Abstract
Microorganisms, virus, weeds, parasitic plants, insects, and nematodes are among the enemies that induce severe economic losses to agrarian production. Farmers have been forced to combat these enemies using different methods, including mechanical and agronomic strategies, since the beginning of agriculture. The development of agriculture, due to an increased request for food production, which is a consequence to the rapid and noteworthy growth of the world’s population, requires the use of more efficient methods to strongly elevate the yield production. Thus, in the last five-to-six decades, a massive and extensive use of chemicals has occurred in agriculture, resulting in heavy negative consequences, such as the increase in environmental pollution and risks for human and animal health. These problems increased with the repetition of treatments, which is due to resistance that natural enemies developed against this massive use of pesticides. There are new control strategies under investigation to develop products, namely biopesticides, with high efficacy and selectivity but based on natural products which are not toxic, and which are biodegradable in a short time. This review is focused on the microbial and plant metabolites with nematocidal activity with potential applications in suitable formulations in greenhouses and fields.
Key Contribution:
Nematodes are among the pests that attack agrarian, forest, and ornamental plants, causing heavy economic and environmental problems. Nematode control up until today has been managed using chemical pesticides, which have generated great environmental pollution and risks for human and animal health. The review reported microbial and plant metabolites with nematocidal activity, and highlights their potential for practical application as biopesticides.
1. Introduction
Since ancient times, agriculture has been developed to produce food to satisfy the human needs. This request became an emergency in parallel with the increasing world population, which could reach to almost 10 billion by 2050 [1,2]. Unfortunately, this request was negatively affected by a strong reduction in natural resources, the diffused environmental pollution, and noteworthy climate changes [3,4]. In addition to these factors, farmers are forced to combat the natural enemies of agrarian plants, such as pathogens, bacteria, fungi, viruses, weeds, parasitic plants, dangerous insect, and nematodes [5,6,7]. The spread and survival of these enemies has been controlled using different methods, including mechanic and agronomy strategies. However, in the last five-to-six decades, a massive and extensive use of chemicals has occurred, with heavy consequences in terms of environmental pollution and risks to human and animal health. These problems have increased with the repetition of treatments, which are due to the resistance that enemies have developed against the pesticides used for a long time [5,8]. Thus, several multidisciplinary research groups have investigated new control strategies to develop products with high efficacy and selectivity against agrarian pests but based on natural products which are not toxic, and which are biodegradable in a short time [8,9,10,11]. Regarding the previous reviews on metabolites with nematocidal activity isolated from natural sources, SciFinder research was used to locate the article of Lorenzen and Anke (1998) [12], which describes some metabolites with insecticidal and nematocidal activities together with several natural compounds with cytotoxic, antitumoral, antiviral, and phytotoxic activities. Another review extensively describes the insecticidal activity of fungal metabolites, while only a short paragraph is dedicated to the fungal compounds with nematocidal activity. In particular, the peptides produced by Omphalotus spp. [13] are reported. In addition, a review only describes the fungal metabolites belonging to the azophilone family, with several different biological activities with very few compounds showing nematocidal activity [14]. Similar content is reported by Shen et al. (2015) [15], but in terms treating benzenediol lactones with a variable structures and belonging to family of fungal polyketides. Mao et al. (2014) [16] describes natural dibenzo-α-pyrones produced by fungi, mycobionts, plants, and animal feces which exhibit a variety of biological activities including nematocidal properties. Similar content was previously described by Ghisalberti (2002) [17]. Another review only describes the saponins isolated from Medicago sativa L., alfalfa, which is the most known plant species within the Medicago genus, and their nematocidal activity against different nematodes species [18]. Another review deals with extracts or secondary metabolites of the Mexican flora that showed biological activity against dangerous insects or parasitic nematodes [19]. A review about the metabolites produced by extremophilic fungi and belonging to different classes of natural compounds reported them as having different biological activities, including a nematocidal one [20]. One of the last biocontrol methods reported to combat nematodes is based on the changes in soil microbial community, the release of nematocidal compounds, and the induction of plant defenses. This strategy essentially uses biochar-based soil amendments, which is ecofriendly and compatible with a circular economy. However, as biochars induce complex and distinct modes of action, their nature and application regimes should be studied for particular pathogens and their effects must be locally checked [21]. Thus, all previous reviews only partially report about natural compounds with nematocidal activity, as some are restricted to one family of fungal or plant metabolites, some others report metabolites from different natural sources, and they also describe a lot of diverse natural compounds with different biological activities. Others extensively discuss fungal metabolites with insecticidal activity and few compounds with nematocidal activity, and some also cover a short time-span within the literature (2005–2020).
Thus, the present manuscript reports, for the first time, an overview which is focused on the microbial and plant metabolites with nematocidal activity with potential applications in suitable formulations in greenhouses and fields. The results discussed in the different sections were obtained from SciFinder research covering from 1995 to today, and these are chronologically reported in each paragraph.
2. Bacterial Metabolites
This section chronologically describes the source, structure, and biological activity of the bacterial metabolites, most of which showed nematocidal activity together with other interesting biological activities.
The avermectins and milbemycms are closely related 16-membered macrocyclic lactones produced by actinomycetes from the genus Streptomyces. Streptomyces avermentilis synthesized avermectins while Streptomyces hygroscopicus and Streptomyces cyaneogriseus produced milbemycms. Avermectins and milbemycins, are constituted by the identical 16-membered macrocyclic backbones which is fused with a hexahydrobenzofuran unit from C-2 to C-8a and a spiroketal unit from C-17 to C-25. The main difference diffrences between the two groups is the substituent at C-13 of macrolactone that is a disaccharaide in avermectin, whereas that position is unsubstituted in milbemycin. Avermectin B1a and milbemycin D (1 and 2, Figure 1), are representative members of the two groups and thus compound 2 appeared to be the aglyvcone of metabolite 1. The avermectins are produced as a mixture of eight different components designed a A1a, A1b, A2a, A2b, B1a, B1b, B2a, and B2b. The A-components have a methoxy group at the 5-position, whereas the B-components have a hydroxy group; the 1-components have a double bond between the 22- and 23-position, whereas the 2-components have a single bond with a hydroxy group at the 23-position. Finally, the a-components have a secondary butyl side chain at the 25-position, whereas the b-components have an isopropyl substituent at the 25-position. Several different alkyl substituents at C-25 can be also present in both groups. B1 analogues showed, followed by B2 ones, the highest toxicity and breadth of spectrum against Haemonchus contortus, Ostertagia circumcincta; Trichostrongylus axei; Trichostrongylus colubriformis; Cooperia spp.; Oesophagostomum columbianu, with a LD50 values in mice approximatively ranking between 15 and 50 mg/kg. Study on the mode of action of both groups using Caenorhabditis elegans showed that avermectins and milbemycins mediate their nematocidal activity through interaction with the common receptor, which is glutamate-gated chloride channel. Furthermore, during the same investigation, also the EC50 value of 89 nM was determined for milbemycin D, and a LD50 values 1.56 and 32.9 µg/mL were calculated for avermectin B1a based on 2 h exposure for Meloidogyne incognita or Rotylenchulus reniformis [22].
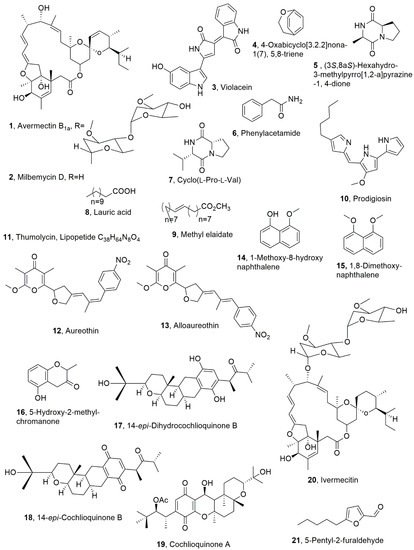
Figure 1.
Metabolites with nematocidal activity produced by Streptomyces avermentilis (1), Streptomyces hygroscopicus and Streptomyces cyaneogriseus (2), several gram-negative bacteria (3), Bacilus strains (4–9), Serriata marcescens (10), Bacillus thuringiensis (11), Streptomyces (12 and 13), Daldinia concentrica (14–16), Neobulgaria pura (17 and 18), Helminthosporium sp. (19), Streptomyces avermitilis (20), and an unidentified species of Dermateaceae (21).
Violacein (3, Figure 1) is a natural violet pigment produced by several gram-negative bacteria, such as Chromobacterium violaceum, Janthinobacterium lividum, Pseudoalteromonas tunicata D2, Collimonas sp., Duganella sp., and Pseudoalteromonas spp. Compound 3 showed different potential pharmacological applications, such as in antibacterial, antitrypanocidal, anti-ulcerogenic, and anticancer drugs [23]. Violacein (3) was involved in oxidative stress resistance in C. violaceum [24]. Violacein (3), when produced by J. lividum, seemed to be involved in the natural defense of amphibians against fungal disease [25]. Furthermore, the marine bacterium Pseudoalteromonas tunicate produced compound 3, which could act as an antipredator defense mechanism against protozoan grazers [26]. Other biological activities of violacein (3) include antioxidant, leishmanicidal, antifungal, and antiviral activities. Violacein (3) showed nematocidal activity against Caenorhabditis elegans with LC50 > 30 mM. In addition, the bacterial accumulation in the nematode intestine, which determined tissue damage and apoptosis, was induced by compound 3 and using Escherichia coli. This process occur also in nematodes, such as C. elegans, which activate a well-defined innate immune system to defend against pathogens. This defense mechanism, studied with compound 3 isolated from marine bacterium Microbulbifer sp. D250, employs the DAF-2/DAF-16 insulin/IGF-1 signaling (IIS) component to modulate sensitivity to violacein-mediated killing. On the basis of these results, violacein appeared to be a potential antinematode [27].
