Bioactive Metabolite Production in the Genus Pyrenophora (Pleosporaceae, Pleosporales)
Abstract
1. Introduction
2. Biology, Pathogenicity, and Toxin Production of Pyrenophora spp.
2.1. Pyrenophora teres
2.1.1. Biology and Pathogenicity of Pyrenophora teres
2.1.2. Phytotoxins Produced by Pyrenophora teres
2.2. Pyrenophora tritici-repentis
2.2.1. Biology and Pathogenicity of P. tritici-repentis
2.2.2. Phytotoxins Produced by Pyrenophora tritici-repentis
2.3. Pyrenophora semeniperda
2.3.1. Biology and Pathogenicity of Pyrenophora semeniperda
2.3.2. Phytotoxins Produced by Pyrenophora semeniperda
2.4. Other Pyrenophora spp.
2.4.1. Biology and Pathogenicity of other Pyrenophora Species
2.4.2. Phytotoxins Produced by other Pyrenophora spp.
3. Classification of the Toxins Produced by Pyrenophora spp. according to Their Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Index Fungorum. Available online: http://www.indexfungorum.org (accessed on 16 July 2022).
- Zhang, G.; Berbee, M.L. Pyrenophora phylogenetics inferred from ITS and glyceradehyde-3-phosphate dehydrogenase gene sequences. Mycologia 2001, 93, 1048–1063. [Google Scholar] [CrossRef]
- Kodsueb, R.; Dhanasekaran, V.; Aptroot, A.; Lumyong, S.; McKenzie, E.H.; Hyde, K.D.; Jeewon, R. The family Pleosporaceae: Intergeneric relationships and phylogenetic perspectives based on sequence analyses of partial 28S rDNA. Mycologia 2006, 98, 571–583. [Google Scholar] [CrossRef]
- Ariyawansa, H.A.; Thambugala, K.M.; Manamgoda, D.S.; Jayawardena, R.; Camporesi, E.; Boonmee, S.; Wanasinghe, D.N.; Phookamsak, R.; Hongsanan, S.; Singtripop, C.; et al. Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers. 2015, 71, 85–139. [Google Scholar] [CrossRef]
- Ciuffetti, L.M.; Manning, V.A.; Pandelova, I.; Faris, J.D.; Friesen, T.L.; Strelkov, S.E.; Weber, G.L.; Goodwin, S.B.; Wolpert, T.J.; Figueroa, M. Pyrenophora tritici-repentis: A plant pathogenic fungus with global impact. In Genomics of Plant-Associated Fungi: Monocot Pathogens; Dean, R.A., Lichens-Park, A., Kole, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–39. [Google Scholar]
- Clare, S.J.; Wyatt, N.A.; Brueggeman, R.S.; Friesen, T.L. Research advances in the Pyrenophora teres–barley interaction. Mol. Plant Pathol. 2020, 21, 272–288. [Google Scholar] [CrossRef]
- Backes, A.; Guerriero, G.; Ait Barka, E.; Jacquard, C. Pyrenophora teres: Taxonomy, morphology, interaction with barley, and mode of control. Front. Plant Sci. 2021, 12, 614951. [Google Scholar] [CrossRef]
- Meyer, S.E.; Nelson, D.L.; Clement, S.; Beckstead, J. Cheatgrass (Bromus tectorum) biocontrol using indigenous fungal pathogens. In Proceedings-Shrublands under Fire: Disturbance and Recovery in a Changing World, Cedar City, UT, USA, 6–8 June 2006; Kitchen, S.G., Pendleton, R.L., Monaco, T.A., Vernon, J.C., Eds.; Proc. RMRS-P-52.; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2008; pp. 61–67. [Google Scholar]
- Meyer, S.E.; Beckstead, J.; Pearce, J. Community ecology of fungal pathogens on Bromus tectorum. In Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US: Causes, Consequences, and Management Implications; Germino, M.J., Chambers, J.C., Brown, C.S., Eds.; Series on Environmental Management; Springer: Berlin/Heidelberg, Germany, 2016; pp. 193–221. [Google Scholar]
- Akhavan, A.; Turkington, T.; Askarian, H.; Tekauz, A.; Xi, K.; Tucker, J.R.; Kutcher, H.R.; Strelkov, S.E. Virulence of Pyrenophora teres populations in western Canada. Can. J. Plant Pathol. 2016, 38, 183–196. [Google Scholar] [CrossRef]
- Tekauz, A. A numerical scale to classify reactions of barley to Pyrenophora teres. Can. J. Plant Pathol. 1985, 7, 181–183. [Google Scholar] [CrossRef]
- Liu, Z.; Ellwood, S.R.; Oliver, R.P.; Friesen, T.L. Pyrenophora teres: Profile of an increasingly damaging barley pathogen. Mol. Plant Pathol. 2011, 12, 1–19. [Google Scholar] [CrossRef]
- Moolhuijzen, P.M.; Muria-Gonzalez, M.J.; Syme, R.; Rawlinson, C.; See, P.T.; Moffat, C.S.; Ellwood, S.R. Expansion and conservation of biosynthetic gene clusters in pathogenic Pyrenophora spp. Toxins 2020, 12, 242. [Google Scholar] [CrossRef]
- Smedegård-Petersen, V. Isolation of two toxins produced by Pyrenophora teres and their significance in disease development of net-spot blotch of barley. Physiol. Plant Pathol. 1977, 10, 203–211. [Google Scholar] [CrossRef]
- Bach, E.; Christensen, S.; Dalgaard, L.; Larsen, P.O.; Olsen, C.E.; Smedegård-Petersen, V. Structures, properties and relationship to the aspergillomarasmines of toxins produced by Pyrenophora teres. Physiol. Plant Pathol. 1979, 14, 41–46. [Google Scholar] [CrossRef]
- Friis, P.; Olsen, C.E.; Møller, B.L. Toxin production in Pyrenophora teres, the ascomycete causing the net-spot blotch disease of barley (Hordeum vulgare L.). J. Biol. Chem. 1991, 266, 13329–13335. [Google Scholar] [CrossRef]
- Haenni, A.L.; Robert, M.; Vetter, W.; Roux, L.; Barbier, M.; Lederer, E. Structure chimique des aspergillomarasmines A et B. Helv. Chim. Acta 1965, 48, 729–750. [Google Scholar] [CrossRef]
