Bites by Non-Native Reptiles in France: Species, Circumstances and Outcome
Abstract
:1. Introduction
2. Results
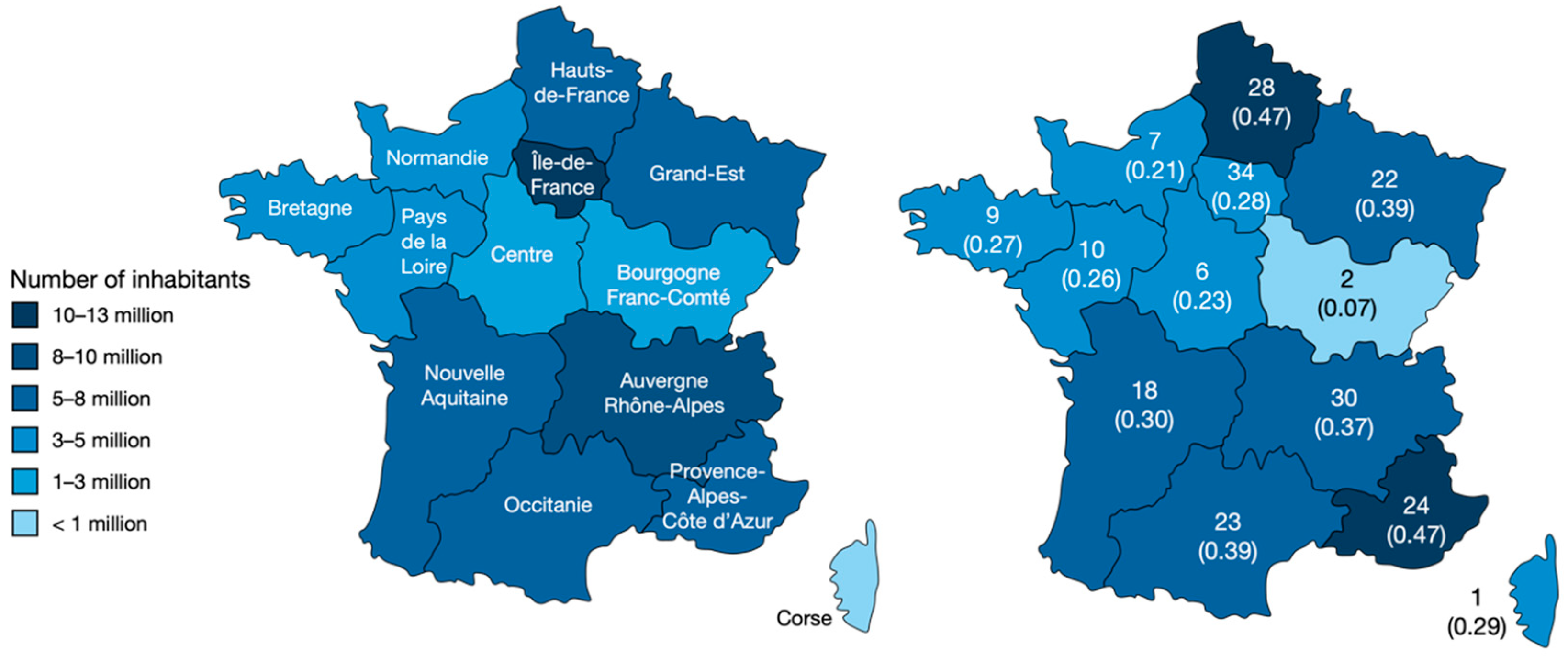
2.1. Sociodemographics
2.2. Circumstances of the Exposure
2.3. Species Involved
2.4. Clinical Aspects
2.5. Care Management
3. Discussion
4. Limitations
5. Conclusions
6. Materials and Methods
6.1. Selection of Cases
6.2. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamb, T.; de Haro, L.; Lonati, D.; Brvar, M.; Eddleston, M. Antivenom for European vipera species envenoming. Clin. Toxicol. 2017, 55, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Paolino, G.; Di Nicola, M.R.; Pontara, A.; Didona, D.; Moliterni, E.; Mercuri, S.R.; Grano, M.; Borgianni, N.; Kumar, R.; Pampena, R. Vipera snakebite in Europe: A systematic review of a neglected disease. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2247–2260. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.M.; Strine, C.; Hughes, A.C. Thousands of reptile species threatened by under-regulated global trade. Nat. Commun. 2020, 11, 4738. [Google Scholar] [CrossRef] [PubMed]
- Persson, H.E.; Sjöberg, G.K.; Haines, J.A.; de Garbino, J.P. Poisoning severity score. Grading of acute poisoning. J. Toxicol. Clin. Toxicol. 1998, 36, 205–213. [Google Scholar] [CrossRef]
- Jollivet, V.; Hamel, J.F.; de Haro, L.; Labadie, M.; Sapori, J.M.; Cordier, L.; Villa, A.; Nisse, P.; Puskarczyk, E.; Berthelon, L.; et al. European viper envenomation recorded by French Poison Control Centers: A clinical assessment and management study. Toxicon 2015, 108, 97–103. [Google Scholar] [CrossRef]
- Malina, T.; Krecsák, L.; Korsós, Z.; Takács, Z. Snakebites in Hungary—Epidemiological and clinical aspects over the past 36 years. Toxicon 2008, 51, 943–951. [Google Scholar] [CrossRef]