Here, 4-Oxabicyclo[3.2.2]nona-1(7), 5,8-triene was isolated together with the well-known compounds, such as (3S,8aS)-hexahydro-3-methylpyrro[1,2-a]pyrazine-1,4-dione, phenylacetamide, cyclo(L-Pro-L-Val), lauric acid, and methyl elaidate (4–9, Figure 1), from the culture filtrates of Bacillus strain SMrs28, which was obtained from the rhizosphere soil of the toxic plant Stellera chamaejasme [28]. In preliminary tests, the bacterial culture filtrates showed strong nematocidal activity against the pinewood nematode Bursaphelenchus xylophilus, which is a major threat to forestry leading to a billion US dollars of economic losses per year, and the tuber rot nematode Ditylenchus destructor [29]. In fact, compounds 4–6 showed toxicity against both nematode B. xylophilus and D. destructor, with a LC50 values of 904.12, 451.26, and 232.98 μg/mL and 1594.0, 366.62, and 206.38 μg/mL at 72 h, respectively [29].
Prodigiosin (10, Figure 1) is a red pigment produced from a few bacteria species, such as Serratia, Pseudomonas, and Streptomyces. Its isolation and structural characterization was first reported when compound 10 was obtained from massive culture filtrates of Serratia sp. KH-95b [30]. Prodigiosin (10), isolated from Serratia marcescens, was tested against nematodes at their juvenile stage and showed an effect against juvenile stages of Radopholus similis and Meloidogyne javanica at low concentrations (LC50 values, 83 and 79 μg/mL, respectively) as compared with the positive control of copper sulphate (LC50 values, 380 and 280 μg/mL, respectively). The pigment also exhibited inhibition on nematode egg-hatching ability [31].
Thumolycin (11, Figure 1), which is a lipopeptide, was isolated from Bacillus thuringiensis, which is a bacterium widely used as a bio-insecticide. Thumolycin has a molecular weight of 696.51 Da and a predicted molecular formula of C38H64N8O4, but its structure has not yet been determined. Compound 11 showed a broad spectrum of antimicrobial and nematocidal activities. In particular, C. elegans was significantly inhibited by thumolycin (11), which, when tested at a higher concentration, induced a higher mortality in this nematode. When C. elegans was treated with 600 U of thumolycin, less than 10% nematodes survived. These experiments indicated that thumolycin has inhibitory activity against a wide range of bacteria and effective nematocidal activity [32].
Aureothin and alloaureothin (12 and 13, Figure 1) were isolated from an endophytic bacterium strain, which was identified as Streptomyces sp. AE170020, and appeared to be a rich source of bioactive secondary metabolites with potential as environmentally benign agents. In fact, both metabolites 12 and 13 showed a significant nematocidal activity suppressing the growth, reproduction, and behavior of B. xylophilus. Pine trees are one of the most important forest plants in ecosystems, as they are widespread in natural reserves, parks, and urban ornamental landscapes, and are also source of wood with high economic value for different practical uses. Unfortunately, pine is affected by different pests, such as the pathogen fungus Sphaeropsis sanipea, a producer of phytotoxins [33], and by pine wood nematode (PWN). The B. xylophilus PWN is the causal agent of most serious pine wilt diseases. In in vivo experiments, extracts of a strain of Streptomyces sp. AE170020 significantly suppressed the development of pine wilt disease in 4-year-old plants of Pinus densifora. The LC50 values of aureothin (12) on different life stages of B. xylophilus (J2s, J3s, and J4s/adults) were 0.81, 1.15, and 1.54 μg/mL, respectively, while the values of alloaureothin (13) were 0.83, 1.10, and 1.47 μg/mL, respectively. Compared with the positive control abamectin, both compounds showed higher nematocidal activity against B. xylophilus at all tested life stages, and exhibited similar mortality rates. Thus, compounds 12 and 13 represent an important tool to combat B. xylophilus, and could be proposed for a suitable bioformulation to develop a natural nematicide [34]
3. Fungal Metabolites
This section chronologically reports the source, structure, and biological activity of the fungal metabolites, most of which showed nematocidal activity together with other interesting biological activities.
Some studies were carried out on five Arthropotrys strains to examine their ability to produce metabolites with nematocidal activity. In fact, the well-known linoleic acid was isolated from Arthrobotrys conoides and Arthrobotrys oligospora [35]. A lot of fungi belonging to Ascomycetes Pyrenomycetes and Discomycetes genera were the object of a similar investigation. A total of 29 isolates belonging to 18 genera produced metabolites with nematocidal activity against C. elegans, out of a total of 267 extracts of culture filtrates of the different stains examined [35]. Here, 1-Methoxy-8-hydroxynaphthalene, 1,8-dimethoxynaphthalene, and 5-hydroxy-2-methyl chromanone (14–16, Figure 1) were isolated from Daldinia concentrica [36]. Both naphthalene derivatives (14 and 15) showed nematocidal activity against C. elegans with LD50 values of 10 and 25 μg/mL, respectively, in addition to cytotoxic and antimicrobial effects, while the chromanone 16 had no nematocidal activity [35].
Here, 14-Epi-dihydrocochlioquinone B and 14-epi-cochlioquinone B (17 and 18, Figure 1) were isolated from Neobulgaria pura [37] and showed nematocidal activity towards C. elegans and Meloidogyne incognita [35]. Furthermore, the close cochlioquinone A (19, Figure 1), which was produced by a Helminthosporium species, competed for the ivermectin binding site on the membrane receptor in nematodes [38]. Ivermectin is the didroderivative of averctim, which as abovereported was originally isolated from soil in Japan as a part of a collaborative program to select microorganisms on the basis of novel microbiological characteristics and a wide variety of pharmacological and chemotherapeutic assays [39].
Here, 5-Pentyl-2-furaldehyde (21, Figure 1), was isolated as a metabolite with nematocidal activity from the culture filtrates of an unidentified species of the Dermateaceae family. The strain was collected in Australia. Compound 21 was also isolated from Irpex lacteus, which is a wood-inhabiting basidiomycete [40], and showed moderate activity against Aphelenchoides besseyi, M. incognita, and C. elegans with LD50 values 60 of and 75 μg/mL when tested on the penultimate and last nematode, respectively [40].
Lachnumon (I), lachnumol A, mycorrhizin A, chloromycorrhizin A, and (1’-E)-dechloromycorrhizin A and its Z-isomer (22–27, Figure 2) were isolated from Lachnum papyraceum, which was collected in southern Germany. All the metabolites showed nematocidal activity against C. elegans but appeared almost inactive towards M. incognita [41,42]. The LD50 values recorded for compounds 22–27 were 25, 5, 1, 100, 2, and 2 μg/mL, respectively [35]. Furthermore, the effect the of substitution of chlorine, which is present in compounds 22–25, with bromine was investigated by growing the fungus in a medium containing CaC12 with an excess of CaBr2. From the organic extract of these culture filtrates, lachnumol A and mycorrhizin A (23 and 24) were isolated in traces while the other were absent. Furthermore, six derivatives of mellein were highlighted by analytical HPLC [42,43]. They were identified as 6-hydroxymellein, 4-chloro-6-hydroxymellein, 6-methoxymellein, 4-bromo-6-hydroxmellein, 4-chloro-6-methoxymellein 6,7-dihydroxymellein, and 4-chloro-6,7-dihydroxymellein (28–34, Figure 2). All the mellein analogues showed no nematocidal activity except the moderate effect exhibited by compound 34 against C. elegans [35]. Hydroxymellein (28), which was previously isolated together with mycorrhizin A (24) and gilmicolin from cultures of Gilmaniella humicola, was proposed to be a biosynthetic precursor of mycorrhizin A and gilmicolin [44,45]. On the basis of these results, the hypothesized steps leading from 6-hydroxymellein to lachnumols and mycorrhizins seemed almost blocked, and it seemed that 6-hydroxymellein, which accumulates, could be transformed into derivatives 28–34. Here, 4-Chloro- and 4-bromo-derivatives (29 and 31) were also synthesized. In another experiment, CaBr2 was later added to the medium, and other different metabolites were produced as bromo-containing analogues. They were lachnumons B1 and B2 (39 and 39, Figure 2) and mycorrhizins B1 and B2 (40 and 41, Figure 2) [41,46,47], together with mycorrhizinol (46, Figure 2), which was previously isolated from Gilmaniella humicola [43]. In addition, other metabolites were isolated, such as papyracons A-G (35–37 and 42–45, Figure 2) and metabolite 47 (Figure 2). The last compound (47), which is trisubstituted 4H-sprobenzofuran-7-(5H)-one, was identified as (1′S,2′R,5S)-5-hydroxy-2′-((R)-1-hydroxyethyl)-2-methyl-4H-spiro[benzofuran-6,1′-cyclopropan]-7(5H)-one. All five bromo-analogues (31 and 38–41) bear the bromine in the side chain. The brominated and the chlorinated compounds had very similar biological activities however, brominated lachnumons (38 and 39) or mycorrhizins (40 and 41) were slightly less active than their corresponding chlorinated analogues. All rnycorrhizins exhibited high activities against C. elegans with LD50 values recorded for compounds 38–41, 46 and 35–37, and 42–45 of 50, 25, 2 and 5, 100, and 25, 50, and 50, and 50, 50, 50, and 50 μg/mL, respectively, but are practically inactive against M. incognita [35].
