Fibrinogenolysis in Venom-Induced Consumption Coagulopathy after Viperidae Snakebites: A Pilot Study
Abstract
:1. Introduction
2. Results
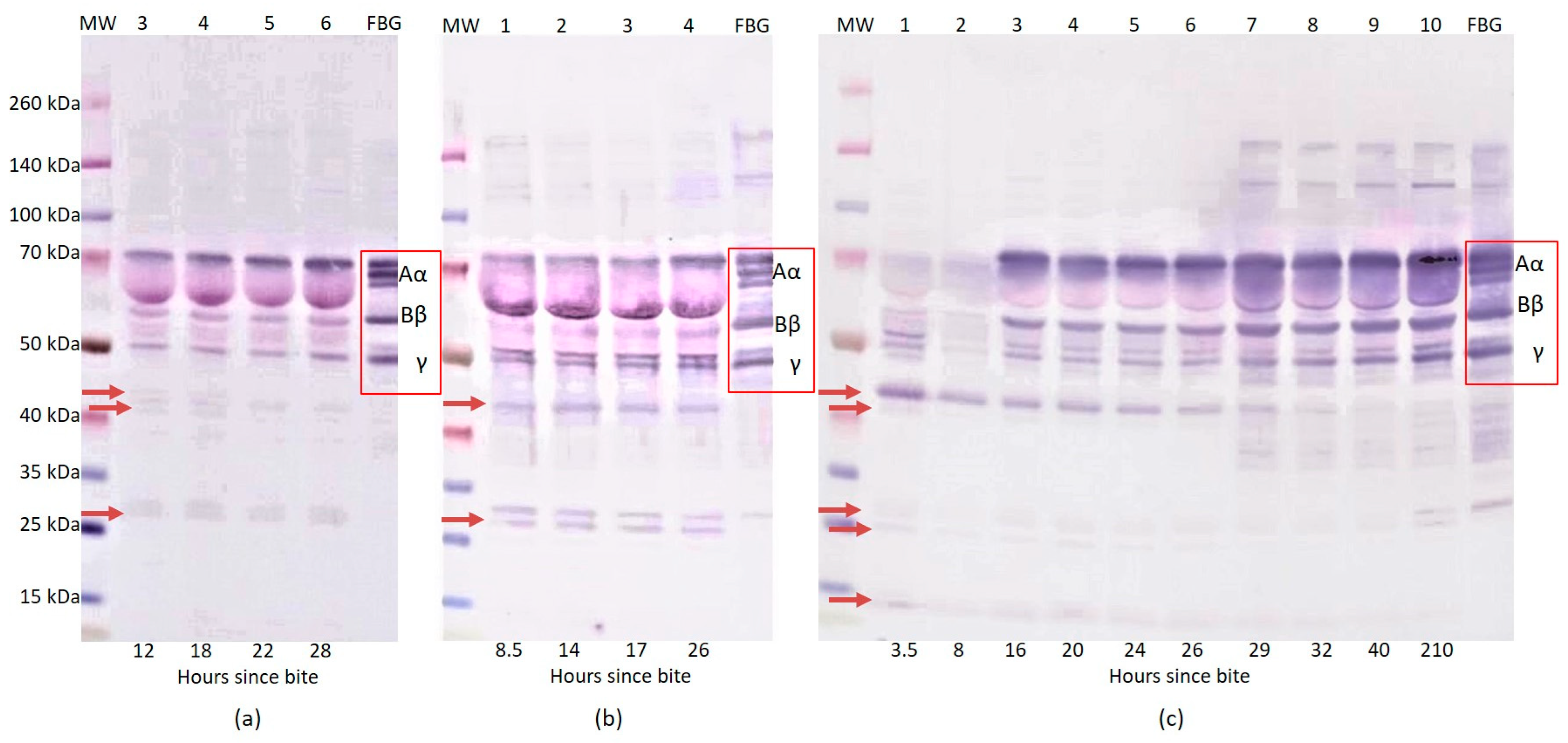
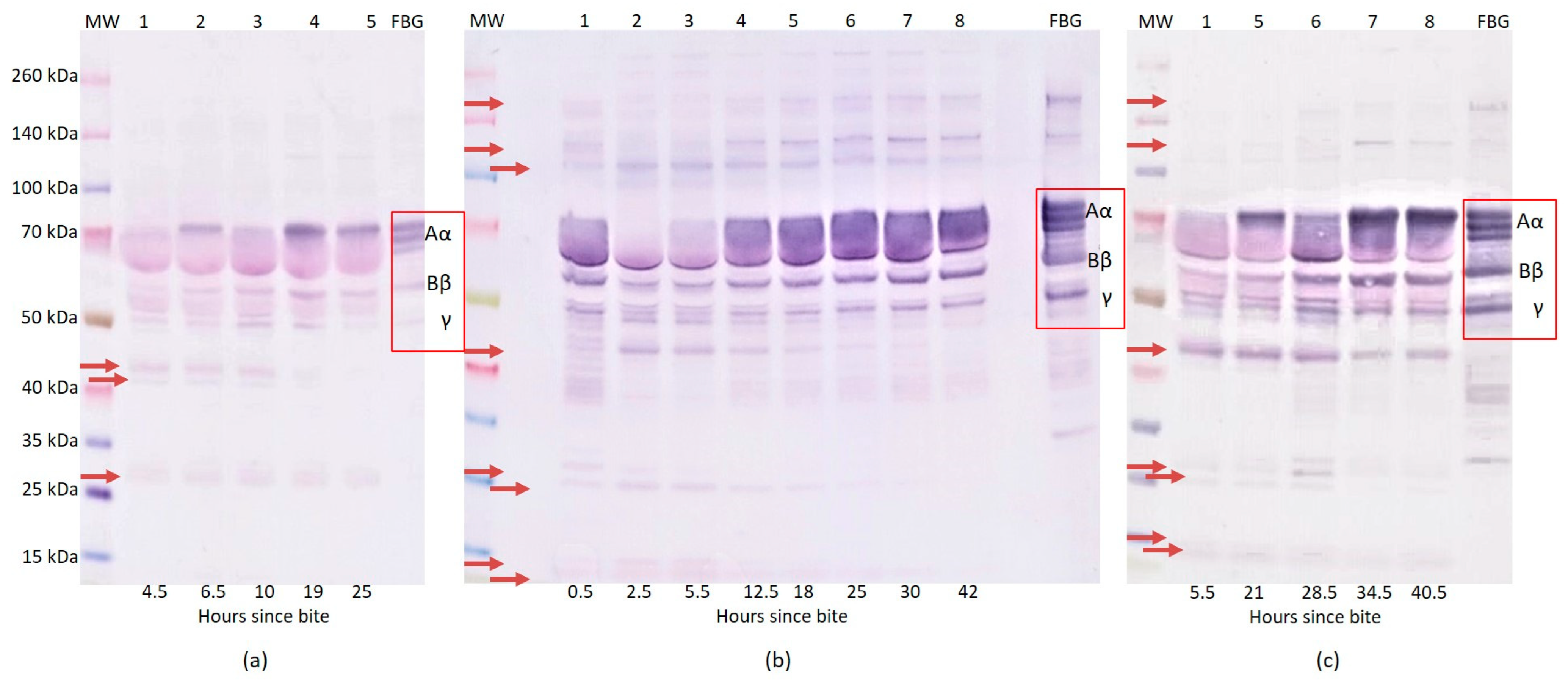
2.1. Western Blot Analysis of Fibrinogen
2.2. Changes in Fibrinogen Levels
| Hours Since Bite | 4.5 | 6.5 | 10 | 19 | 25 |
|---|---|---|---|---|---|
| PT/INR | 1.61 | >10 | 6.22 | 1.22 | 1.11 |
| APTT (s) | 31.1 | >180 | >180.0 | 27.9 | 26.9 |
| TT (s) | 52.4 | >180 | >180.0 | 18.3 | 15.8 |
| AT III (% activity) | 89 | 95 | 87 | 87 | 96 |
| FBG Claus (g/L) | <0.01 | <0.01 | <0.01 | 0.24 | 1.02 |
| D-dimers (μg/L) | 4550 | 6470 | 9874 | 5019 | 3414 |
| PLT (×109/L) | NA | NA | NA | 234 | NA |
| Antivenom (vials) | 3 | ||||
| Sample No. | 1 | 2 | 3 | 4 | 5 |
| Hours Since Bite | 0.5 | 2.5 | 5.5 | 12.5 | 18 | 25 | 30 | 42 |
|---|---|---|---|---|---|---|---|---|
| PT/INR | 1.23 | >10 | >10 | 1.55 | 1.33 | 1.07 | 1.07 | 1.03 |
| APTT (s) | 45.8 | >180 | >180 | 34.2 | 29.2 | 24.9 | 24.3 | 25 |
| TT (s) | 23.4 | >180 | >180 | 32.6 | NA | 18.6 | 17 | 15.7 |
| AT III (% activity) | 85 | 90 | 93 | 83 | NA | 93 | 90 | 90 |
| FBG Claus (g/L) | 0.48 | <0.10 | 0.1 | 0.18 | 0.41 | 0.81 | 1.62 | 2.07 |
| D-dimers (μg/L) | 13,431 | 10,351 | 10,615 | 11,505 | NA | 16,440 | 8834 | 4428 |
| PLT (×109/L) | 225 | 182 | 244 | 198 | 201 | NA | 166 | 155 |
| Antivenom (vials) | 3 | 3 | ||||||
| FFP TU | 3 | |||||||
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Hours Since Bite | 5.5 | 9.0 | 13.0 | 17.0 | 21.0 | 28.5 | 34.5 | 40.5 |
|---|---|---|---|---|---|---|---|---|
| PT/INR | 1.47 | 1.39 | 1.23 | 1.16 | 1.14 | 1.12 | 1.16 | 1.13 |
| APTT (s) | 37 | 39 | 36.2 | 33.2 | 32.6 | 34.3 | 36.6 | 31.6 |
| TT (s) | 23 | 21.1 | 17.4 | 15.3 | 13.4 | 13.9 | 13.9 | 13.3 |