- Valenta, J.; Stach, Z.; Michalek, P. Exotic snake bites in the Czech Republic—Epidemiological and clinical aspects during 15-year period (1999–2013). Clin. Toxicol. 2014, 52, 258–264. [Google Scholar] [CrossRef]
- Schaper, A.; Desel, H.; Ebbecke, M.; De Haro, L.; Deters, M.; Hentschel, H.; Hermanns-Clausen, M.; Langer, C. Bites and stings by exotic pets in Europe: An 11 year analysis of 404 cases from Northeastern Germany and Southeastern France. Clin. Toxicol. 2009, 47, 39–43. [Google Scholar] [CrossRef]
- Crook, V.; Van der Henst, E. Mettre Un Terme Au Trafic d’espèces Sauvages Sur Internet Dans l’UE. Le Commerce En Ligne de Reptiles et d’oiseaux En Belgique et Aux Pays-Bas; WWF Belgique: Brussels, Belgium, 2020. [Google Scholar]
- Warrick, B.J.; Boyer, L.V.; Seifert, S.A. Non-native (exotic) snake envenomations in the U.S., 2005–2011. Toxins 2014, 6, 2899–2911. [Google Scholar] [CrossRef] [Green Version]
- Köppel, C.; Martens, F. Clinical experience in the therapy of bites from exotic snakes in Berlin. Hum. Exp. Toxicol. 1992, 11, 549–552. [Google Scholar] [CrossRef]
- de Haro, L. Injury and Envenomation by Exotic Snakes and Other Venomous Pets in Europe. In Clinical Toxinology; Saunders: Philadelphia, PA, USA, 2018; pp. 471–483. ISBN 9789401774383. [Google Scholar]
- Isbister, G.K.; Brown, S.G.A. Bites in Australian snake handlers—Australian Snakebite Project (ASP-15). QJM 2012, 105, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Clapson, P.; Debien, B.; de Haro, L. Morsures et Piqûres Par Animaux Venimeux En France Métropolitaine. Urgences 2008 2008, 537–546. Available online: https://www.sfmu.org/upload/70_formation/02_eformation/02_congres/Urgences/urgences2008/donnees/pdf/053_debien.pdf (accessed on 19 August 2022).