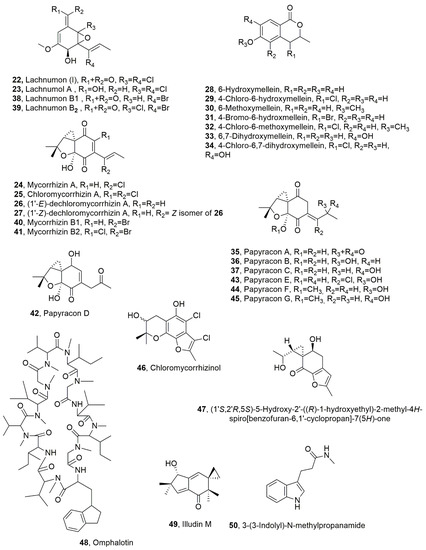
Figure 2.
Metabolites produced by Lachnum papyraceum and in part also from Gilmaniella humicola (22–34, 35–37, 38–41, 42–45, and 47) and by Omphalotus olearius (48–50).
Omphalotin (48, Figure 2), which is a cyclic peptide, was isolated together with sesquiterpene illudin M and the 3-(3-indolyl)-N-methylpropanamide (49 and 50, Figure 2) from the cultures of Omphalotus olearius. This fungus is a wood-inhabiting basidiomycete that spreads worldwide, particularly on olive, oak, and chestnut trees. Omphalotin (48) tested against M. incognita and C. elegans was more active than nematicide ivermectin, which is now commercialized, with LD50 18.95 and 0.57 μg/mL values. Compounds 49 and 50 were inactive against both nematode up to 100 μg/mL [48].
A metabolite, named MK7924 (51, Figure 3), with nematocidal activity, was isolated from the culture filtrates of Coronophora gregaria L2495. Here, MK7924 (51), which is a highly methylated polyketide bearing two mannose residues, was related to the antibiotics TMC-151s [49,50], TMC154s [51], TMC-171s [51], and roselipins which are inhibitors of diacylglycerol acyltransferase [52,53,54]. Although compound 51 did not show antibacterial activity against B. subtilis, S. aureus, E coli, or P. aeruginosa, it exhibited antifungal activity against Aspergillus niger and nematocidal activity against C. elegans [55].
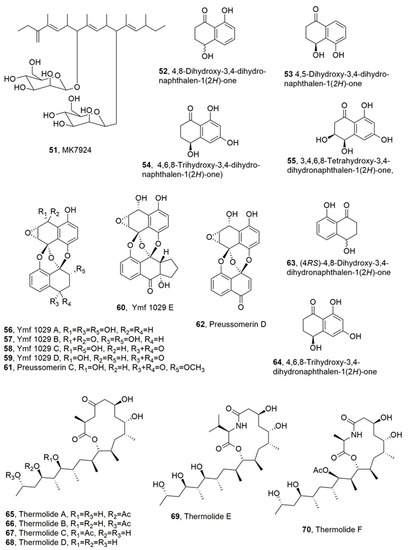
Figure 3.
Metabolites produced by Coronophora gregaria (51), Coronophora gregaria (52–55), unidentified freshwater fungus YMF 1.01029 (56–64), and Talaromyces thermophilus (65–70).
Four naphthalenones, namely 4,8-dihydroxy-3,4-dihydronaphthalen-1(2H)-one, 4,5-dihydroxy-3,4-dihydronaphthalen-1(2H)-one, 4,6,8-trihydroxy-3,4-dihydronaphtha- len-1(2H)-one), and 3,4,6,8-tetrahydroxy-3,4-dihydronaphthalen-1(2H)-one, also known as cis-4-hydroxyscytalone, (52–55, Figure 3) were isolated from the culture filtrates of Caryospora callicarpa [56]. The fungus was collected from a freshwater habitat in Yunnan Province, China. All four metabolites showed noteworthy nematocidal activity against B. xylophilus, which is both a plant-parasitic and fungal-feeding nematode that causes multimillion dollar loses to pine forests, especially in some Asian countries [57,58]. Compound 55 was more active than compound 52 and was, in turn, more active than metabolite 54. The least toxic appeared to be compound 53. Furthermore, compound 55, having a 3-hydroxy group, was more active than the other compounds. Compounds 53 and 54, bearing a 5- and a 6-hydroxy group, respectively, showed a tendency to reduce activity. The activity of metabolites 52–55 significantly increased with the length of the exposure times at the same concentration. In fact, they showed higher antinematodal activity against B. xylophilus in evaluations at 36 h than at 12 and 24 h exposure [56]. The LD50 values, depending on the three different exposure times (12, 24, and 36 h) were 540.2, 436.6, and 209.0 μg/mL for compound 52, 1169.8, 461.3, and 229.6 μg/mL for compound 53, 1011.6, 522.5, and 220.3 μg/mL for compound 54, and 854, 468, and 206.1 μg/mL for compound 55. These results suggested that the action modes of these compounds were systemic, rather than being contact poisons or anti-feedants [59].
Five preussomerin analogues, named ymf 1029A A, B, C, D, and E (56–60, Figure 3), were isolated together with the known preussomerin C, preussomerin D, (4RS)-4,8-dihydroxy-3,4-dihydronaphthalen-1(2H)-one, and 4,6,8-trihydroxy-3,4-dihydronaph- thalen-1(2H)-one (61–64, Figure 3), from the liquid cultures of an unidentified freshwater fungus YMF 1.01029. This fungus was obtained from the split of decaying branches of an unidentified tree near Lake Fuxian in Yunnan Province, China. All the isolated compounds were tested against B. xylophilus, showing weak nematocidal activity, with IC50 values between 100 and 200 µg/mL at the 24 h time point. Among compounds 56–64, preussomerin D (62) exhibited the most potent toxicity, while the two naphthalenones, metabolites 63 and 64, showed the weakest activity. All compounds had weaker activity when compared with the commercial nematicide avermectin. The bis-spirobisnaphthalene pharmacophore appears to be an important structural feature to impart high activity, because all tested bis-spirobisnaphthalene metabolites showed a stronger nematocidal activity than naphthalenone 63 and 64 [60].
Thermolides A–F (65–70, Figure 3), which belong to a class of PKSNRPS hybrid metabolites constituted by a 13-membered lactam-bearing macrolactone, were isolated from a thermophilic fungus Talaromyces thermophilus. Macrocyclic PKS-NRPS hybrid metabolites are a unique family of natural products, essentially produced by bacteria with broad and outstanding biological activities. All the metabolites 65–68 were assayed against three types of nematodes, including the root-knot nematode M. incognita, pine-wood nematode B. siylopilus, and free-living nematode Panagrellus redivevus [61]. Compounds 65 and 66 showed the strongest activities against all the worms, with LC50 values ranging from 0.5–1.0 µg/mL, similar to those of the avermectin used as control, while compound 67 and 68 had, respectively, moderate and weak inhibitory effect on the same organisms [62]. The gene ThmABCE from this fungus is fundamental for thermolide synthesis. Furthermore, a heterologous and engineered expression of the Thm genes in Aspergillus nidulans and E. coli induced a strongly increased yield not only in thermolide production, but also in that of different esterified analogues, such as butyryl- (thermolides J and K) hexanoyl-, and octanyl-derivatives or mixed thermolides. In addition, thermolides L and M were also obtained via genome mining-based combinatorial biosynthesis, and represent the first L-phenylalanine-based thermolides [63].
Palmariol B, 4-hydroxymellein, alternariol 9-methyl ether, and botrallin (71–74, Figure 4) were isolated from the endophytic fungus Hyalodendriella sp. All the compounds were assayed for antibacterial, antifungal, antinematodal, and acetylcholinesterase inhibitory activities. The antimicrobial activity was tested against bacteria, such as B. subtilis, Pseudomonas lachrymans, Ralstonia solanacearum, and Xanthomonas vesicatoria, and against the fungus Magnaporthe oryzae. Compounds 71–74 showed activity against C. elegans with IC50 values of 56.21, 86.86, 93.99, and 84.51 μg/mL, respectively. Here, 4-Hydroxymellein (72) had the strongest antibacterial activity. Palmariol B (71) showed stronger antimicrobial, antinematodal, and acetylcholinesterase inhibitory activities than alternariol 9-methyl ether (73). This last result suggested that the chlorine substitution at position 2 may be a structural feature important for bioactivity [64].
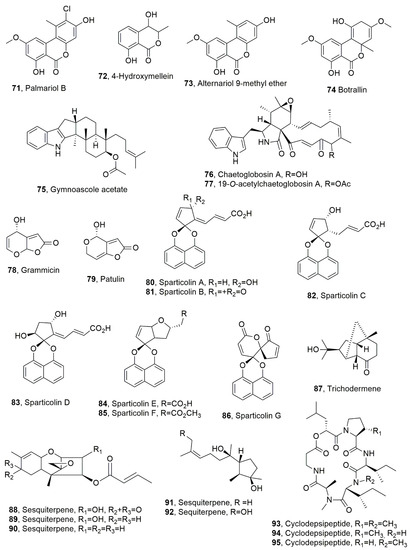
Figure 4.
Metabolites produced by Hyalodendriella sp (71–74), Gymnoascus reessii za-130 (75), Ijuhya vitellina (76 and 77), Xylaria grammica (78), Penicillium griseofulvum, Penicillium expansum (79), a new species of Dothideomycetes (80–86), and Trichoderma longibrachiatum (87–92 and 93–95).
Gymnoascole acetate (75, Figure 4) was isolated from Gymnoascus reessii za-130, which was obtained from the rhizosphere of tomato plants infected by the root-knot nematode M. incognita. Gymnoascole acetate (75) showed strong toxicity against M. incognita second-stage juvenile (J2) viability, while exposure to its solution of 36 μg/mL for 24 h determined 100% paralysis of J2 stage (EC50 = 47.5 μg/mL) [65]. Chaetoglobosin A and its derivate 19-O-acetylchaetoglobosin A (76 and 77, Figure 4, which were produced by Ijuhya vitellina (Ascomycota, Hypocreales, Bionectriaceae), found in wheat fields in Turkey, showed significant nematocidal activity against Heterodera filipjevi, which was paralyzed when both compounds were tested at 50 and 100 μg/mL. In addition, at 300 μg/mL, chaetoglobosin A had higher toxic effects and caused nematode mortality of I. vitellina destructively-parasitizing eggs inside cysts of nematode. The parasitism was also reproduced in in vitro studies [66].