- Arai, K.; Ashikawa, N.; Nakakita, Y.; Matsuura, A.; Ashizawa, N.; Munekata, M. Aspergillomarasmine A and B, potent microbial inhibitors of endothelin-converting enzyme. Biosci. Biotechnol. Biochem 1993, 57, 1944–1945. [Google Scholar] [CrossRef][Green Version]
- Liao, D.; Yang, S.; Wang, J.; Zhang, J.; Hong, B.; Wu, F.; Lei, X. Total synthesis and structural reassignment of aspergillomarasmine A. Angew. Chem. 2016, 128, 4363–4367. [Google Scholar] [CrossRef]
- Albu, S.A.; Koteva, K.; King, A.M.; Al-Karmi, S.; Wright, G.D.; Capretta, A. Total synthesis of aspergillomarasmine A and related compounds: A sulfamidate approach enables exploration of structure–activity relationships. Angew. Chem. 2016, 128, 13453–13456. [Google Scholar] [CrossRef]
- Koteva, K.; King, A.M.; Capretta, A.; Wright, G.D. Total synthesis and activity of the metallo-β-lactamase inhibitor aspergillomarasmine A. Angew. Chem. Int. Ed. 2016, 55, 2210–2212. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Bai, Y.; Guo, Q.; Zhou, J.; Lei, X. Total syntheses of natural metallophores staphylopine and aspergillomarasmine A. J. Org. Chem. 2017, 82, 13643–13648. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, J.; Saifuddin, M.; Cruiming, G.; Tepper, P.G.; Poelarends, G.J. Chemoenzymatic asymmetric synthesis of the metallo-β-lactamase inhibitor aspergillomarasmine A and related aminocarboxylic acids. Nat. Catal. 2018, 1, 186–191. [Google Scholar] [CrossRef]
- King, A.M.; Reid-Yu, S.A.; Wang, W.; King, D.T.; De Pascale, G.; Strynadka, N.C.; Walsh, T.R.; Coombes, B.K.; Wright, G.D. Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 2014, 510, 503–506. [Google Scholar] [CrossRef]
- Sychantha, D.; Rotondo, C.M.; Tehrani, K.H.; Martin, N.I.; Wright, G.D. Aspergillomarasmine A inhibits metallo-β-lactamases by selectively sequestering Zn2+. J. Biol. Chem. 2021, 297, 100918. [Google Scholar] [CrossRef] [PubMed]
- Weiergang, I.; Jørgensen, H.L.; Møller, I.M.; Friis, P.; Smedegaard-Petersen, V. Correlation between sensitivity of barley to Pyrenophora teres toxins and susceptibility to the fungus. Physiol. Mol. Plant Pathol. 2002, 60, 121–129. [Google Scholar] [CrossRef]
- Sarpeleh, A.; Tate, M.E.; Wallwork, H.; Catcheside, D.; Able, A.J. Characterisation of low molecular weight phytotoxins isolated from Pyrenophora teres. Physiol. Mol. Plant Pathol. 2008, 73, 154–162. [Google Scholar] [CrossRef]
- Ballio, A.; Bottalico, A.; Buonocore, V.; Carilli, A.; Di Vittorio, V.; Graniti, A. Production and isolation of aspergillomarasmin B (lycomarasmic acid) from cultures of Colletotrichum gloeosporioides Penz. (Gloeosporium olivarum Aim.). Phytopathol. Mediterr. 1969, 8, 187–196. [Google Scholar]
- Nukina, M.; Ikeda, M.; Sassa, T. Two new pyrenolides, fungal morphogenic substances produced by Pyrenophora teres (Diedicke) Drechsler. Agric. Biol. Chem. 1980, 44, 2761–2762. [Google Scholar] [CrossRef][Green Version]
- Nukina, M.; Sassa, T.; Ikeda, M. A new fungal morphogenic substance, pyrenolide A from Pyrenophora teres. Tetrahedron Lett. 1980, 21, 301–302. [Google Scholar] [CrossRef]
- Venkatasubbaiah, P.; Chilton, W.S. Phytotoxins of Ascochyta hyalospora, causal agent of lambsquarters leaf spot. J. Nat. Prod. 1992, 55, 461–467. [Google Scholar] [CrossRef]
- Greve, H.; Schupp, P.J.; Eguereva, E.; Kehraus, S.; König, G.M. Ten-membered lactones from the marine-derived fungus Curvularia sp. J. Nat. Prod. 2008, 71, 1651–1653. [Google Scholar] [CrossRef]
- Asaoka, M.; Naito, S.; Takei, H. Total synthesis of (±)-pyrenolide B. Tetrahedron Lett. 1985, 26, 2103–2106. [Google Scholar] [CrossRef]
- Suzuki, S.; Tanaka, A.; Yamashita, K. Synthesis and biological activity of (+)-pyrenolide B. Agric. Biol. Chem. 1987, 51, 3095–3098. [Google Scholar]
- Moricz, A.; Gassmann, E.; Bienz, S.; Hesse, M. Synthesis of (±)-pyrenolide B. Helv. Chim. Acta 1995, 78, 663–669. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Prowse, K.S. The singlet oxygen conversion of oxazoles to triamides. Application in the synthesis of (±)-pyrenolide C. Assignment of stereochemistry. Tetrahedron 1992, 48, 8199–8212. [Google Scholar] [CrossRef]
- Nukina, M.; Hirota, H. Pyrenolide D, a new cytotoxic fungal metabolite from Pyrenophora teres. Biosci. Biotechnol. Biochem. 1992, 56, 1158–1159. [Google Scholar] [CrossRef]
- Engstrom, K.M.; Mendoza, M.R.; Navarro-Villalobos, M.; Gin, D.Y. Total synthesis of (+)-pyrenolide D. Angew. Chem. Int. Ed. 2001, 40, 1128–1130. [Google Scholar] [CrossRef]
- Thirupathi, B.; Reddy, P.P.; Mohapatra, D.K. A carbohydrate-based total syntheses of (+)-pyrenolide D and (−)-4-epi-pyrenolide D. J. Org. Chem. 2011, 76, 9835–9840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, J.; Du, Y. A concise total synthesis of (+)-pyrenolide D. Tetrahedron Lett. 2013, 54, 3278–3280. [Google Scholar] [CrossRef]
- Markovič, M.; Lopatka, P.; Koóš, P.; Gracza, T. Asymmetric formal synthesis of (+)-pyrenolide D. Synthesis 2014, 46, 817–821. [Google Scholar]
- Ogawa, Y.; Kato, M.; Sasaki, I.; Sugimura, H. Total synthesis of (+)-pyrenolide D. J. Org. Chem. 2018, 83, 12315–12319. [Google Scholar] [CrossRef]