- Spyres, M.B.; Ruha, A.-M.; Seifert, S.; Onisko, N.; Padilla-Jones, A.; Smith, E.A. Occupational snake bites: A prospective case series of patients reported to the ToxIC North American Snakebite Registry. J. Med. Toxicol. 2016, 12, 365–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FEDIAF. Facts & Figures 2019 European Overview; FEDIAF: Brussels, Belgium, 2019. [Google Scholar]
- Toland, E.; Bando, M.; Hamers, M.; Cadenas, V.; Laidlaw, R.; Martínez-Silvestre, A.; van der Wielen, P. Turning negatives into positives for pet trading and keeping: A review of positive lists. Animals 2020, 10, 2371. [Google Scholar] [CrossRef]
- Fry, B.G.; Casewell, N.R.; Wüster, W.; Vidal, N.; Young, B.; Jackson, T.N.W. The structural and functional diversification of the toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef]
- Weinstein, S.A.; Warrell, D.A.; White, J.; Keyler, D.E. “Venomous” Bites from Non-Venomous Snakes; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9780123877321. [Google Scholar]
- McCarty, V.O.; Cox, R.A.; Haglund, B. Death caused by a constricting snake—An infant death. J. Forensic Sci. 1989, 34, 239–243. [Google Scholar] [CrossRef]
- Omalu, B.I.; Dominick, J.T.; Uhrich, T.G.; Wecht, C.H. Letter to the editor. Child Abus. Negl. 2003, 27, 989–991. [Google Scholar] [CrossRef]
- Ineich, I.; Goyffon, M. Un cas d’envenimation par un colubridae aglyphe opisthodonte du cameroun, thrasops flavigularis (Hallowell, 1852). In Faut-Il Interdire la Detention de Certaines Couleuvres non Opisth; 2006. [Google Scholar]
- Kuch, U.; Mebs, D. Envenomations by colubrid snakes in Africa, Europe, and the Middle East. J. Toxicol.—Toxin Rev. 2002, 21, 159–179. [Google Scholar] [CrossRef]
- Broadley, D.G.; Wallach, V. Review of the dispholidini, with the description of a new genus and species from Tanzania (Serpentes, Colubridae). Bull. Nat. Hist. Mus. Zool. Ser. 2002, 68, 57–74. [Google Scholar] [CrossRef]
- Weinstein, S.A.; Keyler, D.E. Local envenoming by the western hognose snake (Heterodon Nasicus): A case report and review of medically significant heterodon bites. Toxicon 2009, 54, 354–360. [Google Scholar] [CrossRef]
- Weinstein, S.A.; Griffin, R.; Ismail, A.K. Non-front-fanged colubroid (colubrid) snakebites: Three cases of local envenoming by the mangrove or ringed cat-eyed snake (Boiga dendrophila; Colubridae, Colubrinae), the Western beaked snake (Rhamphiophis oxyrhynchus; Lamprophiidae, Psammophinae) and the rain forest cat-eyed snake (Leptodeira frenata; Dipsadidae). Clin. Toxicol. 2014, 52, 277–282. [Google Scholar]
- Wagener, M.; Naidoo, M.; Aldous, C. Wound infection secondary to snakebite. S. Afr. Med. J. 2017, 107, 315–319. [Google Scholar] [CrossRef] [Green Version]
- Brenes-Chacón, H.; Ulloa-Gutierrez, R.; Soriano-Fallas, A.; Camacho-Badilla, K.; Valverde-Muñoz, K.; Ávila-Agüero, M.L. Bacterial infections associated with viperidae snakebites in children: A 14-year experience at the Hospital Nacional de Niños de Costa Rica. Am. J. Trop. Med. Hyg. 2019, 100, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Talan, D.A.; Citron, D.M.; Abrahamian, F.M.; Moran, G.J.; Goldstein, E.J. Bacteriologic analysis of infected dog and cat bites. Emergency medicine animal bite infection study group. N. Engl. J. Med. 1999, 340, 85–92. [Google Scholar] [CrossRef]