Grammicin (78, Figure 4), which is a dihydrofuranone, was isolated from Xylaria grammica KCTC 13121BP, showing a strong nematocidal activity against M. incognita. The fungus was isolated from a lichen, Menegazzia sp., which was collected on Giri Mountain in Korea [67]. Compound 78, which was also previously isolated from the same fungus collected from wood in Cameroon and Peru [68], is a structural isomer of the well-known mycotoxin patulin (79, Figure 4). The latter compound (79) was first isolated in 1943 from Penicillium griseofulvum and Penicillium expansum [69] and then, as recently reviewed [70], from several species belonging to not less 30 genera including Penicillium, Aspergillus, Paecilomyces, and Byssochlamys. Compound 79 is the most common mycotoxin found in apples and apple-derived products and other food, and is associated with immunological, neurological and gastrointestinal outcomes with high human health risks [71]. Grammicin (78) showed strong nematocidal activity against M. incognita in J2 juvenile mortality and eggs-hatching inhibition with EC50 values of 15.95 and 5.87 μg/mL, respectively, compared to trans-cinnamaldehyde used as positive control, which showed in both assay EC50 values of 18.34 and 10.50 μg/mL, respectively. The same compound exhibited weak antibacterial effects against several microorganisms responsible for severe crop diseases [67]. Furthermore, it exhibited very low or no cytotoxic activity when assayed against a human first-trimester trophoblast cell line SW.71. Instead, patulin (79), in the same bioassays, showed a weak nematocidal EC50/72 h value of 115.67 μg/mL and strong antibacterial and cytotoxic activities. In addition, compared with trans-cinnamaldehyde, grammicin (78) showed comparable J2 killing activity but a stronger egg-hatching inhibitory effect. These results suggest that grammicin and its fungal producer have potential for biocontrol of root-knot nematode disease in crops [67].
Seven highly oxygenated and differently functionalized spirodioxynaphthalene, named sparticolins A–G (80–86, Figure 4) were isolated from the new species of Dothideomycetes, the ex-type strain of Sparticola junci, which was introduced as a member of the family Sporormiaceae [72]. These fungi are mostly saprobic on dung, but sometimes occur on other substrates, including plant debris, soil, and wood [73]. Sparticolins A–E (80–84) showed only weak toxicity against the nematode C. elegans, while sparticolin F (85) showed a moderate nematocidal activity, and sparticolin G (86) was not tested due to there being an insufficient amount. The LD50 values recorded for compounds 80–85 were as follows: 50, 50, 25, 50, 50, and 12.5 μg/mL, respectively. Sparticolin B (81) inhibited the gram-positive bacteria B. subtilis, Micrococcus luteus, and Staphylococcus aureus, while sparticolin G (86) showed antifungal activities against Schizosaccharomyces pombe and Mucor hiemalis. The latter two compounds (81 and 86) also showed moderate cytotoxicity against seven mammalian cell lines [73].
Six sesquiterpenes (87–79, Figure 4) and cyclodepsipeptides (93–95, Figure 4) were isolated from Trichoderma longibrachiatum, which is a fungus obtained from the root of Suaeda glauca, a highly halophilic plant collected from the intertidal zone of Jiaozhou Bay, Qingdao, China. Compound 87, which possess a rare an original norsesquiterpene tricyclic-6/5/5-[4.3.1.01,6]decane skeleton, was named trichodermene. Compound (87) and the sesquiterpenes 88 and 89 showed significant antifungal activities against Colletotrichum lagenarium, even better than those of the commercial synthetic fungicide carbendazim. A similar activity was exhibited from the same compounds against carbendazim-resistant Botrytis cinerea. The sesquiterpenes 91 and 92 showed nematocidal activity when assayed at 200 μg/mL at J2s lethal rate of M. incognita of 38.2 and 42.7%, respectively. Cyclodepsipeptides 93–95 showed moderate nematocidal activities against the southern root-knot of the same nematode M. incognita with IC50 values of 149.2, 140.6, and 198.7 μg/mL, respectively [74].
4. Plant Metabolites
This section chronologically describes the source, structure, and biological activity of plant metabolites, most of which showed nematocidal activity together with other interesting biological activities.
Twenty-four secondary metabolites were isolated from Bupleurum salicifolium [75], which is a plant native to the western Canary Islands from Gran Canaria to El Hierro, where it is frequently found up to 1000 m above sea level [76]. The plant is highly specialized in biosynthesizing secondary metabolites, principally lignans, coumarins, and flavonols, which all derive from shikimic acid and belong to different classes of natural compounds (Dewick, 2002) [77]. All the metabolites were tested against viruses, gram-positive and gram-negative bacteria, the yeast Candida albicans, the nematodes G. pallida and G. rostochiensis, the insect Spodoptera littoralis, and the crustacean Artemia salina. These compounds were also tested against tumoral and non-tumoral cell lines. In particular, considering the limited amount available, only dibenzyl-butyrolactone, lignans, such as guayarol, buplerol, matairesinol and its dimethyl ether, bursehernin, pliviatolide, thujaplicatin, methyl ether (96–102, Figure 5) and 2-chloro-matairesinol, nortrachelogenin, nortrachelogenin triacetate, and 2-hydroxy-thujaplicatin-methyl ether (103–106, Figure 5) were tested on potato cyst nematode hatching using G. pallida and G. rostochiensis. After 14 days, all the compounds assayed stimulated the hatching of more juveniles than distilled water (negative control). In particular, matairesinol and bursehernin (98 and 100) significantly reduced hatching by 70% and 55%, respectively, when compared to the positive control agent. The HID recorded for bursehernin (100) was 16.42 μg/mL, while no differences were observed in the inhibition of G. pallida or G. rostochiensis. These results suggested that the presence of a methylene-dioxy group in the aromatic ring B of the dibenzyl-butyrolactone skeleton plays a significant role to impart nematostatic activity. When the methylene-dioxy group was substituted by a methoxy and a hydroxyl group, as in buplerol (97), or two hydroxy groups, as in guayarol (96), a significant reduction in the activity was observed. Furthermore, in compounds lacking the methylenedioxy group, the activity increased according to the number of free hydroxy groups present, as observed in compounds 96 > 98 > 97 > 105. Nortrachelogenin triacetate (105) bears an acetyl group at position 2 in the lactone ring, which could be a consistent steric hindrance between this compound and the receptor on the nematode eggshell whose existence was hypothesized by Atkinson and Taylor (1980; 1983) [78,79]. None of the compounds tested showed nematocidal activity when tested on second-stage juveniles of G. pallida and G. rostochiensis [75]. Successively, some of the same authors tested 22 aromatic derivatives and the conjugated carbonyl compound t-3-penten-2-one for nematocidal activity against the same 2 nematodes, namely G. pallida and G. rostochiensis. Among all the compounds assayed, nine showed high toxicity on the infective stages (second instar juveniles) of the nematodes, with a LC50 ranking from 2 × 10−6 to 1.26 × 10−3 M. As expected, the toxicity is due to the presence of a conjugated carbonyl system [80].

Figure 5.
Metabolites produced by Bupleurum salicifolium (96–102 and 103–106), plants of the Poaceae family (107–110), Apiaceae, Rutaceae, Asteraceae, and Fabaceae (111–113), Stellera chamaejasme (114–121) and Tagetes spp., Azadirachta indica, and Capsicum frutescens (122).
The cyclic hydroxamic acids are common secondary metabolites found in plants of the Poaceae family, such as corn, wheat, and rye, and known for the allelopathy of rye (Secale cereale). The latter plant is well-known for its allelopathic activity. Some of cyclic hydroxamic acids, such as DIBOA (2,4-dihydroxy-(2H)-1,4-benzoxazin-3(4H)-one), DIMBOA (2,4-hydroxy-7-methoxy-(2H)-1,4-benzoxazin-3(4H)-one) (107 and 108, Figure 5), and their degradation products BOA (benzoxazolin-2(3H)-one) and MBOA (6-methoxy-benzoxazolin-2(3H)-one) (109 and 110, Figure 5) are commercially available and, thus, were used to test their toxicity against M. incognita second-stage juveniles (J2) and eggs and mixed-stages of Xiphinema americanum (X. americanum). The LC50 value of 74.3 μg/mL for DIBOA was recorded when assayed against M. incognita eggs after 168 h exposure, while there was no possible recorded any value for the other compounds. In the assay on M. incognita J2 mortality, the LD50 values were 20.9, 46.1, and 49.2 μg/mL for compound 107, 108, and 110, respectively. For compound 108, no value was measured. In the assay against X. americanum, the LD50 values recorded after 24 h of exposure were 18.4 and 48.3 μg/mL for compounds 107 and 108, respectively, while compounds 109 and 110 had no effect on nematode mortality. These results showed that X. americanum was more sensitive to DIBOA and DIMBOA (107 and 108) than M. incognita J2, while eggs of M. incognita were less sensitive to the hydroxamic acids than J2. Only DIBOA (107) resulted in a 50% reduction in egg hatching; MBOA (110) was not toxic to X. americanum or M. incognita eggs but was toxic to M. incognita J2. Furthermore, BOA (109) was the least toxic hydroxamic acid tested and did not reduce M. incognita egg hatching after 1 week of exposure or increase X. americanum mortality after 24 h of exposure. These results showed that the presence of 4-hydroxy-2H-1,4-oxazin-3(4H)-one is determinant of the imparted toxicity, as this activity was strongly reduced or lost when this residue was substituted by oxazol-2(3H)-one [81].