- Coval, S.J.; Hradil, C.M.; Lu, H.S.; Clardy, J.; Satouri, S.; Strobel, G.A. Pyrenoline-A and-B, two new phytotoxins from Pyrenophora teres. Tetrahedron Lett. 1990, 31, 2117–2120. [Google Scholar] [CrossRef]
- Benali, D.; Lyamani, A.; Zaid, A.; Samih, M.; Haloui, N. Cinétique de production de toxines de type pyrenoline A et pyrenoline B par des isolats marocains de Pyrenophora teres. Phytopathol. Mediterr. 1995, 34, 120–125. [Google Scholar]
- Engström, K.; Brishammar, S.; Svensson, C.; Bengtsson, M.; Andersson, R. Anthraquinones from some Drechslera species and Bipolaris sorokiniana. Mycol. Res. 1993, 97, 381–384. [Google Scholar] [CrossRef]
- Wakuliński, W.; Kachlicki, P.; Sobiczewski, P.; Schollenberger, M.; Zamorski, C.; Łotocka, B.; Sarova, J. Catenarin production by isolates of Pyrenophora tritici-repentis (Died.) Drechsler and its antimicrobial activity. J. Phytopathol. 2003, 151, 74–79. [Google Scholar] [CrossRef]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Phenolic glucosides and chromane analogs from the insect fungus Conoideocrella krungchingensis BCC53666. Tetrahedron 2019, 75, 3463–3471. [Google Scholar] [CrossRef]
- Anslow, W.K.; Raistrick, H. Synthesis of catenarin (1:4:5:7-tetrahydroxy-2-methylanthraquinone), a metabolic product of species of Helminthosporium. Biochem. 1941, 35, 1006–1010. [Google Scholar]
- Chandrasenan, K.; Neelakantan, S.; Seshadri, T.R. A new synthesis of catenarin and erythroglaucin. Proc. Indian Natl. Sci. Acad. 1960, 51, 296–300. [Google Scholar] [CrossRef]
- Bouras, N.; Strelkov, S.E. The anthraquinone catenarin is phytotoxic and produced in leaves and kernels of wheat infected by Pyrenophora tritici-repentis. Physiol. Mol. Plant Pathol. 2008, 72, 87–95. [Google Scholar] [CrossRef]
- Martorell, M.; Castro, N.; Victoriano, M.; Capó, X.; Tejada, S.; Vitalini, S.; Pezzani, R.; Sureda, A. An update of anthraquinone derivatives emodin, diacerein, and catenarin in diabetes. Evid. Based Complementary Altern. Med. 2021, 2021, 3313419. [Google Scholar] [CrossRef]
- Mehrabi, R.; Bahkali, A.H.; Abd-Elsalam, K.A.; Moslem, M.; M’Barek, S.B.; Gohari, A.M.; Jashni, M.K.; Stergiopoulos, I.; Kema, G.H.J.; de Wit, P.J.G.M. Horizontal gene and chromosome transfer in plant pathogenic fungi affecting host range. FEMS Microbiol. Rev. 2011, 35, 542–554. [Google Scholar] [CrossRef]
- Friesen, T.L.; Stukenbrock, E.H.; Liu, Z.H.; Meinhardt, S.; Ling, H.; Faris, J.D.; Rasmussen, J.B.; Solomon, P.S.; McDonald, B.A.; Oliver, R.P. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat. Genet. 2006, 38, 953–956. [Google Scholar] [CrossRef]
- Antoni, E.A.; Rybak, K.; Tucker, M.P.; Hane, J.K.; Solomon, P.S.; Drenth, A.; Shankar, M.; Oliver, R.P. Ubiquity of ToxA and absence of ToxB in Australian populations of Pyrenophora tritici-repentis. Australas. Plant Pathol. 2010, 39, 63–68. [Google Scholar] [CrossRef]
- Leisova-Syobodova, L.; Hanzalova, A.; Kucera, L. Expansion and variability of the Ptr Tox A gene in populations of Pyrenophora tritici-repentis and Pyrenophora teres. J. Plant Pathol. 2010, 92, 729–735. [Google Scholar]
- Rawlinson, C.; See, P.T.; Moolhuijzen, P.; Li, H.; Moffat, C.S.; Chooi, Y.H.; Oliver, R.P. The identification and deletion of the polyketide synthase-nonribosomal peptide synthase gene responsible for the production of the phytotoxic triticone A/B in the wheat fungal pathogen Pyrenophora tritici-repentis. Environ. Microbiol. 2019, 21, 4875–4886. [Google Scholar] [PubMed]
- Ballance, G.M.; Lamari, L.; Bernier, C.C. Purification and characterization of a host-selective necrosis toxin from Pyrenophora tritici-repentis. Physiol. Mol. Plant Pathol. 1989, 35, 203–213. [Google Scholar] [CrossRef]
- Strelkov, S.E.; Lamari, L.; Ballance, G.M. Characterization of a host-specific protein toxin (Ptr ToxB) from Pyrenophora tritici-repentis. Mol. Plant-Microbe Interact. 1999, 12, 728–732. [Google Scholar] [CrossRef]
- Pandelova, I.; Figueroa, M.; Wilhelm, L.J.; Manning, V.A.; Mankaney, A.N.; Mockler, T.C.; Ciuffetti, L.M. Host-selective toxins of Pyrenophora tritici-repentis induce common responses associated with host susceptibility. PLoS ONE 2012, 7, e40240. [Google Scholar]
- Andrie, R.M.; Schoch, C.L.; Hedges, R.; Spatafora, J.W.; Ciuffetti, L.M. Homologs of ToxB, a host-selective toxin gene from Pyrenophora tritici-repentis, are present in the genome of sister-species Pyrenophora bromi and other members of the Ascomycota. Fungal Genet. Biol. 2008, 45, 363–377. [Google Scholar]
- Effertz, R.J.; Meinhardt, S.W.; Anderson, J.A.; Jordahl, J.G.; Francl, L.J. Identification of a chlorosis-inducing toxin from Pyrenophora tritici-repentis and the chromosomal location of an insensitivity locus in wheat. Phytopathology 2002, 92, 527–533. [Google Scholar]
- Betts, M.F.; Manning, V.A.; Cardwell, K.B.; Pandelova, I.; Ciuffetti, L.M. The importance of the N-terminus for activity of Ptr ToxB, a chlorosis-inducing host-selective toxin produced by Pyrenophora tritici-repentis. Physiol. Mol. Plant Pathol. 2011, 75, 138–145. [Google Scholar]