- Larréché, S.; Mion, G.; Clapson, P.; Debien, B.; Wybrecht, D.; Goyffon, M. Neurotoxines ophidiennes. Ann. Françaises D’anesth. Réanim. 2008, 27, 310–316. [Google Scholar] [CrossRef]
- Chippaux, J.P. Envenimations et empoisonnements par les animaux venimeux ou veneneux. III. Envenimations par elapidae. Med. Trop. 2007, 67, 9–12. [Google Scholar]
- Dijkema, G.H.; Pat, J.J.; Steffens, M.G. Scrotal necrosis after cobra (Naja annulifera) envenomation. Urol. Case Rep. 2021, 39, 101844. [Google Scholar] [CrossRef] [PubMed]
- Yeh, H.; Gao, S.-Y.; Lin, C.-C. Wound infections from Taiwan cobra (Naja atra) bites: Determining bacteriology, antibiotic susceptibility, and the use of antibiotics—A cobra BITE study. Toxins 2021, 13, 183. [Google Scholar] [CrossRef]
- Chippaux, J.P. Envenimations et intoxications par les animaux venimeux ou veneneux II. Envenimations par viperidae. Med. Trop. 2006, 66, 423–428. [Google Scholar]
- Chu, E.R.; Weinstein, S.A.; White, J.; Warrell, D.A. Venom ophthalmia caused by venoms of spitting elapid and other snakes: Report of ten cases with review of epidemiology, clinical features, pathophysiology and management. Toxicon 2010, 56, 259–272. [Google Scholar] [CrossRef]
- Pourreau, F.; Pinsard, M.; Goyffon, M.; Plasse, F.; Desport, E.; Thierry, A.; Touchard, G.; Bridoux, F. Bilateral renal cortical necrosis with end-stage renal failure following envenoming by Proatheris superciliaris: A case report. Toxicon 2014, 84, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Rojnuckarin, P.; Banjongkit, S.; Chantawibun, W.; Akkawat, B.; Juntiang, J.; Noiphrom, J.; Pakmanee, N.; Intragumtornchai, T. Green pit viper (Trimeresurus albolabris and T. macrops) venom antigenaemia and kinetics in humans. Trop. Dr. 2007, 37, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Hutton, R.A.; Looareesuwan, S.; Ho, M.; Silamut, K.; Chanthavanich, P.; Karbwang, J.; Supanaranond, W.; Vejcho, S.; Viravan, C.; Phillips, R.E.; et al. Arboreal green pit vipers (Genus Trimeresurus) of South-East Asia: Bites by T. Albolabris and T. Macrops in Thailand and a review of the literature. Trans. R. Soc. Trop. Med. Hyg. 1990, 84, 866–874. [Google Scholar] [CrossRef]
- Boels, D.; Harry, P.; de Haro, L.; Quistinic, P.; Clerc, M.-A.; Lourdais, O. La Banque des Sérums Antivenimeux (BSA) et la prise en charge des envenimations par serpents exotiques en France. Urgence Prat. 2009, 94, 41–44. [Google Scholar]
- Darsonval, A.; Boels, D.; Clerc, M.-A.; De Haro, L.; Penot-Ragon, C.; Moal, F.; Quistinic, P.; Lourdais, O.; Harry, P. Création et organisation d’une banque des serums antivenimeux en France. Presse Méd. 2010, 39, 865–870. [Google Scholar] [CrossRef]
- World Health Organization. Snakebite Envenoming: A Strategy for Prevention and Control; World Health Organization: Geneva, Switzerland, 2019.