Coumarins are a large group of naturally occurring compounds widely distributed in the Apiaceae, Rutacea, Asteraceae, and Fabaceae plant families, and are known for their phytotoxic, fungitoxic, insecticidal, antibiotic, and nematocidal activity [82]. Furanocoumarins showed nematocidal activity. In particular, 8-geranylpsoralen, imperatorin, and heraclenin (111–113, Figure 5) exhibited nematocidal activity against B. xylophilus and Panegrellus redivivus. The LD50 values recorded for compounds 111–113 after 72 h of exposure were 188.3, 161.7, and 114.7 μg/mL and 117.5, 179.0, and 184.7 μg/mL when assayed against B. xylophilus and P. redivivus, respectively [82].
Ruixianglangdusu B, umbelliferone, chamaejasmenin C, daphnoretin 7-methoxyneochaejasmin A, (+)-chamaejasmine, chamaechromone, and isosikokianin A (114–120, Figure 5) were isolated from the organic extract of Stellera chamaejasme L. roots, which showed significant nematocidal activity against B. xylophilus and Bursaphelenchus mucronatus [83]. The eight metabolites were tested against J2s of B. xylophilus and B. mucronatus. The LC50 values recorded for compound 114–121 depending on the exposure time (24, 48, and 72 h) when assayed against B. xylophilus were as follows: 227.4, 71.6, and 15.7 μM; 1.3 × 107, 5.7 × 107 and 3.3 μM; 47.8, 3.1 and 2.7 μM; 1.7 × 104, 1.1 × 104 and 65.3 μM; <0.001, 3.4 and 167.3 μM; 16.5, 8.8, and 4.7 μM; 0.7, 10.3 and 36.7 μM; 147.7, 385.2 and 2.2 × 102. Similarly, the LC50 values recorded at exposure of 12, 24 and 72 h for compound 114–121 when assayed against B. mucronatus were as follows: 1.8 × 103, 160.2 and 0.6 μM; 2.6 × 103, 851 and 33.4 μM; 2.3 × 106, 169.9 and 3.1 μM; 463.5, 156.7 and 0.05 μM; 1.8 × 104, 384.2 and 151.1 μM; 1.8 × 103, 1.6 × 103, 5.1 × 103 μM; 327, 5.7, 0.003 μM; 2.6 × 104, 32.5 and 2.3 μM. These results showed that chamaejasmenin C (117) and (+)-chamaejasmin (119) showed significant toxicity against B. xylophilus at a concentration of 100 µM at 72 h. Umbelliferone (115), daphnoretin (116) and chamaechromone (120) exhibited moderate activity at a concentration of 800 µM at 72 h, while compound 114, 118 and 121 showed weak activity when tested in the same conditions. The nematocidal activities of the eight purified compounds against B. mucronatus were similarly observed at 72 h after treatment but the toxicity values of compounds 117 and 120 were highest at a concentration of 400 µM. The nematocidal activity of compound 119 was strongest against B. mucronatus at the lowest test concentration, while the most toxic compounds were 114, 116, and 120, with LC50 values ranging from 0.003 to 0.6 µM, which were comparable with that of the lambda cyhalothrin (LC50 = 1.1 µM) used as the positive commercial control [83].
Medium-chain fatty acids and phenolic acids were the main component of the organic extract of Picria fel-terrae. The plant extract showed toxicity against free-living nematode C. elegans and the parasitic nematode Haemonchus contortus, killing C. elegans adults and inhibiting the motility of 48 exsheathed L3 of H. contortus. The same extract had minimal cytotoxic activity in mammalian cell 49 culture [84].
A screening was carried out carried out for 790 plant metabolites, including those obtained from Tagetes spp., Azadirachta indica, and Capsicum frutescens, and involved testing their nematocidal activity against C. elegans. A total of 10 compounds proved to be toxic, 3 of which were further evaluated for their inhibitory activities against egg hatching of C. elegans and J2 M. incognita and the wild nematode N2 L4 eggs. Only 1,4-naphthoquinone (122, Figure 5) appeared to be an active compound that could not only kill N2 L4 nematodes (LC50 42.26 ± 2.53 μg/mL), and inhibit egg hatching of N2 (LC50 34.83 ± 0.58 μg/mL), but also showed toxicity on more than 50% of M. incognita at a concentration of less than 50 μg/mL (LC50 33.51 ± 0.21 μg/mL). The results obtained using C. elegans demonstrated that compound 122 could influence reactive oxygen production, superoxide dismutase activity, and the heat-shock transcription factor (HSF)-1 pathway, suggesting that compound 122 stimulated significant oxidative stress [85].
Deguelin (123, Figure 6) rotenone and other rotenoids, such as β-rotenolone (12aβ-hydroxyrotenone), tephrosin (12aα-hydroxydeguelin), 12aR-hydroxyrotenone, and dehydrorotenone, are flavonoids which were extracted from the family of Leguminosae (e.g., Lonchocarpus, Derris, Cassia, and Tephrosia) [86,87,88,89,90]. In fact, resins extracted from the roots of Lonchocarpus utiliz (cube), Lonchocarpus urucu (barbasco), Derris elliptica (tuba plant), or Derris involuta (jewel vine) were shown to contain rotenoids, and have been used in many countries as natural insecticides, acaricides, and/or piscicides [91,92]. Denguilin, when assayed against H. contortus, showed inhibitory activity with IC50 values, depending from the time of exposure (24, 48, and 72 h), of 81, 54, and 21 μM for exsheathed L3 mortality and 11.39, 25.4, and 0.004 μM for L4 mortality, respectively. Rotenoid 123 enhanced oxidative phosphorylation in mitochondria. In fact, in both parasitic (H. contortus) and free-living (C. elegans) nematodes, measurements of oxidative phosphorylation in response to deguelin (123, Figure 6) treatment resulted in a decrease in oxygen consumption. Thus, the toxicity of this compound could be ascribed to its ability to modulate the oxidative phosphorylation process in nematodes [93].
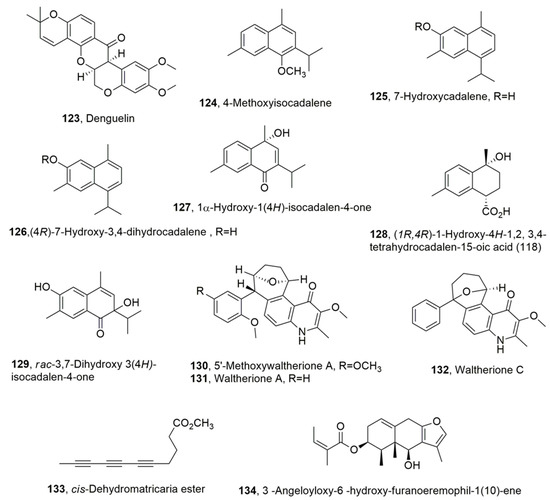
Figure 6.
Metabolites produced by the Leguminosae family (123), Heterotheca inuloides (124–129), Waltheria indica (130–132), Tanacetum falconeri (133), and Senecio sinuatos (134).
The acetone extract of Heterotheca inuloides showed strong toxicity against Nacobbus aberrans (Tylenchida: Pratylenchidae), which is one of the main plant-parasitic nematodes species that affects crops in Mexico, with consequent heavy economic losses. Sesquiterpenes, belonging to cadinene subgroup, such as 4-methoxyisocadalene, 7-hydroxycadalene, (4R)-7-hydroxy-3,4-dihydrocadalene, 1𝛼-hydroxy-1(4H)-isocadalen -4-one, (1R,4R)-1-hydroxy-4H-1,2,3,4-tetrahydrocadalen-15-oic acid, and rac-3,7-dihydroxy 3(4H)-isocadalen-4-one (112–129, Figure 6), were isolated from the extract of the dried H. inuloides flowers. The natural cadinenes and some hemisynthetic analogs, such as (1S,4R)-7-hydroxycalamenane, (1S,4R)-7-acetoxy-3,4-dihydrocadalene, and (1S,4R)-7-benzoiloxy-3,4-dihydrocadalene, mansonone C, 7-acetoxycadalene, 7-benzoiloxycadalene, and acetyl and benzoyl derivatives of compound 116 (124 and 125, Figure 6) prepared from 124, 125, and 126, respectively, by conventional chemical procedures [94,95]. All the natural and hemisynthetic compounds were tested on the immobility and mortality of Nacobbus aberrans at the J2 stage. Compounds 125, 126, and their derivatives (1S,4R)-7-hydroxy calamenane, mansonone C, 7-acetoxycadalene, and (4R)-7-acetoxy-3,4dihydrocadalene, (1S,4R)-7-acetoxy-3,4-dihydrocadalene and (1S,4R)-7-benzoiloxy-3,4-dihydrocadalene showed after 36 h exposure LC50 values of 31.30, 26.30, 25.39, 21.92, 42.31, 36.19, 31.08, and 111.37 mg/L, respectively. Among all the compounds tested, nematodes were more susceptible to hydroxylated (125–129) and quinone (monsonone) compounds, whereas the remaining compounds showed moderate or no activity. The presence of the hydroxyl group seemed to be an important structural feature for nematocidal activity [96].
Suitable formulation of the ethyl acetate extract of W. indica, which was collected in Vietnam, reduced the formation of galls and the egg masses of M. incognita on the tomato roots in a dose-dependent manner. Here, 5′-Methoxywaltherione A, waltherione A waltherione C, three 4-quinolone alkaloids (130–132, Figure 6), were isolated from this extract, and exhibited strong nematocidal activity against the same organism. In particular, when assayed against Meloidogyne arenaria, Meloidogyne hapla, M. incognita, and B. xylophilus, the compound 130–132 at 72 h exposure, in comparison abamectin used as posto itive control, showed EC50 values of 0.25, 0.63, and 10.67 μg/mL; 0.09, 1.74 and 19.79 μg/mL, 0.09, 0.27 and 16.59 μg/mL, and 2.13, 3.54 and 790.85 μg/mL, respectively. Furthermore, the plant extract formulation significantly reduced gall formation on the roots of melon plants and the population density of nematodes in soil compared with the untreated control. Here, 5-Methoxywaltherione A and waltherione A (130 and 131) induced high mortality in the juvenile stage of all nematodes tested. The order of efficacy of the three compounds was 130 > 131 > 132. Waltherione C (132) exhibited significant nematocidal activity against only root-knot nematodes [97].