- Shi, G.; Kariyawasam, G.; Liu, S.; Leng, Y.; Zhong, S.; Ali, S.; Moolhuijzen, P.; Moffat, C.S.; Rasmussen, J.B.; Friesen, T.L.; et al. A conserved hypothetical gene is required but not sufficient for Ptr ToxC production in Pyrenophora tritici-repentis. Mol. Plant-Microbe Interact. 2022, 35, 336–348. [Google Scholar] [CrossRef]
- Hallock, Y.F.; Lu, H.S.; Clardy, J.; Strobel, G.A.; Sugawara, F.; Samsoedin, R.; Yoshida, S. Triticones, spirocyclic lactams from the fungal plant pathogen Drechslera tritici-repentis. J. Nat. Prod. 1993, 56, 747–754. [Google Scholar] [CrossRef]
- Sugawara, F.; Takahashi, N.; Strobel, G.A.; Strobel, S.A.; Lu, H.S.; Clardy, J. Triticones A and B, novel phytotoxins from the plant pathogenic fungus Drechslera tritici-repentis. J. Am. Chem. Soc. 1988, 110, 4086–4087. [Google Scholar] [CrossRef]
- Kenfield, D.; Strobel, S.; Sugawara, F.; Berglund, D.; Strobel, G. Triticone A: A novel bioactive lactam with potential as a molecular probe. Biochem. Biophys. Res. Commun. 1988, 157, 174–182. [Google Scholar] [CrossRef]
- Shinohara, C.; Chikanishi, T.; Nakashima, S.; Hashimoto, A.; Hamanaka, A.; Endo, A.; Hasumi, K. Enhancement of fibrinolytic activity of vascular endothelial cells by chaetoglobosin A, crinipellin B, geodin and triticone B. J. Antibiot. 2000, 53, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Hilario, F.; Polinário, G.; de Amorim, M.R.; de Sousa Batista, V.; do Nascimento, N.M., Jr.; Araújo, A.R.; Baubab, T.M.; Dos Santos, L.C. Spirocyclic lactams and curvulinic acid derivatives from the endophytic fungus Curvularia lunata and their antibacterial and antifungal activities. Fitoterapia 2020, 141, 104466. [Google Scholar] [CrossRef] [PubMed]
- Bouras, N.; Strelkov, S.E. Influence of carbon source on growth and mycotoxin production by isolates of Pyrenophora tritici-repentis from wheat. Can. J. Microbiol. 2010, 56, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.E.; Quinney, D.; Nelson, D.L.; Weaver, J. Impact of the pathogen Pyrenophora semeniperda on Bromus tectorum seedbank dynamics in North American cold deserts. Weed Res. 2007, 47, 54–62. [Google Scholar] [CrossRef]
- Paul, A.R. The production of Pyrenophora semeniperda in culture. Trans. Br. Mycol. Soc. 1969, 52, 373–379. [Google Scholar] [CrossRef]
- Medd, R.W.; Murray, G.M.; Pickering, D.I. Review of the epidemiology and economic importance of Pyrenophora semeniperda. Australas. Plant Pathol. 2003, 32, 539–550. [Google Scholar] [CrossRef]
- Medd, R.W.; Campbell, M.A. Grass seed infection following inundation with Pyrenophora semeniperda. Biocontrol. Sci. Technol. 2005, 15, 21–36. [Google Scholar] [CrossRef]
- Beckstead, J.; Meyer, S.E.; Molder, C.J.; Smith, C. A race for survival: Can Bromus tectorum seeds escape Pyrenophora semeniperda-caused mortality by germinating quickly? Ann. Bot. 2007, 99, 907–914. [Google Scholar] [CrossRef]
- Allen, P.S.; Finch-Boekweg, H.; Meyer, S.E. A proposed mechanism for high pathogen-caused mortality in the seed bank of an invasive annual grass. Fungal Ecol. 2018, 35, 108–115. [Google Scholar] [CrossRef]
- Meyer, S.E.; Stewart, T.E.; Clement, S. The quick and the deadly: Growth vs virulence in a seed bank pathogen. New Phytol. 2010, 187, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Evidente, A.; Meyer, S.; Nicholson, J.; Muñoz, A. Effect of strain and cultural conditions on the production of cytochalasin B by the potential mycoherbicide Pyrenophora semeniperda (Pleosporaceae, Pleosporales). Biocontrol. Sci. Technol. 2014, 24, 53–64. [Google Scholar] [CrossRef]
- Meyer, S.E.; Masi, M.; Clement, S.; Davis, T.L.; Beckstead, J. Mycelial growth rate and toxin production in the seed pathogen Pyrenophora semeniperda: Resource trade-offs and temporally varying selection. Plant Pathol. 2015, 64, 1450–1460. [Google Scholar] [CrossRef]
- Boose, D.; Harrison, S.; Clement, S.; Meyer, S. Population genetic structure of the seed pathogen Pyrenophora semeniperda on Bromus tectorum in western North America. Mycologia 2011, 103, 85–93. [Google Scholar] [CrossRef]
- Coleman, C.E.; Meyer, S.E.; Ricks, N. Mating system complexity and cryptic speciation in the seed bank pathogen Pyrenophora semeniperda. Plant Pathol. 2019, 68, 369–382. [Google Scholar] [CrossRef]
- Beckstead, J.; Meyer, S.E.; Reinhart, K.O.; Bergen, K.M.; Holden, S.R.; Boekweg, H.F. Factors affecting host range in a generalist seed pathogen of semi-arid shrublands. Plant Ecol. 2014, 15, 427–440. [Google Scholar] [CrossRef]
- Beckstead, J.; Meyer, S.E.; Ishizuka, T.S.; McEvoy, K.M.; Coleman, C.E. Lack of host specialization on winter annual grasses in the fungal seed bank pathogen Pyrenophora semeniperda. PLoS ONE 2016, 11, e0151058. [Google Scholar] [CrossRef]
- Soliai, M.M.; Meyer, S.E.; Udall, J.A.; Elzinga, D.E.; Hermansen, R.A.; Bodily, P.M.; Hart, A.A.; Coleman, C.E. De novo genome assembly of the fungal plant pathogen Pyrenophora semeniperda. PLoS ONE 2014, 9, e87045. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Motta, A. Cytochalasins Z1, Z2 and Z3, three 24-oxa[14]cytochalasans produced by Pyrenophora semeniperda. Phytochemistry 2002, 60, 45–53. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Armstrong, J.J.; Speake, R.N.; Turner, W.B. The cytochalasins, a new class of biologically active mould metabolites. Chem. Comm. 1967, 26–27. [Google Scholar] [CrossRef]