- Warrell, D.A. Commissioned article: Management of exotic snakebites. QJM 2009, 102, 593–601. [Google Scholar] [CrossRef] [Green Version]

| Variable | Total | PSS * 0/1 | PSS * 2/3 | p |
|---|---|---|---|---|
| Age (y.o.) | 211 | 27.5 ± 15.8 | 37.8 ± 13.3 | <0.001 |
| Sex | 0.039 | |||
| Male | 140 | 115 | 25 | |
| Female | 78 | 72 | 6 | |
| Venomous animal | <0.001 | |||
| Yes | 42 | 18 | 24 | |
| No | 168 | 161 | 7 | |
| Time of bite | N.S. | |||
| Day | 120 | 101 | 19 | |
| Evening | 72 | 62 | 10 | |
| Deep night | 23 | 21 | 2 | |
| Activity | N.S. | |||
| Feeding, nursing | 41 | 31 | 10 | |
| Handling | 34 | 31 | 3 | |
| Context | <0.01 | |||
| Private | 187 | 166 | 21 | |
| Occupational | 24 | 15 | 9 | |
| Location | 0.022 | |||
| At home | 186 | 164 | 22 | |
| At work | 20 | 13 | 7 | |
| Pet shop, fair… | 5 | 5 | 0 |
| Species | No. of Bites n = 218 | PSS * 0 n = 98 | PSS 1 n = 89 | PSS 2 n = 23 | PSS 3 n = 8 |
|---|---|---|---|---|---|
| Lizards | 7 | 4 | 2 | 1 | |
| “Exotic lizard” | 1 | 1 | |||
| Pogona vitticeps | 1 | 1 | |||
| Heloderma suspectum | 1 | 1 | |||
| Varanus sp. (incl. V. exanthematicus) | 3 | 1 | 1 | ||
| Iguana iguana | 1 | 1 | |||
| Snakes | 211 | 94 | 87 | 22 | 8 |
| “Exotic snake” | 1 | 1 | |||
| “Snake from Guyana” | 1 | 1 | |||
| Elapidae | 8 | 1 | 2 | 4 | 1 |
| Aspidelaps lumbricus infuscates | 1 | 1 | |||
| “African naja” | 1 | 1 | |||
| Naja mossambica | 1 | 1 | |||
| Naja annulifera | 1 | 1 | |||
| Naja atra | 1 | 1 | |||
| Naja naja | 2 | 1 | 1 | ||
| Oxyuranus microlepidotus | 1 | 1 | |||
| Viperidae: Viperinae | 5 | 1 | 3 | 1 | |
| Bitis nasicornis | 1 | 1 | |||
| Cerastes vipera | 1 | 1 | |||
| Cerastes cerastes | 1 | 1 | |||
| Daboia palestinae | 1 | 1 | |||
| Proatheris supercialiaris | 1 | 1 | |||
| Viperidae: Crotalinae | 28 | 4 | 10 | 9 | |
| Agkistrodon contortrix | 2 | 1 | 1 | ||
| Bothriechis schlegelii | 1 | 1 | |||
| Bothriopsis taeniata | 1 | 1 | |||
| Bothrops asper | 1 | 1 | |||
| Bothrops atrox | 1 | 1 | |||
| Bothrops moojeni | 1 | 1 | |||
| Crotalus sp. | 2 | 1 | 1 | ||
| Crotalus atrox | 1 | 1 | |||
| Crotalus adamanteus | 1 | 1 | |||
| Crotalus durissus (incl. C. d. durissus and unicolor) | 3 | 1 | 1 | 1 | |
| Crotalus polystictus | 1 | 1 | |||
| Crotalus viridis oreganus | 1 | 1 | |||
| Trimeresurus albolabris | 6 | 1 | 4 | 1 | |
| Trimeresurus flavomaculatus | 2 | 1 | 1 | ||
| Trimeresurus schultzei | 1 | 1 | |||
| Trimeresurus trigonocephalus | 2 | 1 | 1 | ||
| Trimeresurus venustus | 1 | 1 | |||
| Pythonidae | 69 | 37 | 3 | 1 | |
| Malayophython reticulatus | 1 | 1 | |||
| Morelia sp. | 2 | 2 | |||
| Morelia spilota (incl. M. s. cheyeni and M. s. macdowelli) | 2 | 2 | |||
| Morelia viridis | 1 | 1 | |||