Furthermore, cis-Dehydromatricaria ester (133, Figure 6) was isolated for the first time from Tanacetum falconeri, collected in Astore (Daosai), Pakistan. This organic extract showed nematocidal and insecticidal activity. Compound 133 showed strong nematocidal activity against M. incognita. The EC50 values recorded, at exposure times of 24, 36, and 72 h, were 3.4, 0.18, and 0.04 mg/L, respectively [98].
Additionally, 3β-Angeloyloxy-6β-hydroxyfuranoeremophil-1(10)-ene (134, Figure 6), the main secondary metabolite extracted from the roots of Senecio sinuatos, showed nematocidal activity against the second-stage juveniles (J2) of M. incognita and N. aberrans. Compound 134 was alkaline hydrolyzed to produce a derivative which, in turn, was differently esterified with anhydride acetic, benzoic acid, 2-nitrobenzoic acid, 2-bromobenzoic acid, 4-nitrobenzoic acid, 4-bromobenzoic acid, and 4-methoxybenzoic acid to produce the corresponding 6-O-acetyl ester and benzoyl esters. All compounds and the corresponding benzoic acids were tested for nematocidal activity against M. incognita and N. aberrans J2 using fluopyram as a positive control. In particular, the benzoyl esters possess more nematocidal activity than the corresponding free benzoic acids, while compound 134 had nematocidal activity against M. incognita when assayed at 10 μg/mL, and this effect was more nematostatic as the concentration decreased at the most effective time of 72 h [99].
5. Conclusions
Nematodes are one of several enemies that induce severe economic losses in agrarian production, forcing farmers to use different methods to prevent their growth and diffusion. Among these methods, the last five-to-six decades have seen a massive and extensive use of chemicals, with heavy negative consequences, such as an increase in environmental pollution and risks for human and animal health. A negative effect of the use of chemicals in agriculture is also their noteworthy contribution to climate change. The development of new control strategies based on natural products with high efficacy and selectivity has become an emergency. This review reports, for the first time, a complete overview of the microbial and plant metabolites with nematocidal activity and, thus, they are a potential applications in suitable formulations in greenhouses and fields. All the results described are summarized in Table 1. The compounds selected for their efficacy and specific nematocidal activity should be investigated firstly for their human, animal, and environmental toxicological effects. Then, for the promising compounds, a total, convenient, and ecofriendly synthesis should be developed for their large production at an industrial level.

Table 1.
Microbial and plant metabolites.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Abbreviations
EC50, 50% effective concentration; IC50, 50% inhibit concentration; IGF-1, insulin-like growth factor, HID, hatch inhibiting dose; HPLC, high performance liquid chromatography; J, juvenile; LC50, lethal concentration 50; LD30, lethal dose 30; LD50, lethal dose 50; NRPS, non-ribosomal peptide synthetases; PKS, polyketide synthases; U, the minimum concentration that could lead to a visible zone of Erwinia herbicola inhibition was defined as one unit, U.
References
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations(FAO). Future of Food and Agriculture-Trends and Challenges; Food and Agriculture Organization of the United Nations(FAO): Rome, Italy, 2017. [Google Scholar]
- Rosegrant, M.W.; Cline, S.A. Global food security: Challenges and policies. Science 2003, 302, 1917–1919. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Caicedo, J.C.; Villamizar, S. Xanthomonas citri ssp. citri pathogenicity, a review. Citrus Res. Dev. Biotech. 2021, 135. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Si, J.; Han, Z.; Chen, D. Action mechanisms of effectors in plant-pathogen interaction. Int. J. Mol. Sci. 2022, 23, 6758. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Lavermicocca, P.; Lonigro, S.L.; Evidente, A.; Andolfi, A. Bacteriocin production by Pseudomonas syringae pv. ciccaronei NCPPB2355. Isolation and partial characterization of the antimicrobial compound. J. Appl. Microbiol. 1999, 86, 257–265. [Google Scholar]
- Evidente, A.; Abouzeid, A.M.; Andolfi, A.; Cimmino, A. Recent achievements in the bio-control of Orobanche infesting important crops in the Mediterranean basin. J. Agric. Sci. Technol. 2011, 1, 461–483. [Google Scholar]
- Pires, D.; Vicente, C.S.; Menéndez, E.; Faria, J.M.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Lorenzen, K.; Anke, K. Basidiomycetes as a source for new bioactive natural products. In Current Organic Chemistry; Mori, K., Ed.; Bentham Science Publisher: Miami, FL, USA, 1998; pp. 329–364. [Google Scholar]
- Anke, H.; Sterner, O. Insecticidal and nematocidal metabolites from fungi. In The Mycota; Osiewacz, H.D., Ed.; Springer: Berlin, Germany, 2002; Volume 10, pp. 109–127. [Google Scholar]
- Osmanova, N.; Schultze, W.; Ayoub, N. Azaphilones: A class of fungal metabolites with diverse biological activities. Phytochem. Rev. 2010, 9, 315–342. [Google Scholar] [CrossRef]
- Shen, W.; Mao, H.; Huang, Q.; Dong, J. Benzenediol lactones: A class of fungal metabolites with diverse structural features and biological activities. Eur. J. Med. Chem. 2015, 97, 747–777. [Google Scholar] [CrossRef]
- Mao, Z.; Sun, W.; Fu, L.; Luo, H.; Lai, D.; Zhou, L. Natural dibenzo-α-pyrones and their bioactivities. Molecules 2014, 19, 5088–5108. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Secondary metabolites with antinematodal activity. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2002; Volume 26, pp. 425–506. [Google Scholar]
- D’addabbo, T.; Carbonara, T.; Leonetti, P.; Radicci, V.; Tava, A.; Avato, P. Control of plant parasitic nematodes with active saponins and biomass from Medicago sativa. Phytochem. Rev. 2011, 10, 503–519. [Google Scholar] [CrossRef]
- Hernández-Carlos, B.; Gamboa-Angulo, M. Insecticidal and nematicidal contributions of Mexican flora in the search for safer biopesticides. Molecules 2019, 24, 897. [Google Scholar] [CrossRef] [PubMed]
- Ibrar, M.; Ullah, M.W.; Manan, S.; Farooq, U.; Rafiq, M.; Hasan, F. Fungi from the extremes of life: An untapped treasure for bioactive compounds. Appl. Microbiol. Biotechnol. 2020, 104, 2777–2801. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Martínez-Gómez, Á.; Fenoll, C.; Escobar, C. The use of biochar for plant pathogen control. Phytopathology 2021, 111, 1490–1499. [Google Scholar] [CrossRef]
- Snoop, W.; Mrozik, H.; Fisher, M.H. Structure and activity of avermectins and milbemycins in animal health. Vet. Parsitol. 1995, 59, 139–156. [Google Scholar]
- Hoshino, T. Violacein and related tryptophan metabolites produced by Chromobacterium violaceum: Biosynthetic mechanism and pathway for construction of violacein core. Appl. Microbiol. Biotechnol. 2011, 91, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Konzen, M.; De Marco, D.; Cordova, C.A.; Vieira, T.O.; Antonio, R.V.; Creczynski-Pasa, T.B. Antioxidant properties of violacein: Possible relation on its biological function. Bioorg. Med. Chem. 2006, 14, 8307–8313. [Google Scholar] [CrossRef]
- Becker, M.H.; Brucker, R.M.; Schwantes, C.R.; Harris, R.N.; Minbiole, K.P. The bacterially produced metabolite violacein is associated with survival of amphibians infected with a lethal fungus. Appl. Environ. Microbiol. 2009, 75, 6635–6638. [Google Scholar] [CrossRef]
- Matz, C.; Webb, J.S.; Schupp, P.J.; Phang, S.Y.; Penesyan, A.; Egan, S.; Steinberg, P.; Kjelleberg, S. Marine biofilm bacteria evade eukaryotic predation by targeted chemical defense. PLoS ONE 2008, 3, e2744. [Google Scholar] [CrossRef]
- Ballestriero, F.; Daim, M.; Penesyan, A.; Nappi, J.; Schleheck, D.; Bazzicalupo, P.; Di Schiavi, E.; Egan, S. Antinematode activity of violacein and the role of the insulin/IGF-1 pathway in controlling violacein sensitivity in Caenorhabditis elegans. PLoS ONE 2014, 9, e109201. [Google Scholar] [CrossRef]
- Zeng, L.; Jin, H.; Lu, D.; Yang, X.; Pan, L.; Cui, H.; He, X.; Qiu, H.; Qin, B. Isolation and identification of chemical constituents from the bacterium Bacillus sp. and their nematicidal activities. J. Basic Microbiol. 2015, 55, 1239–1244. [Google Scholar] [CrossRef]