- Scherlach, K.; Boettger, D.; Remme, N.; Hertweck, C. The chemistry and biology of cytochalasans. Nat. Prod. Rep. 2010, 27, 869–886. [Google Scholar] [CrossRef]
- Campbell, M.A.; Medd, R.W.; Brown, J.B. Optimizing conditions for growth and sporulation of Pyrenophora semeniperda. Plant Pathol. 2003, 52, 448–454. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Andolfi, A.; Cimmino, A.; Evidente, A. Spirostaphylotrichin W, a spirocyclic γ-lactam isolated from liquid culture of Pyrenophora semeniperda, a potential mycoherbicide for cheatgrass (Bromus tectorum) biocontrol. Tetrahedron 2014, 70, 1497–1501. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Andolfi, A.; Evidente, A. Pyrenophoric acid, a phytotoxic sesquiterpenoid penta-2,4-dienoic acid produced by a potential mycoherbicide, Pyrenophora semeniperda. J. Nat. Prod. 2014, 77, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Application of Mosher’s method for absolute configuration assignment to bioactive plants and fungi metabolites. J. Pharm. Biomed. Anal. 2017, 144, 59–89. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Cimmino, A.; Clement, S.; Black, B.; Evidente, A. Pyrenophoric acids B and C, two new phytotoxic sesquiterpenoids produced by Pyrenophora semeniperda. J. Agric. Food Chem. 2014, 62, 10304–10311. [Google Scholar] [CrossRef]
- Lozano-Juste, J.; Masi, M.; Cimmino, A.; Clement, S.; Fernández, M.A.; Antoni, R.; Meyer, S.; Rodriguez, P.L.; Evidente, A. The fungal sesquiterpenoid pyrenophoric acid B uses the plant ABA biosynthetic pathway to inhibit seed germination. J. Exp. Bot. 2019, 70, 5487–5494. [Google Scholar] [CrossRef]
- da Rosa, C.R.; Martinelli, J.A.; Federizzi, L.C.; Bocchese, C.A. Quantification of conidia produced by Pyrenophora chaetomioides on dead leaves of Avena sativa under field condition. Fitopatol. Bras. 2003, 28, 319–322. [Google Scholar]
- Chen, H.; Xue, L.; White, J.F.; Kamran, M.; Li, C. Identification and characterization of Pyrenophora species causing leaf spot on oat (Avena sativa) in western China. Plant Pathol. 2022, 71, 566–577. [Google Scholar] [CrossRef]
- Lam, A. Drechslera siccans from ryegrass fields in England and Wales. Trans. Br. Mycol. Soc. 1984, 83, 305–311. [Google Scholar] [CrossRef]
- Wiewióra, B.; Żurek, G.; Żurek, M. Endophyte-mediated disease resistance in wild populations of perennial ryegrass (Lolium perenne). Fungal Ecol. 2015, 15, 1–8. [Google Scholar] [CrossRef]
- Osterhage, C.; König, G.M.; Höller, U.; Wright, A.D. Rare sesquiterpenes from the algicolous fungus Drechslera dematioidea. J. Nat. Prod. 2002, 65, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, R.A. Marielliottia, a new genus of cereal and grass parasites segregated from Drechslera. Can. J. Bot. 1998, 76, 1558–1569. [Google Scholar]
- Jones, E.B.G.; Sakayaroj, J.; Suetrong, S.; Somrithipol, S.; Pang, K.L. Classification of marine Ascomycota, anamorphic taxa and Basidiomycota. Fungal Divers. 2009, 35, 187. [Google Scholar]
- Nozoe, S.; Hirai, K.; Tsuda, K.; Ishibashi, K.; Shirasaka, M.; Grove, J.F. The structure of pyrenophorin. Tetrahedron Lett. 1965, 6, 4675–4677. [Google Scholar] [CrossRef]
- Sugawara, F.; Strobel, G.A. (−)-Dihydropyrenophorin, a novel and selective phytotoxin produced by Drechslera avenae. Plant Sci. 1986, 43, 1–5. [Google Scholar] [CrossRef]
- Lerario, P.; Graniti, A. Attività fitotossica della pirenoforina e sua produzione nelle colture di Pyrenophora avenae Ito et Kurib. Phytopathol. Mediterr. 1985, 24, 280–283. [Google Scholar]
- McMullin, D.R.; Green, B.D.; Miller, J.D. Antifungal sesquiterpenoids and macrolides from an endophytic Lophodermium species of Pinus strobus. Phytochemistry Lett. 2015, 14, 148–152. [Google Scholar] [CrossRef]
- Yu, H.; Sperlich, J.; Höfert, S.P.; Janiak, C.; Teusch, N.; Stuhldreier, F.; Wesselborg, S.; Wang, C.; Kassack, M.U.; Dai, H.; et al. Azaphilone pigments and macrodiolides from the coprophilous fungus Coniella fragariae. Fitoterapia 2019, 137, 104249. [Google Scholar] [CrossRef]
- Ramakrishna, K.; Sreenivasulu, R.; Vidavalur, S.; Jagan Mohan Reddy, B. Stereoselective total synthesis of (-)-pyrenophorin. Lett. Org. Chem. 2016, 13, 693–697. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.; Krohn, K.; Egold, H.; Draeger, S.; Schulz, B. Diversity of antimicrobial pyrenophorol derivatives from an endophytic fungus, Phoma sp. Eur. J. Org. Chem. 2008, 2008, 4320–4328. [Google Scholar] [CrossRef]
- Kastanias, M.A.; Chrysayi-Tokousbalides, M. Herbicidal potential of pyrenophorol isolated from a Drechslera avenae pathotype. Pest Manag. Sci. 2000, 56, 227–232. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, U.S.; Reddy, B.S. Stereoselective total synthesis of (−)-pyrenophorol. Tetrahedron Lett. 2009, 50, 5984–5986. [Google Scholar] [CrossRef]
- Raistrick, H.; Robinson, R.; Todd, A.R. Studies in the biochemistry of micro-organisms: (a) On the production of hydroxyanthraquinones by species of Helminthosporium. (b) Isolation of tritisporin, a new metabolic product of Helminthosporium tritici-vulgaris Nisikado. (c) The molecular constitution of catenarin. Biochem. J. 1934, 28, 559–572. [Google Scholar] [PubMed]
