Lethal and Sublethal Toxicity Assessment of Cyclosporin C (a Fungal Toxin) against Plutella xylostella (L.)
Abstract
:1. Introduction
2. Results
2.1. Toxicity of Cyclosporin C against P. xylostella
2.1.1. Concentration–Mortality Response of P. xylostella to Cyclosporin C
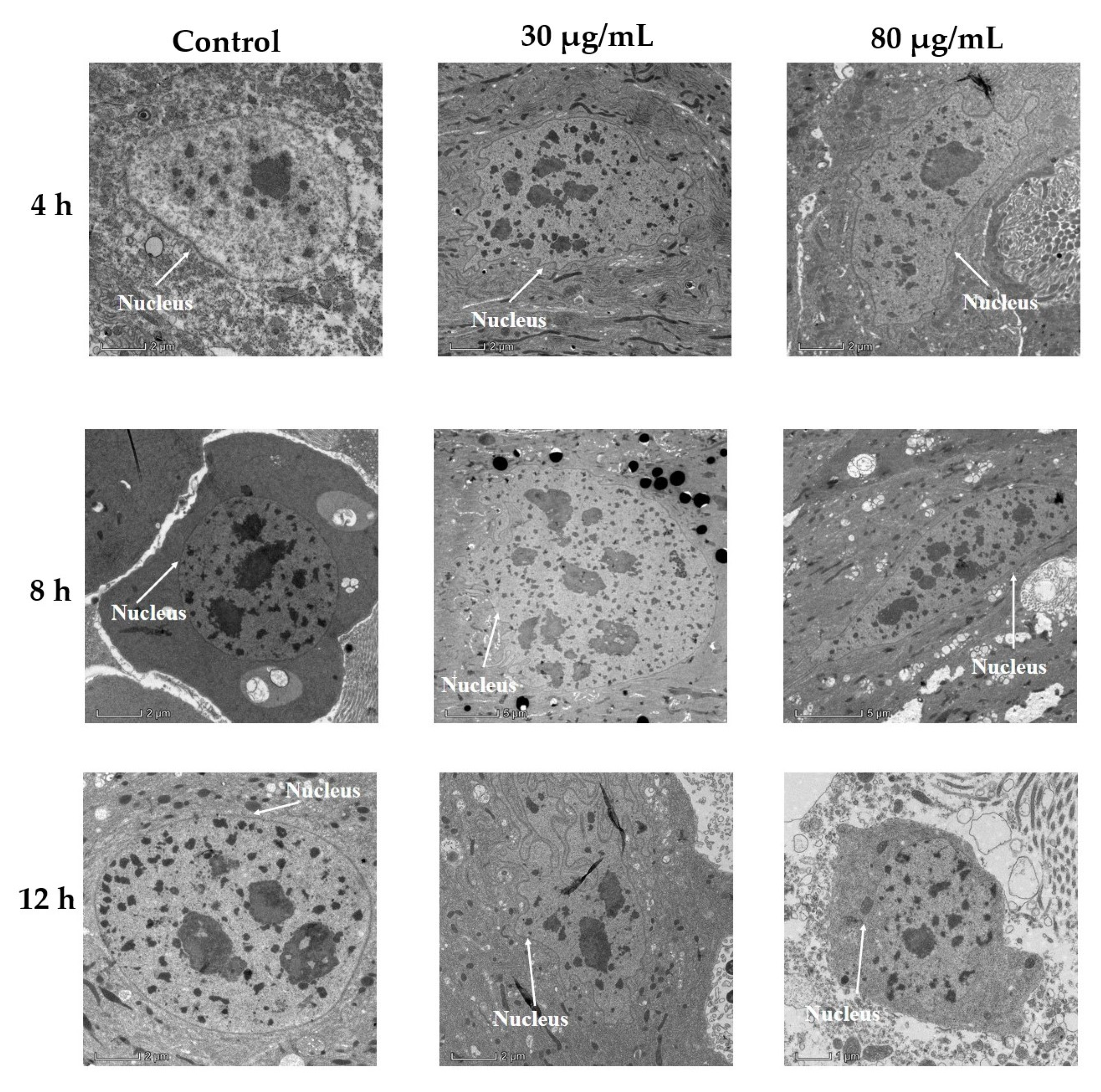
2.1.2. Transmission Electron Microscopic Examination of P. xylostella Midgut following Cyclosporin C Treatment
2.2. Sublethal Effects of Cyclosporin C against P. xylostella
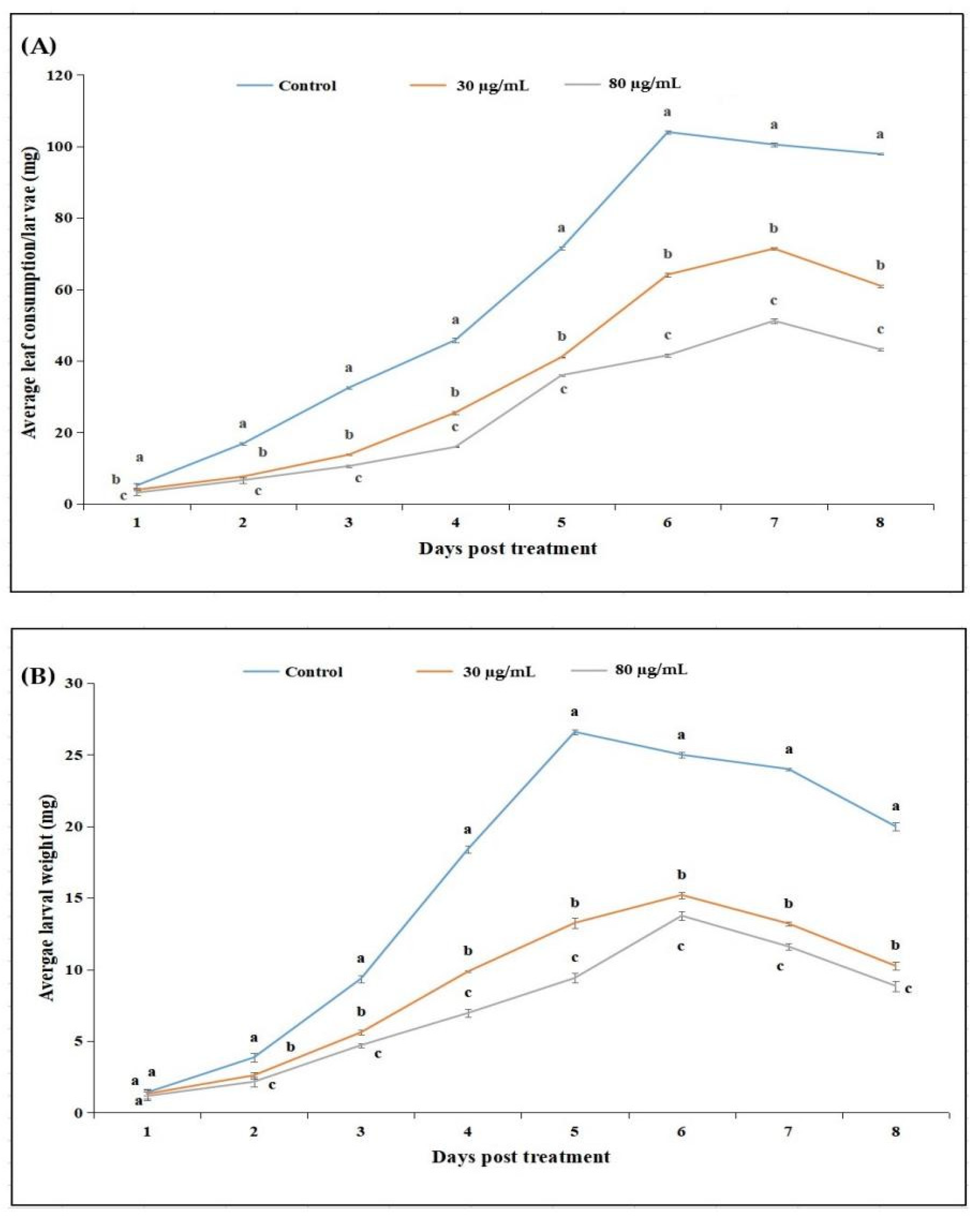
2.2.1. Effect of Cyclosporin C on Feeding and Larval Weight of P. xylostella
2.2.2. Sublethal Toxicity of Cyclosporin C
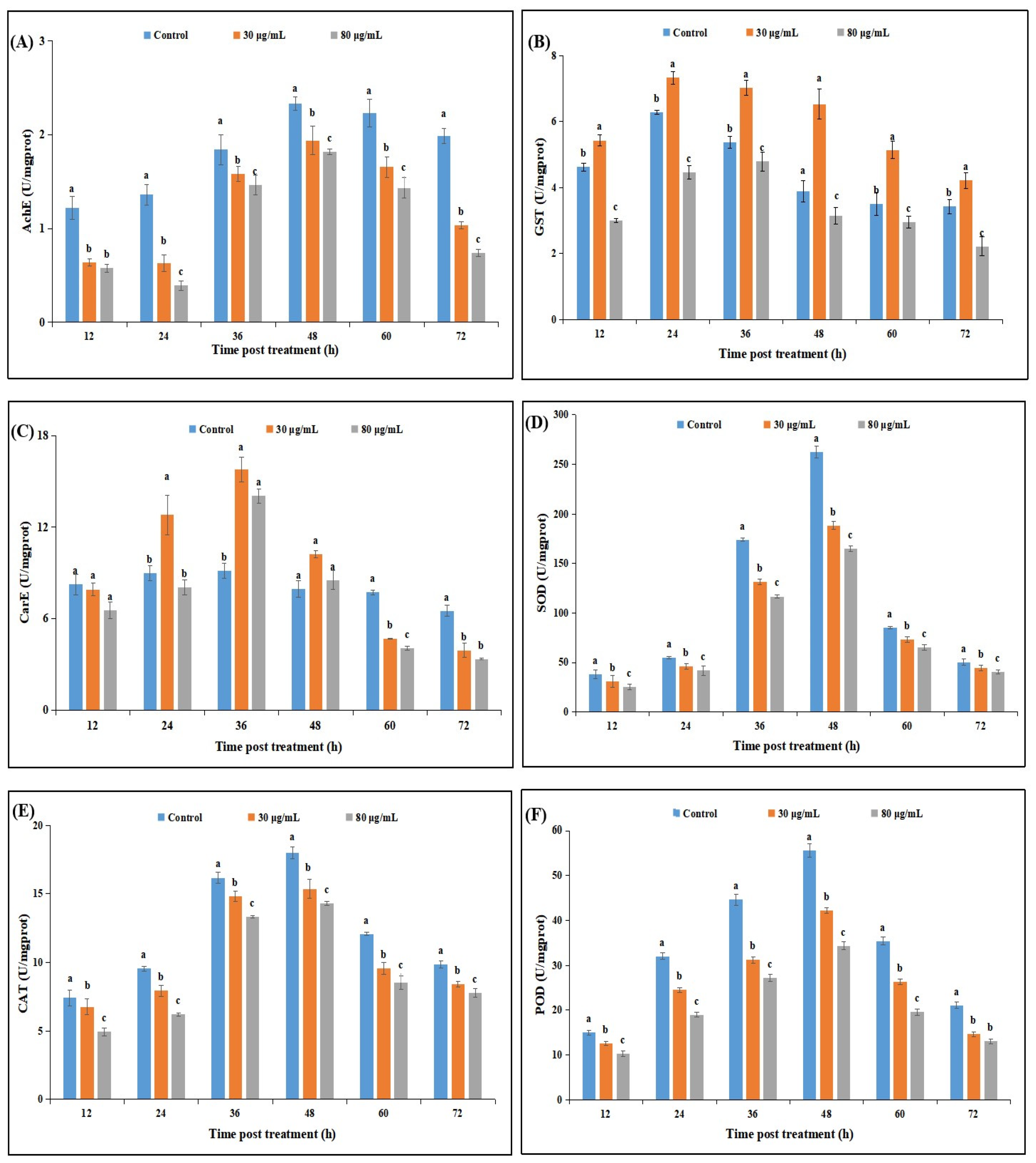
2.2.3. Effect of Cyclosporin C Treatment on Neurophysiological and Antioxidant Enzyme Activities of P. xylostella
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plutella xylostella and Cyclosporin C