- Kosaka, H.; Aikawa, T.; Ogura, N.; Tabata, K.; Kiyohara, T. Pine wilt disease caused by the pine wood nematode: The induced resistance of pine trees by the avirulent isolates of nematode. Eur. J. Plant Pathol. 2001, 107, 667–675. [Google Scholar] [CrossRef]
- Song, M.J.; Bae, J.; Lee, D.S.; Kim, C.H.; Kim, J.S.; Kim, S.W.; Hong, S.I. Purification and characterization of prodigiosin produced by integrated bioreactor from Serratia sp. KH-95. JBB 2006, 101, 157–161. [Google Scholar]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Chandrakant, N.; Laxmikant, S.; Satish, P. Nematicidal activity of microbial pigment from Serratia marcescens. Nat. Prod. Res. 2014, 28, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Zeng, Z.; Xue, B.; Deng, Y.; Sun, M.; Tang, Y.-J.; Ruana, L. Bacillus thuringiensis produces the lipopeptide thumolycin to antagonize microbes and nematodes. Microbiol. Res. 2018, 215, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Cabras, A.; Mannoni, M.A.; Serra, S.; Andolfi, A.; Fiore, M.; Evidente, A. Occurrence, isolation and biological activity of phytotoxic metabolites produced in vitro by Sphaeropsis sapinea, pathogenic fungus of Pinus radiata. Eur. J. Plant Pathol. 2006, 115, 187–193. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, J.H.; Liu, M.J.; Jin, C.Z.; Park, D.; Kim, J.; Sung, B.-H.; Kim, C.J.; Son, K.-H. New discovery on the nematode activity of aureothin and alloaureothin isolated from endophytic bacteria Streptomyces sp. AE170020. Sci. Rep. 2022, 12, 3947. [Google Scholar] [CrossRef]
- Anke, H.; Stadler, M.; Mayer, A.; Sterner, O. Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Can. J. Bot. 1995, 73, 932–939. [Google Scholar] [CrossRef]
- Dasenbrock, J. Isolierung und Strukturautlclarung neuer Wirkstoffe aus Hijheren Pilzen. Ph.D. Thesis, University of Bonn, Bonn, Germany, 1994. [Google Scholar]
- Lorenzen, K.; Anke, T.; Anders, U.; Hindermayr, H.; Hansske, F. 14-Epidihydrocochlioquinone B, and 14-epicochlioquinone B, antibiotics from fermentations of the Ascomycete Neobulgaria pura: Structure elucidation and effects on platelet aggregation. Z. Naturforsch. 1994, 49C, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, J.M.; Frazier, E.G.; Bergstrom, A.R.; Williamson, J.M.; Liesch, J.M.; Goetz, M.A. Cochlioquinone A, a nematocidal agent which competes for specific [3H]ivermectin binding sites. J. Antibiot. 1990, 43, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.C. Ivermectin: An update. Parasitol. Today 1985, 1, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Wada, K.; Munakata, K. New nematicidal metabolites from a fungus, Irpex lacteus. Agric. Biol. Chem. 1981, 45, 1527–1529. [Google Scholar]
- Stadler, M.; Anke, H.; Sterner, O. Metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. VII. Structure determination of brominated lachnumon and mycorrhizin A derivatives. J. Antibiot. 1995, 48, 158–161. [Google Scholar]
- Stadler, M.; Anke, H.; Sterner, O. Metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. 111. Production of novel isocoumarin derivatives. Isolation and biological activities. J. Antibiot. 1995, 48, 261–266. [Google Scholar] [CrossRef]
- Stadler, M.; Anke, H.; Sterner, O. Metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceurn (Karst.) Karst. IV. Structural elucidation of novel isocoumarin derivatives. J. Antibiot. 1995, 48, 267–270. [Google Scholar]
- Chexal, K.K.; Tamm, C. Biosynthesis of mikrolin. Helv. Chim. Acta 1978, 61, 2002–2018. [Google Scholar] [CrossRef]
- Chexal, K.K.; Tamm, C.; Clardy, J.; Hirotsu, K. Gilmicolin and mycorrhizinol, two new metabolites of Gilmaniella humicola Barron. Helv. Chim. Acta 1979, 62, 1130–1142. [Google Scholar] [CrossRef]
- Stadler, M.; Anke, H.; Sterner, O. Metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. V. Production, isolation and biological activities of bromine-containing mycorrhizin and lachnumon derivatives and four additional new bioactive metabolites. J. Antibiot. 1995, 48, 149–153. [Google Scholar]
- Stadler, M.; Anke, H.; Sterner, O. Metabolites with nematicidal and antimicrobial activities from the Ascomycete Lachnum papyraceum (Karst.) Karst. VI. Structure determination of non-halogenated metabolites structurally related to mycorrhizin A. J. Antibiot. 1995, 48, 154–157. [Google Scholar]
- Mayer, A.; Anke, H.; Sterner, O. Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius I. Fermentation and biological activity. Nat. Prod. Lett. 1997, 10, 25–32. [Google Scholar] [CrossRef]
- Kohno, J.; Nishio, M.; Sukarai, M.; Kawano, K.; Hiramatsu, H.; Kameda, N.; Kish, N.; Yamashita, T.; Okuda, T.; Komatsubara, S. Isolation and structure determination of TMC-151s: Novel polyketide antibiotics from Gliocladium catenulatum Gilman & Abbott TC 1280. Tetrahedron 1999, 55, 7771–7786. [Google Scholar]
- Kohno, J.; Nishio, M.; Kish, M.N.; Komatsubara, S. Biosynthesis of the fungal polyketide antibiotics TMC151s: Origin of the carbon skeleton. J. Antibiot. 2000, 53, 1301–1304. [Google Scholar] [CrossRef]
- Kohno, J.; Asai, Y.; Nishio, M.; Sakurai, M.; Kawano, K.; Hiramatsu, H.; Kameda, N.; Kishi, N.; Okuda, T.; Komatsubara, S. TMC-171A, B, C and TMC-154, novel polyketide antibiotics produced by Gliocladium sp. TC 1304 and TC 1282. J. Antibiot. 1999, 52, 1114–1123. [Google Scholar]
- Omura, S.; Tomoda, H.; Tabata, N.; Ohyama, Y.; Abe, T.; Namikoshi, M. Roselipins, novel fungal metabolites having a highly methylated fatty acid modified with a monnose and an arabinitol. J. Antibiot. 1999, 52, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Tomoda, H.; Ohyama, Y.; Abe, T.; Tabata, N.; Namikoshi, M.; Yamaguchi, Y.; Masuma, R.; Omura, S. Roselipins, inhibitors of diacylglycerol acyltransferase, produced by Gliocladium roseum KF-1040. J. Antibiot. 1999, 52, 689–694. [Google Scholar] [CrossRef] [PubMed]
- Tabata, N.; Ohyama, Y.; Tomoda, H.; Abe, T.; Namikoshi, M.; Omura, S. Structure elucidation of roselipins, inhibitors of diacylglycerol acyltransferase, produced by Gliocladium roseum KF-1040. J. Antibiot. 1999, 52, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Kanda, M.; Utagawa, M.; Chiba, N.; Ohtani, H.; Mikawa, T. MK7924, a novel metabolite with nematocidal activity from Coronophora gregaria. J. Antibiot. 2003, 56, 652–654. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.; Dong, J.; Wang, L.; Zhou, W.; Li, L.; He, H.; Liu, H.; Zhang, K. Screening and isolation of antinematodal metabolites against Bursaphelenchus xylophilus produced by fungi. Ann. Microbiol. 2008, 58, 375–380. [Google Scholar] [CrossRef]
- Mamiya, Y. The pine wood nematode. In Plant and Insect Nematodes; Nickle, W.R., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1984. [Google Scholar]
- Sutherland, J.R.; Webster, J.M. Nematode pests of forest trees. In Plant-Parasitic Nematodes in Temperate Agriculture; Evans, K., Trudgill, D.L., Webster, J.M., Eds.; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Ferris, H.; Zheng, L. Plant sources of Chinese Herbal Remedies: Effectis on Pratylenchus vulnus and Meloidogyne javanica. J. Nematol. 1999, 31, 241–263. [Google Scholar] [PubMed]
- Dong, J.Y.; Song, H.C.; Li, J.H.; Tang, Y.S.; Sun, R.; Wang, L.; Zhou, Y.P.; Wang, M.L.; Shen, K.Z.; Wang, C.R.; et al. Ymf 1029A-E, preussomerin analogues from the fresh-water-derived fungus YMF 1.01029. J. Nat. Prod. 2008, 71, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.S.; Niu, X.M.; Wang, Y.L.; Guo, J.P.; Pan, W.Z.; Huang, X.-W.; Zhang, K.-Q. Isolation of putative biosynthetic intermediates of prenylated indole alkaloids from a thermophilic fungus Talaromyces thermophilus. Org. Lett. 2010, 12, 4356–4359. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.P.; Zhu, C.Y.; Zhang, C.P.; Chu, Y.S.; Wang, Y.L.; Zhang, J.-X.; Wu, D.-K.; Zhang, K.-Q.; Niu, X.-M. Thermolides, potent nematocidal PKS-NRPS hybrid metabolites from thermophilic fungus Talaromyces thermophilus. J. Am. Chem. Soc. 2012, 134, 20306–20309. [Google Scholar] [CrossRef]
- Zhang, J.M.; Wang, H.H.; Liu, X.; Hu, C.H.; Zou, Y. Heterologous and engineered biosynthesis of nematocidal polyketide-nonribosomal peptide hybrid macrolactone from extreme thermophilic fungi. J. Am. Chem. Soc. 2020, 142, 1957–1965. [Google Scholar] [CrossRef]