| AT III (% activity) | 62 | 65 | 69 | 62 | 64 | 67 | 63 | 69 |
| FBG Claus (g/L) | 1.31 | 1.32 | 1.75 | 2.17 | 2.45 | 2.41 | 2.36 | 2.53 |
| D-dimers (μg/L) | 8217 | >6400 | >6400 | >6400 | 5189 | 2314 | 1438 | 1276 |
| PLT (×109/L) | 88 | NA | NA | 46 | 39 | 30 | 21 | 48 |
| PLT (TU) | 1 | |||||||
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
2.3. Changes in Other Hemocoagulation Parameters
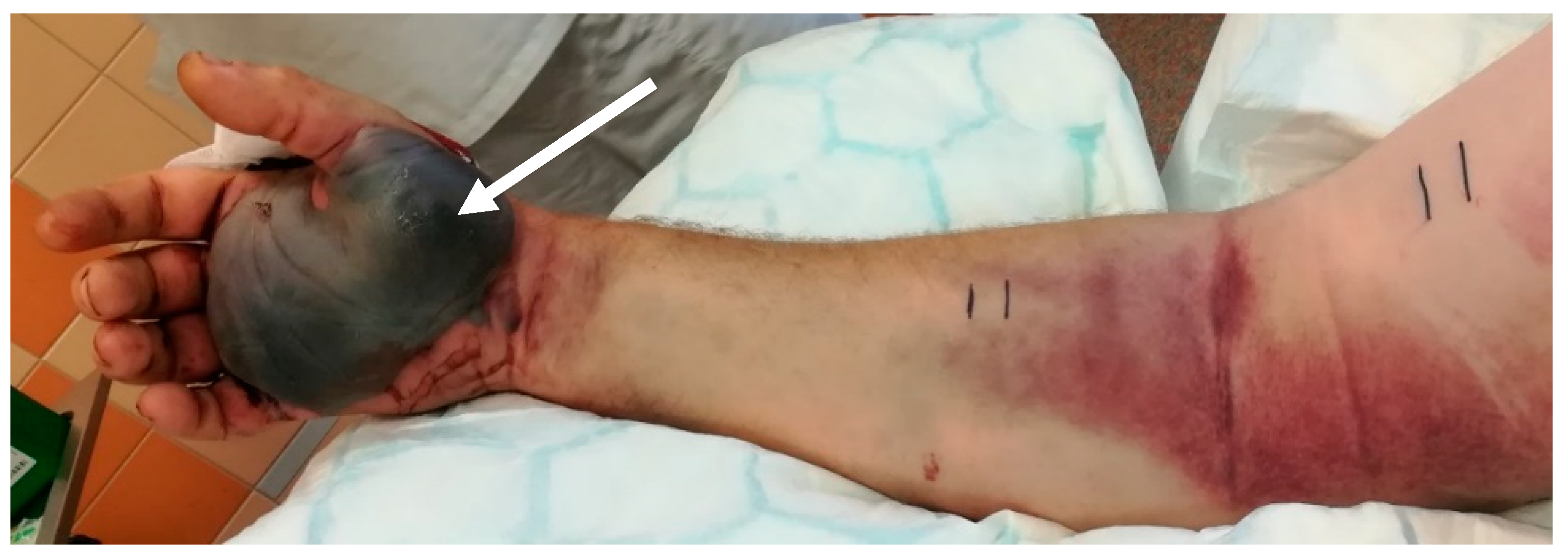
2.4. Clinical Course
3. Discussion
4. Conclusions
5. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- White, J. Snake venom and coagulopathy. Toxicon 2005, 45, 951–967. [Google Scholar] [CrossRef]
- Al-Sadawi, M.; Mohamadpour, M.; Zhyvotovska, A.; Ahmad, T.; Schechter, J.; Soliman, Y.; McFarlane, S.I. Cerebrovascular accident and snake envenomation: A scoping study. Int. J. Clin. Res. Trials 2019, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Noutsos, T.; Currie, B.J.; Wijewickrama, E.S.; Isbister, G.K. Snakebite associated thrombotic microangiopathy and recommendations for clinical practice. Toxins 2022, 14, 57. [Google Scholar] [CrossRef]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef]
- Berling, I.; Isbister, G.K. Hematologic effect and complications of snake envenoming. Transfus. Med. Rev. 2015, 29, 82–89. [Google Scholar] [CrossRef]
- Mosesson, M.W.; Siebenlist, K.R.; Meh, D.A. The structure and biological features of fibrinogen and fibrin. Ann. N. Y. Acad. Sci. 2001, 936, 11–30. [Google Scholar] [CrossRef]
- Valenta, J.; Stach, Z.; Porizka, M.; Michalek, P. Analysis of hemocoagulation tests for prediction of venom-induced consumption coagulopathy development after Viperidae bite. Bratisl. Med. J. 2019, 120, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: Their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Haemostasis 2001, 31, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Markland, F.S., Jr. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef]
