of fusarium-infected and deoxynivalenol-contaminated wheat straw. Soil Biol. Biochem. 2008, 40, 3049–3053. [Google Scholar] [CrossRef]
- Schrader, S.; Kramer, S.; Oldenburg, E.; Weinert, J. Uptake of deoxynivalenol by earthworms from Fusarium-infected wheat straw. Mycotoxin Res. 2009, 25, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Santiveri Morata, F.; Cantero-Martínez, C.; Ojeda Domínguez, L.; Angás Pueyo, P. Técnicas de laboreo del suelo en zonas de secano semi-árido. Agric. Rev. Agropecu. Ganad. 2004, 866, 724–729. [Google Scholar]
- Bockus, W.W.; Shroyer, J.P. The impact of reduced tillage on soilborne plant pathogens. Annu. Rev. Phytopathol. 1998, 36, 485–500. [Google Scholar] [CrossRef]
- Dwyer, L.M.; Ma, B.; Stewart, D.W.; Hayhoe, H.; Balchin, D.; Culley, J.L.B.; Mcgovern, M. Root mass distribution under conventional and conservation tillage. Can. J. Soil Sci. 1996, 76, 23–28. [Google Scholar]
- Ono, E.; Moreno, E.; Ono, M.; Rossi, C.; Saito, G.; Vizoni, É.; Sugiura, Y.; Hirooka, E. Effect of cropping systems and crop successions on fumonisin levels in corn from Northern Paraná State, Brazil. Eur. J. Plant Pathol. 2011, 131, 653–660. [Google Scholar] [CrossRef]
- Ariño, A.; Herrera, M.; Juan, T.; Estopañán, G.; Carramiñana, J.; García, C.; Herrera, A. Influence of agricultural practices on the contamination of Maize by fumonisin mycotoxins. J. Food Prot. 2009, 72, 898–902. [Google Scholar] [CrossRef]
- Marocco, A.; Gavazzi, C.; Pietri, A.; Tabaglio, V. On Fumonisin incidence in monoculture Maize under no-till, conventional tillage and two nitrogen fertilisation levels. J. Sci. Food Agric. 2008, 88, 1217–1221. [Google Scholar] [CrossRef]
- Marocco, A.; Tabaglio, V.; Pietri, A.; Gavazzi, C. Monoculture Maize (Zea mays L.) cropped under conventional tillage, no-tillage and n fertilization: (II) Fumonisin incidence on kernels. Ital. J. Agron. 2009, 4, 69–75. [Google Scholar] [CrossRef]
- Battilani, P.; Pietri, A.; Barbano, C.; Scandolara, A.; Bertuzzi, T.; Marocco, A. Logistic regression modeling of cropping systems to predict fumonisin contamination in Maize. J. Agric. Food Chem. 2008, 56, 10433–10438. [Google Scholar] [CrossRef]
- Madege, R.R.; Audenaert, K.; Kimanya, M.; Tiisekwa, B.; De Meulenaer, B.; Bekaert, B.; Landschoot, S.; Haesaert, G. Control of Fusarium verticillioides (Sacc.) nirenberg and fumonisins by using a combination of crop protection products and fertilization. Toxins 2018, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Blandino, M.; Vanara, F.; Reyneri, A. Influence of nitrogen fertilization on mycotoxin contamination of Maize kernels. Crop Prot. 2008, 27, 222–230. [Google Scholar] [CrossRef]
- Carbas, B.; Simões, D.; Soares, A.; Freitas, A.; Ferreira, B.; Carvalho, A.R.F.; Silva, A.S.; Pinto, T.; Diogo, E.; Andrade, E.; et al. Occurrence of Fusarium spp. in Maize grain harvested in Portugal and accumulation of related mycotoxins during storage. Foods 2021, 10, 375. [Google Scholar] [CrossRef]
- Cao, A.; Santiago, R.; Ramos, A.J.; Marín, S.; Reid, L.M.; Butrón, A. Environmental factors related to fungal infection and fumonisin accumulation during the development and drying of white Maize kernels. Int. J. Food Microbiol. 2013, 164, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in cereals: Occurrence, legislation, factors promoting the appearance and their management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Daou, R.; Joubrane, K.; Maroun, R.G.; Khabbaz, L.R.; Ismail, A.; El Khoury, A. Mycotoxins: Factors influencing production and control strategies. AIMS Agric. Food 2021, 6, 416–447. [Google Scholar] [CrossRef]
- Lanza, F.E.; Zambolim, L.; Costa, R.V.; Figueiredo, J.E.F.; Silva, D.D.; Queiroz, V.A.V.; Guimarães, E.A.; Cota, L.V. Symptomatological aspects associated with fungal incidence and fumonisin levels in corn kernels. Trop. Plant Pathol. 2017, 42, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Comerio, R.M.; Fernández Pinto, V.E.; Vaamonde, G. Influence of water activity on deoxynivalenol accumulation in wheat. Mycotoxin Res. 1999, 15, 24–32. [Google Scholar] [CrossRef]
- Ramirez, M.L.; Chulze, S.; Magan, N. Temperature and water activity effects on growth and temporal deoxynivalenol production by two argentinean strains of Fusarium graminearum on irradiated wheat grain. Int. J. Food Microbiol. 2006, 106, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Parra, R.; Geisen, R.; Magan, N. Modelling the relationship between environmental factors, transcriptional genes and deoxynivalenol mycotoxin production by strains of two Fusarium Species. J. R. Soc. Interface 2011, 8, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Soil Science Division Staff. Soil Survey Manual; Ditzler, C., Scheffe, K., Monger, H.C., Eds.; USDA Handbook No 18; Government Printing Office: Washington, DC, USA, 2017.