- Shujun, J.; Sheng, Q.; Yunzhi, Z. Isolation, purification, identification, and bioassay of helminthosporin with herbicidal activity from Curvularia eragrostidis. Acta Phytophylacica Sin. 2006, 33, 313–318. [Google Scholar]
- Fozia, A.A. Phytochemical Investigation of Aloe turkanensis for Anticancer Activity. Doctoral Dissertation, University of Nairobi, Nairobi, Kenya, 2014. [Google Scholar]
- Augustin, N.; Nuthakki, V.K.; Abdullaha, M.; Hassan, Q.P.; Gandhi, S.G.; Bharate, S.B. Discovery of helminthosporin, an anthraquinone isolated from Rumex abyssinicus Jacq as a dual cholinesterase inhibitor. ACS Omega 2020, 5, 1616–1624. [Google Scholar] [CrossRef] [PubMed]
- Chrysayi-Tokousbalides, M.; Kastanias, M.A. Cynodontin: A fungal metabolite with antifungal properties. J. Agric. Food Chem. 2003, 51, 4920–4923. [Google Scholar] [CrossRef] [PubMed]
- Đorović, J.; Antonijević, M.; Marković, Z. Antioxidative and inhibition potency of cynodontin. J. Serb. Soc. Comput. Mech. 2020, 2020. [Google Scholar] [CrossRef]
- van Eijk, G.W. Chrysophanol and emodin from Drechslera catenaria. Phytochemistry 1974, 13, 650. [Google Scholar] [CrossRef]
- Dussart, F.; Jakubczyk, D. Biosynthesis of rubellins in Ramularia collo-cygni—Genetic basis and pathway proposition. Int. J. Mol. Sci. 2022, 23, 3475. [Google Scholar] [CrossRef] [PubMed]
- Choi, G.J.; Lee, S.W.; Jang, K.S.; Kim, J.S.; Cho, K.Y.; Kim, J.C. Effects of chrysophanol, parietin, and nepodin of Rumex crispus on barley and cucumber powdery mildews. Crop Prot. 2004, 23, 1215–1221. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Singh, B.N.; Sudheer, S.; Kharwar, R.N.; Siddiqui, S.; Abdel-Azeem, A.M.; Fraceto, L.F.; Dashora, K.; Gupta, V.K. Chrysophanol: A natural anthraquinone with multifaceted biotherapeutic potential. Biomolecules 2019, 9, 68. [Google Scholar]
- Su, S.; Wu, J.; Gao, Y.; Luo, Y.; Yang, D.; Wang, P. The pharmacological properties of chrysophanol, the recent advances. Biomed. Pharmacother. 2020, 125, 110002. [Google Scholar] [CrossRef]
- Lin, C.H.; Tseng, H.F.; Hsieh, P.C.; Chiu, V.; Lin, T.Y.; Lan, C.C.; Tzeng, I.S.; Chao, H.N.; Hsu, C.C.; Kuo, C.Y. Nephroprotective role of chrysophanol in hypoxia/reoxygenation-induced renal cell damage via apoptosis, ER stress, and ferroptosis. Biomedicines 2021, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Jadaun, K.S.; Mehan, S.; Sharma, A.; Siddiqui, E.M.; Kumar, S.; Alsuhaymi, N. Neuroprotective effect of chrysophanol as a PI3K/AKT/mTOR signaling inhibitor in an experimental model of autologous blood-induced intracerebral hemorrhage. Curr. Med. Sci. 2022, 2022, 1–18. [Google Scholar] [CrossRef]
- Cui, W.H.; Zhang, H.H.; Qu, Z.M.; Wang, Z.; Zhang, D.J.; Wang, S. Effects of chrysophanol on hippocampal damage and mitochondrial autophagy in mice with cerebral ischemia reperfusion. Int. J. Neurosci. 2022, 132, 613–620. [Google Scholar] [CrossRef]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef]
- Anke, H.; Kolthoum, I.; Laatsch, H. Metabolic products of microorganisms. 192. The anthraquinones of the Aspergillus glaucus group. II. Biological activity. Arch. Microbiol. 1980, 126, 231–236. [Google Scholar] [CrossRef]
- Hasan, H.A.H. Studies on toxigenic fungi in roasted foodstuff (salted seed) and halotolerant activity of emodin-producing Aspergillus wentii. Folia Microbiol. 1998, 43, 383–391. [Google Scholar] [CrossRef]
- Macías, M.; Ulloa, M.; Gamboa, A.; Mata, R. Phytotoxic compounds from the new coprophilous fungus Guanomyces polythrix. J. Nat. Prod. 2000, 63, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Hallock, Y.F.; Clardy, J.; Kenfield, D.S.; Strobel, G. De-O-methyldiaporthin, a phytotoxin from Drechslera siccans. Phytochemistry 1988, 27, 3123–3125. [Google Scholar] [CrossRef]
- Evidente, A.; Andolfi, A.; Vurro, M.; Fracchiolla, M.; Zonno, M.C.; Motta, A. Drazepinone, a trisubstituted tetrahydronaphthofuroazepinone with herbicidal activity produced by Drechslera siccans. Phytochemistry 2005, 66, 715–721. [Google Scholar] [CrossRef]
- Cao, F.; Pan, L.; Gao, W.; Liu, Y.; Zheng, C.; Zhang, Y. Structure revision and protein tyrosine phosphatase inhibitory activity of drazepinone. Mar. Drugs 2021, 19, 714. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.H.; Miyagawa, H.; Ueno, T.; Takenaka, H.; Sung, N.D. Siccanol: Sesterterpene isolated from pathogenic fungus Drechslera siccans. Appl. Biol. Chem. 1996, 39, 241–244. [Google Scholar]
- Chan, J.; Jamison, T.F. Enantioselective synthesis of (−)-terpestacin and structural revision of siccanol using catalytic stereoselective fragment couplings and macrocyclizations. J. Am. Chem. Soc. 2004, 126, 10682–10691. [Google Scholar] [CrossRef]
- Masi, M.; Zonno, M.C.; Boari, A.; Vurro, M.; Evidente, A. Terpestacin, a toxin produced by Phoma exigua var. heteromorpha, the causal agent of a severe foliar disease of oleander (Nerium oleander L.). Nat. Prod. Res. 2022, 36, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K. Studies on antibiotics from Helminthosporium sp. fungi. VII Siccanin, a new antifungal antibiotic produced by Helminthosporium siccans. J. Antibiot. 1962, 15, 161–167. [Google Scholar]