- Senis, Y.A.; Kim, P.Y.; Fuller, G.L.J.; García, A.; Prabhakar, S.; Wilkinson, M.C.; Brittan, H.; Zitzmann, N.; Wait, R.; Warrell, D.A.; et al. Isolation and characterization of cotiaractivase, a novel low molecular weight prothrombin activator from the venom of Bothrops cotiara. Biochim. Biophys. Acta 2006, 1764, 863–871. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Zhou, M.; Wang, L.; Chen, T.; Shaw, C. Molecular characterization of three novel phospholipase A2 proteins from the venom of Atheris chlorechis, Atheris nitschei and Atheris squamigera. Toxins 2016, 8, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCleary, R.J.R.; Kini, R.M. Snake bites and hemostasis/thrombosis. Thromb. Res. 2013, 132, 642–646. [Google Scholar] [CrossRef] [PubMed]
- Rojnuckarin, P. Snake Venom and Hemostasis. In Clinical Toxinology in Asia Pacific and Africa. Toxinology; Gopalakrishnakone, P., Faiz, A., Fernando, R., Gnanathasan, C., Habib, A., Yang, C.C., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 2, pp. 415–435. [Google Scholar] [CrossRef]
- Isbister, G.K. Procoagulant snake toxins: Laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin. Thromb. Hemost. 2009, 35, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venom and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Budzynski, A.Z.; Pandya, V.B.; Rubin, R.N.; Brizuela, B.S.; Soszka, T.; Steward, G.J. Fibrinogenolytic fibrinogenemia after envenomation by Western diamondback rattlesnake (Crotalus atrox). Blood 1984, 63, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paes Leme, A.F.; Prezoto, B.C.; Yamashiro, E.T.; Bertholm, L.A.; Tashima, K.; Klitzke, C.F.; Camargo, A.C.M.; Serrano, S.M.T. Bothrops protease A, a unique highly glycosylated serine proteinase, is a potent, specific fibrinogenolytic agent. J. Thromb. Haemost. 2008, 6, 1363–1372. [Google Scholar] [CrossRef]