- Dastane, N.G. Effective Rainfall in Irrigated Agriculture; Irrigation and Drainage Paper; FAO: Rome, Italy, 1978; Volume 25, ISBN 925100272X. [Google Scholar]
- Borràs-Vallverdú, B.; Ramos, A.J.; Marín, S.; Sanchis, V.; Rodríguez-Bencomo, J.J. Deoxynivalenol degradation in wheat kernels by exposition to ammonia vapours: A tentative strategy for detoxification. Food Control 2020, 118, 107444. [Google Scholar] [CrossRef]
- Belajova, E.; Rauova, D. Single laboratory-validated HPLC methods for determination of ochratoxin A, fumonisin B1 and B2, zearalenone and deoxynivalenol in cereals and cereal-based foods. J. Food Nutr. Res. 2010, 49, 57–68. [Google Scholar]
- Castellá, G.; Bragulat, M.R.; Rubiales, M.V.; Cabañes, F.J. Malachite green agar, a new Selective medium for Fusarium spp. Mycopathologia 1997, 137, 173–178. [Google Scholar] [CrossRef]

| FAO Maturity Group/Cropping System | Fertilization | Tillage System | Average DON Contamination (μg Toxin kg−1 Maize) |
|---|---|---|---|
| 400/SC | 0 N | DD | 440 |
| IT | 2848 | ||
| High N | DD | 566 | |
| IT | 4406 | ||
| 700/LC | 0 N | DD | 654 |
| IT | 791 | ||
| High N | DD | 236 | |
| IT | 2734 |
| SS | df | MS | F | Sig. | |
|---|---|---|---|---|---|
| Crop diversification | 3.697 | 1 | 3.697 | 1.292 | 0.289 |
| N. fert. rate | 2.574 | 1 | 2.574 | 0.900 | 0.371 |
| Tillage system | 19.729 | 1 | 19.729 | 6.897 | 0.030 |
| Crop diversification × N fert. rate | 0.006 | 1 | 0.006 | 0.002 | 0.964 |
| Crop diversification × Tillage system | 3.260 | 1 | 3.260 | 1.140 | 0.317 |
| N fert. Rate × Tillage system | 3.598 | 1 | 3.598 | 1.258 | 0.295 |
| Crop diversification × N fert. Rate × Tillage system | 0.216 | 1 | 0.216 | 0.075 | 0.791 |
| Temperature | DON Concentration (μg DON kg−1 Maize) | ||
|---|---|---|---|
| Day 0 | Day 5 | Day 10 | |
| 15 °C | 1584 1932 | 2367 2983 | 2649 2349 |
| 25 °C | 1584 1932 | 3771 3597 | 3469 4300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borràs-Vallverdú, B.; Ramos, A.J.; Cantero-Martínez, C.; Marín, S.; Sanchis, V.; Fernández-Ortega, J. Influence of Agronomic Factors on Mycotoxin Contamination in Maize and Changes during a 10-Day Harvest-Till-Drying Simulation Period: A Different Perspective. Toxins 2022, 14, 620. https://doi.org/10.3390/toxins14090620
Borràs-Vallverdú B, Ramos AJ, Cantero-Martínez C, Marín S, Sanchis V, Fernández-Ortega J. Influence of Agronomic Factors on Mycotoxin Contamination in Maize and Changes during a 10-Day Harvest-Till-Drying Simulation Period: A Different Perspective. Toxins. 2022; 14(9):620. https://doi.org/10.3390/toxins14090620
Chicago/Turabian StyleBorràs-Vallverdú, Bernat, Antonio J. Ramos, Carlos Cantero-Martínez, Sonia Marín, Vicente Sanchis, and Jesús Fernández-Ortega. 2022. "Influence of Agronomic Factors on Mycotoxin Contamination in Maize and Changes during a 10-Day Harvest-Till-Drying Simulation Period: A Different Perspective" Toxins 14, no. 9: 620. https://doi.org/10.3390/toxins14090620
APA StyleBorràs-Vallverdú, B., Ramos, A. J., Cantero-Martínez, C., Marín, S., Sanchis, V., & Fernández-Ortega, J. (2022). Influence of Agronomic Factors on Mycotoxin Contamination in Maize and Changes during a 10-Day Harvest-Till-Drying Simulation Period: A Different Perspective. Toxins, 14(9), 620. https://doi.org/10.3390/toxins14090620