- Mogi, T.; Kawakami, T.; Arai, H.; Igarashi, Y.; Matsushita, K.; Mori, M.; Shiomi, K.; Ōmura, S.; Harada, S.; Kita, K. Siccanin rediscovered as a species-selective succinate dehydrogenase inhibitor. J. Biochem. 2009, 146, 383–387. [Google Scholar] [CrossRef]
- Trost, B.M.; Shen, H.C.; Surivet, J.P. An enantioselective biomimetic total synthesis of (−)-siccanin. Angew. Chem. 2003, 115, 4073–4077. [Google Scholar] [CrossRef]
- Bills, G.F.; Peláez, F.; Polishook, J.D.; Diez-Matas, M.T.; Harris, G.H.; Clapp, W.H.; Dufresne, C.; Byrne, K.M.; Nallin-Omstead, M.; Jenkins, R.G.; et al. Distribution of zaragozic acids (squalestatins) among filamentous ascomycetes. Mycol. Res. 1994, 98, 733–739. [Google Scholar] [CrossRef]
- Huang, L.; Lingham, R.B.; Harris, G.H.; Singh, S.B.; Dufresne, C.; Nallin-Omstead, M.; Bills, G.F.; Mojena, M.; Sanchez, M.; Karkas, J.D.; et al. New fungal metabolites as potential antihypercholesterolemics and anticancer agents. Can. J. Bot. 1995, 73, 898–906. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Yue, E.W.; La Greca, S.; Nadin, A.; Yang, Z.; Leresche, J.E.; Tsuri, T.; Naniwa, Y.; de Riccardis, F. Synthesis of zaragozic acid A/squalestatin S1. Eur. J. Chem. 1995, 1, 467–494. [Google Scholar] [CrossRef]
- Sarpeleh, A.; Wallwork, H.; Catcheside, D.E.; Tate, M.E.; Able, A.J. Proteinaceous metabolites from Pyrenophora teres contribute to symptom development of barley net blotch. Phytopathology 2007, 97, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Mathieu, V.; Andolfi, A.; Cimmino, A.; Lefranc, F.; Kiss, R.; Evidente, A. In vitro growth inhibitory effects of cytochalasins and derivatives in cancer cells. Planta Med. 2011, 77, 711–717. [Google Scholar] [CrossRef]
- Berestetskiy, A.; Dmitriev, A.; Mitina, G.; Lisker, I.; Andolfi, A.; Evidente, A. Nonenolides and cytochalasins with phytotoxic activity against Cirsium arvense and Sonchus arvensis: A structure–activity relationships study. Phytochemistry 2008, 69, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Berestetskiy, A.; Evidente, A. Production of phytotoxins by Phoma exigua var. exigua, a potential mycoherbicide against perennial thistles. J. Agric. Food Chem. 2008, 56, 6304–6309. [Google Scholar] [CrossRef]
- Sumarah, M.W.; Kesting, J.R.; Sørensen, D.; Miller, J.D. Antifungal metabolites from fungal endophytes of Pinus strobus. Phytochemistry 2011, 72, 1833–1837. [Google Scholar] [CrossRef]
- Chooi, Y.H.; Solomon, P.S. A chemical ecogenomics approach to understand the roles of secondary metabolites in fungal cereal pathogens. Front. Microbiol. 2014, 5, 640. [Google Scholar] [CrossRef]
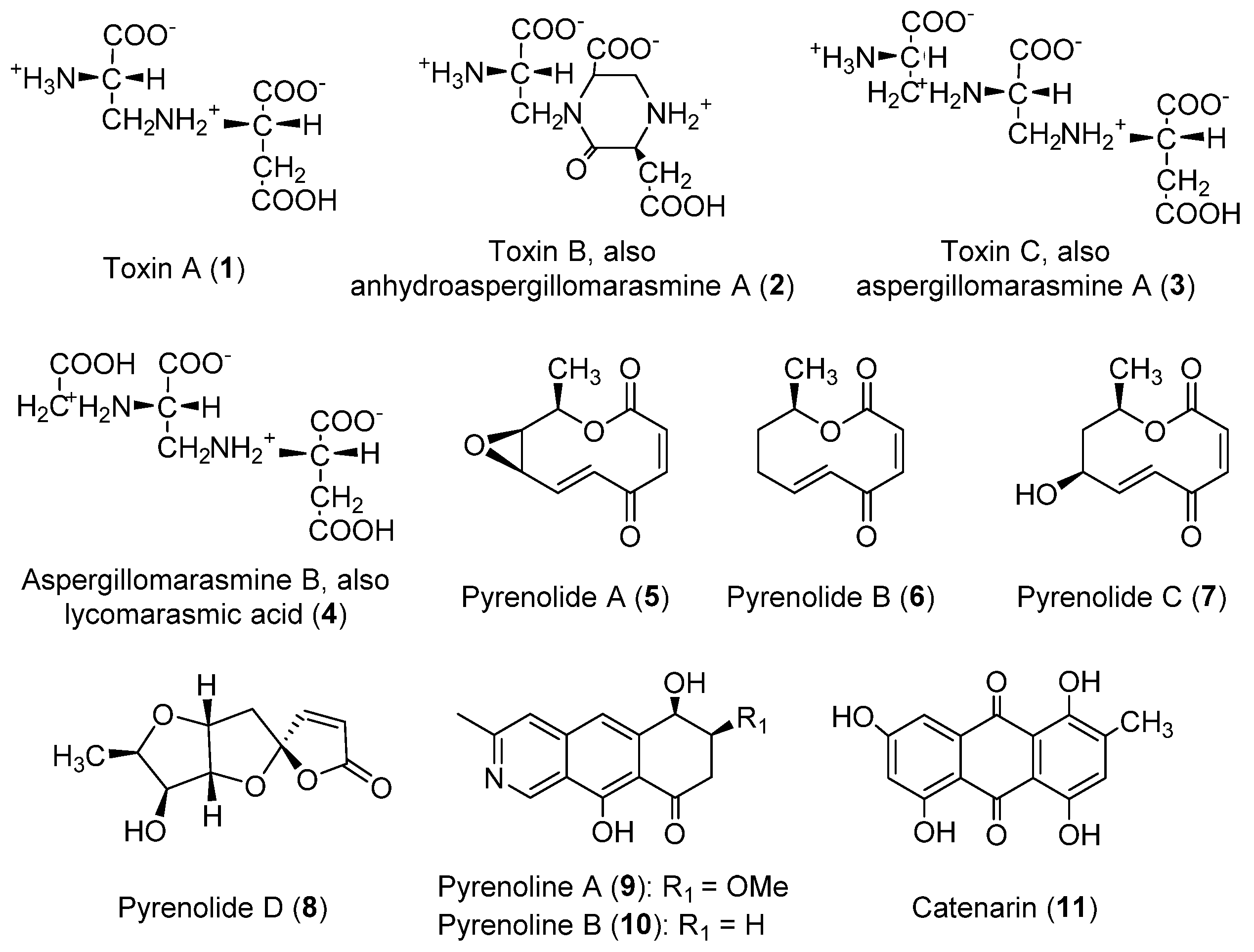
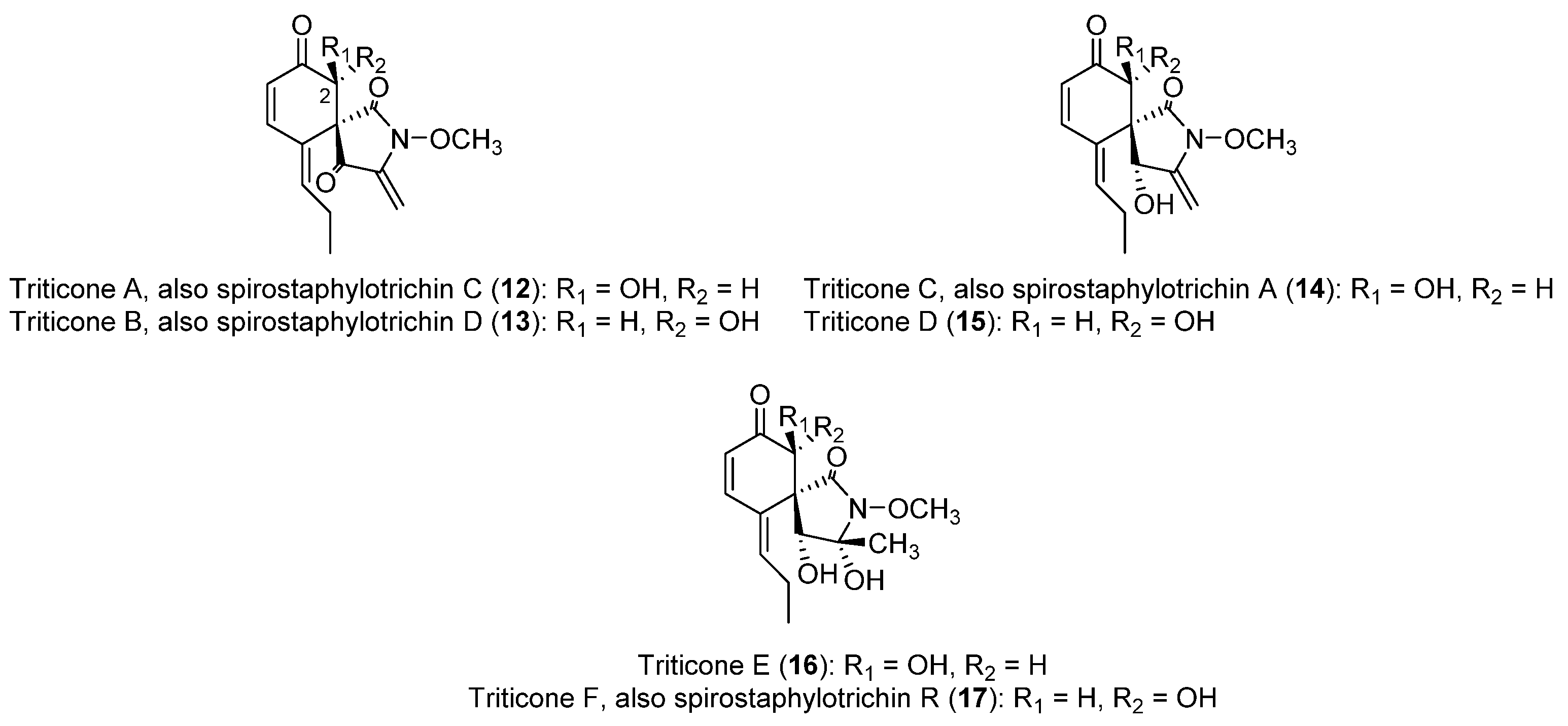
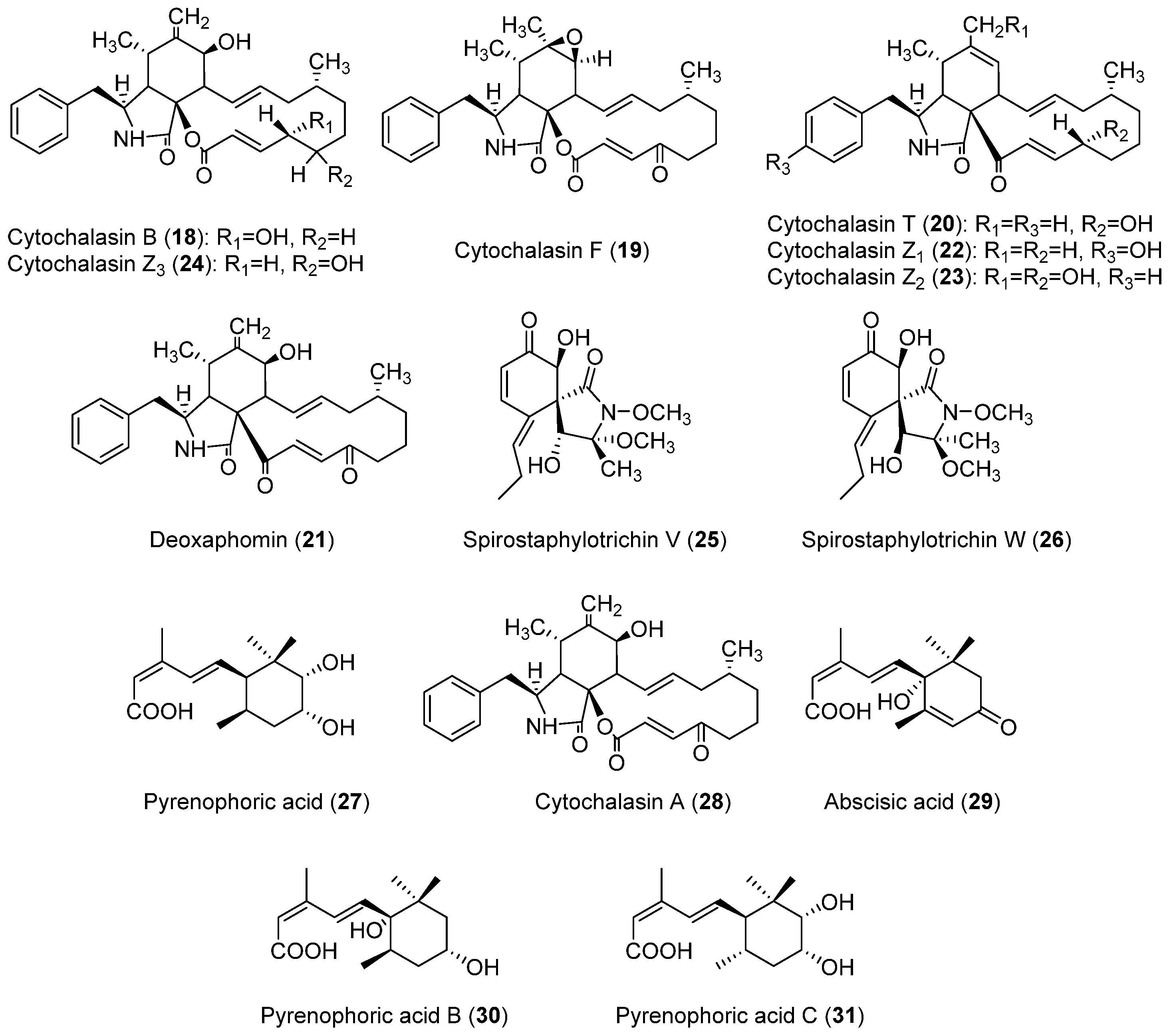
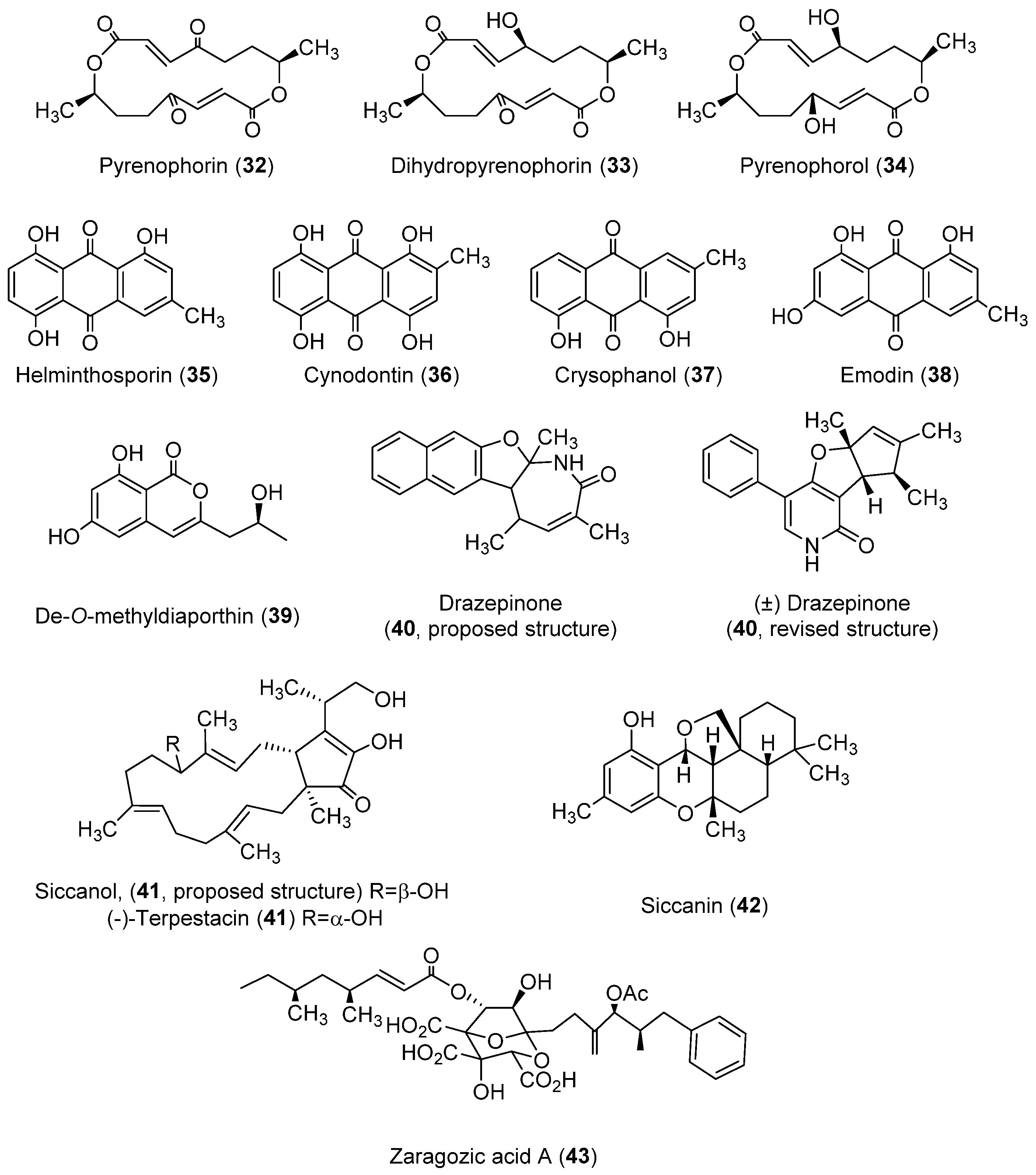
| Class | Compound | Pyrenophora species | Activity | References |
|---|---|---|---|---|
| Amino acid derivatives | Toxin A [N-(2-amino-2-carboxyethyl) aspartic acid] (1, Figure 1) | P. teres | Phytotoxic to barley | [14,15,16,26,27,139] |
| Toxin B [1-(2-amino-2-carboxyethyl)-6- carboxy-3-carboxymethyl-2-piperazinone]; anhydroaspergillomarasmine A (2, Figure 1) | P. teres | Phytotoxic to barley | [14,15,16,26,27,139] | |
| Toxin C [N-[2-(2-amino-2-carboxy ethyl-amino)-2-carboxyethyl] aspartic acid]; aspergillomarasmine A (3, Figure 1) | P. teres | Phytotoxic to barley; reverse of resistance to Gram-negative pathogens | [15,16,24,26,27,139] | |
| Aspergillomarasmine B; lycomarasmic acid (4, Figure 1) | P. teres | Phytotoxic to barley | [27] | |
| Anthraquinones | Catenarin (11, Figure 1) | P. catenaria P. teres P. tritici-repentis | Phytotoxic to wheat; antibacterial; antifungal; cytotoxic; antidiabetic | [45,46,47,50,51,109,115] |
| Chrysophanol (37, Figure 4) | P. catenaria | Antifungal; anti-inflammatory; antiviral; anti-cancer; neuroprotective; anti-cardiovascular disease; antiulcer | [115,117,118,119,120,121,122] | |
| Cynodontin (36, Figure 4) | P. avenae | Antifungal; antioxidant | [45,109,113,114] | |
| Emodin (38, Figure 4) | P. catenaria | Phytotoxic to sunflower, Amaranthus hypochondriacus and Echinochloa crus-galli; antibacterial; anticancer; hepatoprotective; anti-inflammatory; antioxidant; antimicrobial | [115,123,124,125,126] | |
| Helminthosporin (35, Figure 4) | P. avenae P. catenaria | Herbicidal; cytotoxic; inhibition of cholinesterase | [45,109,110,111,112] | |
| Bicyclic sesquiterpene | Siccanol; (-)-terpestacin (41, Figure 4) | D. siccans | Phytotoxic to Lolium multiflorum | [130] |
| Cytochalasans | Cytochalasin A (28, Figure 3) | P. semeniperda | Phytotoxic to Bromus tectorum, Cirsium arvense and Sonchus arvensis; anticancer; antibacterial; antifungal; antiviral | [85,86,91,140,141] |
| Cytochalasin B (18, Figure 3) | P. semeniperda | Phytotoxic to wheat, tomato, B. tectorum, Lilium longiflorum, C. arvense and S. arvensis; algicidal; anticancer; cytotoxic; antiparasital; enzyme inhibition | [77,78,84,85,86,89,91,140,141,142] | |