- Lancellotti, S.; Rutella, S.; De Filippis, V.; Pozzi, N.; Rocca, B.; De Cristofaro, R. Fibrinogen-elongated chain inhibits thrombin-induced platelet response, hindering the interaction with different receptors. J. Biol. Chem. 2008, 283, 30193–30204. [Google Scholar] [CrossRef] [Green Version]
- Maruñak, S.L.; Acosta, O.C.; Leiva, L.C.; Ruiz, R.M.; Aguirre, M.V.; Teibler, P. Mice plasma fibrinogen consumption by thrombin-like enzyme present in rattlesnake venom from the north-east region of Argentina. Medicina 2004, 64, 509–517. [Google Scholar]
- Pizzo, S.V.; Schwartz, M.L.; Hill, R.L.; McKee, P.A. The effect of plasmin on the subunit structure of human fibrinogen. J. Biol. Chem. 1972, 247, 636–645. [Google Scholar] [CrossRef]
- Mosesson, M.W.; Holyst, T.; Hernandez, I.; Siebenlist, K.R. Evidence for covalent linkage between some plasma a2-antiplasmin molecules and Aa chains of circulating fibrinogen. J. Thromb. Haemost. 2013, 11, 995–998. [Google Scholar] [CrossRef]
- Rodrigues, C.R.; Molina Molina, D.A.; de Souza, D.L.N.; Cardenas, J.; Costal-Oliveira, F.; Guerra-Duarte, C.; Chávez-Olórtegui, C. Biological and proteomic characterization of the venom from Peruvian Andes rattlesnake Crotalus durissus. Toxicon 2022, 207, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.B.; Nesheim, M.E. The molecular weights, mass distribution, chain composition, and structure of soluble fibrin degradation products released from a fibrin clot perfused with plasmin. J. Biol. Chem. 1999, 274, 5201–5212. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, C.S.; Enghild, J.J.; Mary, A.; Dobson, J.V.; Achyuthan, K.E. Isolation of a fibrin-binding fragment from blood coagulation factor XIII capable of cross-linking fibrin(ogen). Biochem. J. 1988, 256, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D.; Holada, K.; Kornalik, F.; Simak, J.; Vankova, H.; Müller, D.; Schoenemann, H.; Lange, H.; Herrmann, H.W. Severe coagulopathy after a bite of a green bush viper (Atheris squamiger): Case report and biochemical analysis of the venom. Toxicon 1998, 36, 1333–1340. [Google Scholar] [CrossRef]
| Hours Since Bite | 0.75 | 3.75 | 12 | 18 | 22 | 28 | 36 |
|---|---|---|---|---|---|---|---|
| PT/INR | 0.94 | 1.02 | 1.13 | 1.38 | 1.35 | 1.31 | 1.17 |
| APTT (s) | 26.1 | 27.8 | 27.8 | 36.8 | 34.8 | 33.2 | 32.8 |
| TT (s) | NA | NA | NA | 22.8 | 19.4 | 16.8 | 15.0 |
| AT III (% activity) | 105 | 84 | 74 | 81 | 76 | 77 | 81 |
| FBG Claus (g/L) | 2.6 | 1.6 | 0.3 | 0.2 | 0.3 | 0.3 | 0.94 |
| D-dimers (μg/L) | 210 | 3110 | 18,500 | 5190 | 2226 | 1100 | 691 |
| PLT (×109/L) | 186 | NA | 159 | 180 | 172 | 172 | 180 |
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| Hours Since Bite | 8.5 | 14 | 17 | 26 | 33 | 38 | 44 | 50 |
|---|---|---|---|---|---|---|---|---|
| PT/INR | 1.15 | 1.35 | 1.68 | 1.43 | 1.49 | 1.38 | 1.28 | 1.23 |
| APTT (s) | 29.1 | 33.6 | 38.2 | 31.4 | 36.8 | 35.4 | 33.8 | 30.6 |
| TT (s) | 31.7 | 46.3 | 60.1 | 45.4 | 33.5 | 24.4 | 20.5 | 18.6 |
| AT III (% activity) | 89 | 89 | 89 | 81 | 85 | 88 | 87 | 80 |
| FBG Claus (g/L) | 0.8 | 0.14 | 0.05 | 0.11 | 0.2 | 0.23 | 0.59 | 0.65 |
| D-dimers (μg/L) | 2616 | 4526 | 5096 | 2176 | 890 | 575 | 85 | 476 |
| PLT (×109/L) | 50 | 45 | 44 | 142 | NA | 130 | NA | 121 |
| Antivenom (vials) | 3 | |||||||
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Hours Since Bite | 3.5 | 8 | 16 | 20 | 24 | 26 | 29 | 32 | 40 | 210 |