| Python sp. | 26 | 16 | 10 | ||
| Python molurus | 5 | 3 | 2 | ||
| Python regius | 32 | 17 | 14 | 1 | |
| Boidae | 43 | 24 | 18 | 1 | |
| Boa sp. | 20 | 12 | 8 | ||
| Boa constrictor | 18 | 9 | 8 | 1 | |
| Boa imperator | 4 | 2 | 2 | ||
| Eryx colubrinus | 1 | 1 | |||
| Colubridae | 48 | 27 | 18 | 3 | |
| “Exotic colubrid” | 3 | 1 | 2 | ||
| Elaphe schrenckii | 1 | 1 | |||
| Lampropeltis sp. | 2 | 1 | 1 | ||
| Lampropeltis californiae | 4 | 3 | 1 | ||
| Lampropeltis triangulum (incl. L. t. hondurensis and L. t. campbelli) | 4 | 2 | 2 | ||
| Pantherophis sp. | 1 | 1 | |||
| Pantherophis bairdi | 2 | 1 | 1 | ||
| Pantherophis guttatus | 29 | 17 | 10 | 2 | |
| Pantherophis obsoletus | 1 | 1 | |||
| Thrasops flavigularis | 1 | 1 | |||
| Dipsadidae | 6 | 5 | 1 | 1 | |
| Heterodon nasicus | 7 | 5 | 1 | 1 | |
| Lamprophiidae | 1 | 1 | |||
| Rhamphiophis oxyrhynchus | 1 | 1 |
| Reported Signs or Symptoms | Number of Cases | |
|---|---|---|
| Local signs | Erythema | 12 |
| Pain | 11 | |
| Edema | 10 | |
| Necrosis, blisters | 4 | |
| Ecchymosis | 4 | |
| Bleeding at the skin puncture | 1 | |
| Compartment syndrome | 1 | |
| Systemic signs | Extensive edema | 3 |
| Adenopathy | 3 | |
| Tachycardia | 2 | |
| High blood pressure | 2 | |
| Paresthesia | 1 | |
| Organic acute kidney injury | 1 | |
| Hyperthermia | 1 | |
| Low blood pressure | 1 | |
| Extensive ischemia | 1 | |
| Biological perturbations | Coagulopathy | 3 |
| Increased prothrombin time | 3 | |
| Hyperleukocytosis | 3 | |
| Thrombocytopenia | 2 | |
| Rise in CK | 2 | |
| Hypofibrinogenemia | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Roux, G.; Grenet, G.; Schmitt, C.; French Poison Control Centers Research Group; Larréché, S.; Descatha, A. Bites by Non-Native Reptiles in France: Species, Circumstances and Outcome. Toxins 2022, 14, 570. https://doi.org/10.3390/toxins14080570
Le Roux G, Grenet G, Schmitt C, French Poison Control Centers Research Group, Larréché S, Descatha A. Bites by Non-Native Reptiles in France: Species, Circumstances and Outcome. Toxins. 2022; 14(8):570. https://doi.org/10.3390/toxins14080570
Chicago/Turabian StyleLe Roux, Gaël, Guillaume Grenet, Corinne Schmitt, French Poison Control Centers Research Group, Sébastien Larréché, and Alexis Descatha. 2022. "Bites by Non-Native Reptiles in France: Species, Circumstances and Outcome" Toxins 14, no. 8: 570. https://doi.org/10.3390/toxins14080570
APA StyleLe Roux, G., Grenet, G., Schmitt, C., French Poison Control Centers Research Group, Larréché, S., & Descatha, A. (2022). Bites by Non-Native Reptiles in France: Species, Circumstances and Outcome. Toxins, 14(8), 570. https://doi.org/10.3390/toxins14080570