| Cytochalasin F (19, Figure 3) | P. semeniperda | Phytotoxic to wheat, tomato, B. tectorum, C. arvense and S. arvensis; algicidal; anticancer | [84,85,86,89,91,140,141,142] | |
| Cytochalasin T (20, Figure 3) | P. semeniperda | Phytotoxic to C. arvense and S. arvensis | [84,141] | |
| Cytochalasin Z1 (22, Figure 3) | P. semeniperda | - | [84] | |
| Cytochalasin Z2 (23, Figure 3) | P. semeniperda | Phytotoxic to C. arvense and S. arvensis | [84,141,142] | |
| Cytochalasin Z3 (24, Figure 3) | P. semeniperda | Phytotoxic to wheat, tomato, C. arvense and S. arvensis; anticancer | [84,89,91,140,141,142] | |
| Deoxaphomin (21, Figure 3) | P. semeniperda | Phytotoxic to B. tectorum, s C. arvense and S. arvensis; anticancer | [84,89,91,140,141,142] | |
| Isocoumarin | De-O-methyldiaporthin (39, Figure 4) | D. siccans | Phytotoxic to corn, soybean, Amaranthus spinosus, Digitaria ischaemum and E. crus-galli | [127] |
| Isoquinoline derivatives | Pyrenoline A (9, Figure 1) | P. teres | Phytotoxic to barley, Festuca spp., Agropyron repens and Cynodon dactylon | [43] |
| Pyrenoline B (10, Figure 1) | P. teres | Phytotoxic to barley, oat, Hibiscus sabdariffa and Euphorbia heterophylla | [43] | |
| Macrocyclic compounds | Pyrenophorin (32, Figure 4) | P. avenae | Inhibition of radical growth in oat and non-host plants; antifungal; cytotoxic | [100,102,103,104] |
| Dihydropyrenophorin (33, Figure 4) | P. avenae | Phytotoxic to barley, soybean, wheat, maize, oat, Sorghum halepense and different weeds; antibacterial; antifungal; antialgal | [101,106] | |
| Pyrenophorol (34, Figure 4) | P. avenae | Phytotoxic to oat and tomato; antibacterial; antifungal; antialgal | [106,107,143] | |
| Naphthofuroazepinone | Drazepinone (40, Figure 4) | D. siccans | Phytotoxic to durum wheat and diverse weed species; protein tyrosine phosphatase inhibitor | [128,129] |
| Nonenolides | Pyrenolide A (5, Figure 1) | P. teres | Antifungal | [30] |
| Pyrenolide B (6, Figure 1) | P. teres | Antifungal | [29] | |
| Pyrenolide C (7, Figure 1) | P. teres | Antifungal | [29] | |
| Phenolic compound | Siccanin (42, Figure 4) | D. siccans | Antifungal; succinate dehydrogenase inhibition | [133,134] |
| Proteins | Ptr ToxA | P. tritici-repentis | Phytotoxic to wheat | [57] |
| Ptr ToxB | P. tritici-repentis | Phytotoxic to wheat | [58] | |
| Sesquiterpenoids | Abscisic acid (29, Figure 3) | P. semeniperda | Phytotoxic to B. tectorum | [91,92] |
| Pyrenophoric acid (27, Figure 3) | P. semeniperda | Phytotoxic to B. tectorum | [89,91,92] | |
| Pyrenophoric acid B (30, Figure 3) | P. semeniperda | Phytotoxic to Arabidopsis thaliana and B. tectorum | [91,92] | |
| Pyrenophoric acid C (31, Figure 3) | P. semeniperda | Phytotoxic to B. tectorum | [91,92] | |
| Spirocyclic lactams | Triticone A; spirostaphylotrichin C (12, Figure 2) | P. semeniperda P. tritici-repentis | Phytotoxic to wheat, tomato, oat, and different weed species | [64,65,66,88] |
| Triticone B; spirostaphylotrichin D (13, Figure 2) | P. semeniperda P. tritici-repentis | Phytotoxic to wheat, tomato and different weed species | [64,65,88] | |
| Triticone C; spirostaphylotrichin A (14, Figure 2) | P. semeniperda P. tritici-repentis | Phytotoxic to B. tectorum coleoptiles, weakly to wheat, tomato and different weed species | [64,66,88] | |
| Triticone D (15, Figure 2) | P. tritici-repentis | Weakly phytotoxic to wheat and different weed species | [64,66] | |
| Triticone E (16, Figure 2) | P. semeniperda P. tritici-repentis | Antibacterial | [64,68,88] | |
| Triticone F; spirostaphylotrichin R (17, Figure 2) | P. semeniperda P. tritici-repentis | Antibacterial | [64,68,88] | |
| Spirostaphylotrichin V (25, Figure 3) | P. semeniperda | Weakly phytotoxic to B. tectorum coleoptiles | [88] | |
| Spirostaphylotrichin W (26, Figure 3) | P. semeniperda | Weakly phytotoxic to tomato and B. tectorum coleoptiles | [88] | |
| Spirocyclic lactone | Pyrenolide D (8, Figure 1) | P. teres | Cytotoxic | [37] |
| Squalestatin | Zaragozic acid A; squalestatin S1 (43, Figure 4) | D. biseptata | Squalene synthase inhibition | [136,137] |
| Unknown | Ptr ToxC | P. tritici-repentis | Phytotoxic to wheat | [61] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Zorrilla, J.G.; Meyer, S. Bioactive Metabolite Production in the Genus Pyrenophora (Pleosporaceae, Pleosporales). Toxins 2022, 14, 588. https://doi.org/10.3390/toxins14090588
Masi M, Zorrilla JG, Meyer S. Bioactive Metabolite Production in the Genus Pyrenophora (Pleosporaceae, Pleosporales). Toxins. 2022; 14(9):588. https://doi.org/10.3390/toxins14090588
Chicago/Turabian StyleMasi, Marco, Jesús García Zorrilla, and Susan Meyer. 2022. "Bioactive Metabolite Production in the Genus Pyrenophora (Pleosporaceae, Pleosporales)" Toxins 14, no. 9: 588. https://doi.org/10.3390/toxins14090588
APA StyleMasi, M., Zorrilla, J. G., & Meyer, S. (2022). Bioactive Metabolite Production in the Genus Pyrenophora (Pleosporaceae, Pleosporales). Toxins, 14(9), 588. https://doi.org/10.3390/toxins14090588