- Meng, X.; Mao, Z.; Lou, J.; Xu, L.; Zhong, L.; Peng, Y.; Zhou, L.; Wang, M. Benzopyranones from the endophytic fungus Hyalodendriella sp. Ponipodef12 and their bioactivities. Molecules 2012, 17, 11303–11314. [Google Scholar] [CrossRef]
- Liu, T.; Meyer, S.L.; Chitwood, D.J.; Chauhan, K.R.; Dong, D.; Zhang, T.; Li, J.; Liu, W.C. New nematotoxic indoloditerpenoid produced by Gymnoascus reessii za-130. J. Agric. Food Chem. 2017, 65, 3127–3132. [Google Scholar]
- Ashrafi, S.; Helaly, S.; Schroers, H.J.; Stadler, M.; Richert-Poeggeler, K.R.; Dababat, A.A.; Maier, W. Ijuhya vitellina sp. nov., a novel source for chaetoglobosin A, is a destructive parasite of the cereal cyst nematode Heterodera filipjevi. PLoS ONE 2017, 12, e0180032. [Google Scholar] [CrossRef]
- Kim, T.Y.; Jang, J.Y.; Yu, N.H.; Chi, W.J.; Bae, C.H. Nematicidal activity of grammicin produced by Xylaria grammica KCTC 13121BP against Meloidogyne incognita. Pest Manag. Sci. 2018, 74, 384–391. [Google Scholar] [CrossRef]
- Edwards, R.L.; Maitland, D.J.; Pittayakhajonwut, A.J.S.; Whalley, J. Metabolites of the higher fungi. Part 33. Grammicin, a novel bicyclic C7H6O4 furanopyranol from the fungus Xylaria grammica (Mont.) Fr. J. Chem. Soc. Perkin Trans. 2001, 1, 1296–1299. [Google Scholar] [CrossRef]
- Birkinshaw, J.H.; Michael, S.E.; Bracken, A.; Raistrick, H. Patulin in the common cold collaborative research on a derivative of Penicillium patulum Bainier. II. Biochem. Chem. Lancet 1943, 245, 625. [Google Scholar]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 1, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Puel, O.; Galtie, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef] [PubMed]
- Phukhamsakda, C.; Ariyawansa, H.A.; Phillips, A.J.; Wanasinghe, D.N.; Bhat, D.J.; McKenzie, E.H.C.; Camporesi, E.; Hide, K.D. Additions to Sporormiaceae: Introducing two novel genera, Sparticola and Forliomyces, from Spartium. Cryptogam. Mycol. 2016, 37, 75–97. [Google Scholar]
- Phukhamsakda, C.; Macabeo, A.P.G.; Huch, V.; Cheng, T.; Hyde, K.D. Stadler, M. Sparticolins A-G, biologically active oxidized spirodioxynaphthalene derivatives from the ascomycete. J. Nat. Prod. 2019, 82, 2878–2885. [Google Scholar] [CrossRef]
- Du, F.Y.; Ju, G.L.; Xiao, L.; Zhou, Y.M.; Wu, X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar. Drugs 2020, 18, 165. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Estevez-Braun, A.; Estevez-Reyes, R.; Bazzocchi, I.L.; Moujir, L.; Jimenez, I.A.; Ravelo, A.G.; Gonzalez, A.G. Biological activity of secondary metabolites from Bupleurum salicifolium (Umbelliferae). Experientia 1995, 51, 35–39. [Google Scholar]
- Bramwell, D.; BramwelI, Z. Wíld Flowers of the Canary Islands; Cabíldo Insular de Tenerífe: Santa Cruz de Tenerife, Spain, 1974. [Google Scholar]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Chicester, UK, 2002. [Google Scholar]
- Atkinson, H.J.; Taylor, J.D. Evidence for a calcium-binding site on the eggshell of Globodera rostochiensis with a role in hatching. Ann. Appl. Biol. 1980, 96, 307–315. [Google Scholar] [CrossRef]
- Atkinson, H.J.; Taylor, J.D. A calcium-binding sialoglycoprotein associated with an apparent eggshell membrane of Globodera rostochiensis. Ann. Appl. Biol. 1983, 102, 345–354. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Estevez-Braun, A. Phytonematicidal activity of aromatic compounds related to shikimate pathway. Pestic. Biochem. Phys. 1997, 58, 193–197. [Google Scholar] [CrossRef]
- Zasada, I.A.; Meyer, S.L.F.; Halbrendt, J.M.; Rice, C. Activity of hydroxamic acids from Secale cereale against the plant-parasitic nematodes Meloidogyne incognita and Xiphinema americanum. Phytopathology 2005, 95, 1116–1121. [Google Scholar] [CrossRef]
- Razavi, S.M. Plant counnarins as allelopathic agents. Int. J. Biol. Chem. 2011, 5, 86–90. [Google Scholar] [CrossRef]
- Cui, H.; Jin, H.; Liu, Q.; Yan, Z.; Ding, L.; Qin, B. Nematicidal metabolites from roots of Stellera chamaejasme against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. Pest Manag. Sci. 2014, 70, 827–835. [Google Scholar] [CrossRef]
- Kumarasingha, R.; Karpe, A.V.; Preston, S.; Yeo, T.C.; Lim, D.S.; Tu, C.L.; Kavlene, J.L.; Simpson, J.; Gasser, R.B.; Beale, P.D.; et al. Metabolic profiling and in vitro assessment of anthelmintic fractions of Picria fel-terrae Lour. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 171–178. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, G.; Huang, X.; Wang, Z.; Tan, N. 1, 4-naphthoquinone triggers nematode lethality by inducing oxidative stress and activating insulin/IGF signaling pathway in Caenorhabditis elegans. Molecules 2017, 22, 798. [Google Scholar] [CrossRef]
- Fang, N.; Casida, J.E. Anticancer action of cube’ insecticide: Correlation for rotenoid constituents between inhibition of NADH:ubiquinone oxidoreductase and induced ornithine decarboxylase activities. Proc. Natl. Acad. Sci. USA 1998, 95, 3380–3384. [Google Scholar] [CrossRef]
- Cabizza, M.; Angioni, A.; Melis, M.; Cabras, M.; Tuberoso, C.V.; Cabras, P. Rotenone and rotenoids in cub’e resins, formulations, and residues on olives. J. Agric. Food Chem. 2004, 52, 288–293. [Google Scholar] [CrossRef]
- Wenjie, J.; Fang, Y.; Gan, C.; Wu, Y.; Pang, J. Extraction and purification of deguelin from Derris trifoliata Lour root. Int. J. Agric. Biol. Eng. 2009, 2, 98–103. [Google Scholar]
- Chen, C.S.; Ho, D.R.; Chen, F.Y.; Chen, C.R.; Ke, Y.D.; Su, J.G.J. AKT mediates actinomycin D-induced p53 expression. Oncotarget 2014, 5, 693–703. [Google Scholar] [CrossRef]
- Vats, S.; Kamal, R. Cassia occidentalis L. (a new source of rotenoids): Itsin vitro regulation by feeding precursors and larvicidal efficacy. Plant Cell Tissue Organ Cult. 2014, 116, 403–409. [Google Scholar] [CrossRef]
- Ashack, R.J.; McCarty, L.P.; Malek, R.S.; Goodman, F.R.; Peet, N.P. Evaluation of rotenone and related compounds as antagonists of slow-reacting substance of anaphylaxis. J. Med. Chem. 1980, 23, 1022–1026. [Google Scholar] [CrossRef]
- Okombe Embeya, V.; Lumbu Simbi, J.B.; Stevigny, C.; Vandenput, S.; Pongombo Shongo, C.; Duez, P. Traditional plantbased remedies to control gastrointestinal disorders in livestock in the regions of Kamina and Kaniama (Katanga province, DemocraticRepublic of Congo. J. Ethnopharmacol. 2014, 153, 686–693. [Google Scholar] [CrossRef]
- Preston, S.; Korhonen, P.K.; Mouchiroud, L.; Cornaglia, M.; McGee, S.L.; Young, N.D.; Davis, R.A.; Crawford, S.; Nowell, C.; Ansell, B.R.E.; et al. Deguelin exerts potent nematocidal activity via the mitochondrial respiratory chain. FASEB J. 2017, 31, 4515–4532. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.L.; Rufino-González, Y.; Ponce-Macotela, M.; Delgado, G. In vitro activity of “Mexican Arnica” Heterotheca inuloides Cass natural products and some derivatives against Giardia intestinalis. Parasitology 2015, 142, 576–584. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.L.; Coballase-Urrutia, E.; Sicilia-Argumedo, G.; Ramírez-Apan, T.; Delgado, G. Toxicological evaluation of thenatural products and some semisynthetic derivatives of Heterotheca inuloides Cass (Asteraceae). J. Ethnopharmacol. 2015, 175, 256–265. [Google Scholar] [CrossRef]
- Rodríguez-Chávez, J.L.; Franco-Navarro, F.; Delgado, G. In vitro nematicidal activity of natural and semisynthetic cadinenes from Heterotheca inuloides against the plant-parasitic nematode Nacobbus aberrans (Tylenchida: Pratylenchidae). Pest Manag. Sci. 2019, 75, 1734–1742. [Google Scholar] [CrossRef]
- Jang, J.Y.; Le Dang, Q.; Choi, G.J.; Park, H.W.; Kim, J.C. Control of root-knot nematodes using Waltheria indica producing 4-quinolone alkaloids. Pest Manag. Sci. 2019, 75, 2264–2270. [Google Scholar]
- Ismail, M.; Kowsar, A.; Javed, S.; Choudhary, M.I.; Khan, S.W.; Abbas, Q.; Tang, Y.; Wang, W. The antibacterial, insecticidal and nematocidal cctivities and toxicity studies of Tanacetum falconeri Hook. f. Turk. J. Pharm. Sci. 2021, 18, 744. [Google Scholar] [CrossRef]
- Velasco-Azorsa, R.; Zeferino-Díaz, R.; Alvarado-Rodríguez, J.G.; López-Ruiz, H.; Rojas-Lima, S.; Flores-Castro, K.; Cid del Prado-Vera, I.; Alatorre-Rosas, R.; Tut-Pech, F.; Carrillo-Benítez, M.G.; et al. Nematicidal activity of furanoeremophilenes against Meloidogyne incognita and Nacobbus aberrans. Pest Manag. Sci. 2022, 78, 2571–2580. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).