|---|---|---|---|---|---|---|---|---|---|---|
| PT/INR | >10 | >10 | 3.89 | 4.41 | 4.44 | 2.38 | 1.27 | 1.18 | 1.14 | 1.1 |
| APTT (s) | >180 | >180 | 56.5 | 56.9 | 62 | 42.1 | 31.9 | 32.5 | 27.2 | 31.7 |
| TT (s) | >180 | >180 | 25.2 | 25 | 33.6 | 26 | 16.5 | 16.8 | 14.4 | 12.7 |
| AT III (% activity) | 61 | 58 | 55 | 59 | 55 | 59 | 65 | 66 | 77 | 78 |
| FBG Claus (g/L) | 0.1 | 0.16 | 1.13 | <0.01 | <0.01 | 0.1 | 1.27 | 1.35 | 1.65 | 2.97 |
| D-dimers (μg/L) | 7321 | 4591 | 4269 | 5251 | 2078 | 1817 | 633 | 529 | 274 | 378 |
| PLT (×109/L) | 84 | NA | 174 | NA | NA | NA | 154 | NA | 130 | NA |
| Antivenom (vials) | 4 | 3 | 4 | |||||||
| FFP (TU) | 6 | |||||||||
| Sample No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valenta, J.; Hlavackova, A.; Stach, Z.; Stikarova, J.; Havlicek, M.; Michalek, P. Fibrinogenolysis in Venom-Induced Consumption Coagulopathy after Viperidae Snakebites: A Pilot Study. Toxins 2022, 14, 538. https://doi.org/10.3390/toxins14080538
Valenta J, Hlavackova A, Stach Z, Stikarova J, Havlicek M, Michalek P. Fibrinogenolysis in Venom-Induced Consumption Coagulopathy after Viperidae Snakebites: A Pilot Study. Toxins. 2022; 14(8):538. https://doi.org/10.3390/toxins14080538
Chicago/Turabian StyleValenta, Jiri, Alzbeta Hlavackova, Zdenek Stach, Jana Stikarova, Marek Havlicek, and Pavel Michalek. 2022. "Fibrinogenolysis in Venom-Induced Consumption Coagulopathy after Viperidae Snakebites: A Pilot Study" Toxins 14, no. 8: 538. https://doi.org/10.3390/toxins14080538
APA StyleValenta, J., Hlavackova, A., Stach, Z., Stikarova, J., Havlicek, M., & Michalek, P. (2022). Fibrinogenolysis in Venom-Induced Consumption Coagulopathy after Viperidae Snakebites: A Pilot Study. Toxins, 14(8), 538. https://doi.org/10.3390/toxins14080538