5.2. Toxicity of Cyclosporin C against P. xylostella
5.2.1. Toxicity Assays
5.2.2. Transmission Electron Microscopic Examination of P. xylostella Midgut following Cyclosporin C Treatment
5.3. Antifeedant and Sublethal Toxicity of Cyclosporin C
5.3.1. Antifeedant Activity Assays
5.3.2. Sublethal Toxicity of Cyclosporin C
5.4. Effect of Cyclosporin C on Neurophysiological and Antioxidant Enzyme Activities
5.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, X.S.; Xu, J.; Wang, X.M.; Qiu, B.L.; Cuthbertson, A.G.S.; Du, C.L.; Wu, J.H.; Ali, S. Isaria fumosorosea-based-zero-valent iron nanoparticles affect the growth and survival of sweet potato whitefly, Bemisia tabaci (Gennadius). Pest Manag. Sci. 2019, 75, 2174–2181. [Google Scholar] [CrossRef] [PubMed]
- Goffré, D.; Folgarait, P.J. Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. J. Invertebr. Pathol. 2015, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.F.; Wu, J.H.; Ali, S. Morphological and molecular identification of four Purpureocillium isolates and evaluating their efficacy against the sweet potato whitefly, Bemisia tabaci (Genn.) (Hemiptera: Aleyrodidae). Egypt. J. Biol. Pest. Cont. 2021, 31, 27. [Google Scholar] [CrossRef]
- Amala, U.; Jiji, T.; Naseema, A. Laboratory evaluation of local isolate of entomopathogenic fungus, Paecilomyces lilacinus Thom Samson (ITCC 6064) against adults of melon fruit fly, Bactrocera cucurbitae Coquillett (Diptera; Tephritidae). J. Trop. Agri. 2013, 51, 132–134. [Google Scholar]
- Vilcinskas, A.; Jegorov, A.; Landa, Z.; Götz, P.; Matha, V. Effects of beauverolide L and cyclosporin A on humoral and cellular immune response of the greater wax moth, Galleria mellonella. Comp. Biochem. Physiol. 1999, 122, 83–92. [Google Scholar] [CrossRef]
- Khan, A.; Williams, K.; Nevalaine, H. Testing the nematophagous biological control strain Paecilomyces lilacinus 251 for paecilotoxin production. FEMS Microbiol. Lett. 2003, 227, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Fiolka, M.J. Immuno-suppressive effect of cyclosporin A on insect humoral immune response. J. Invert. Pathol. 2008, 98, 287–292. [Google Scholar] [CrossRef]
- Jegorov, A.; Matha, V.; Weiser, J. Production of cyclosporins by entomogenous fungi. Microbiol. Lett. 1990, 45, 65–69. [Google Scholar]
- Dumas, C.; Ravallec, M.; Matha, V.; Vey, A. Comparative study of the cytological aspects of the mode of action of destruxins and other peptidic fungal metabolites on target epithelial cells. J. Invertebr. Pathol. 1996, 67, 137–146. [Google Scholar] [CrossRef]
- Gillespie, J.P.; Bailey, A.M.; Cobb, B.; Vilcinskas, A. Fungi as elicitors of insect immune responses. Arch. Insect Biochem. Physiol. 2000, 44, 49–68. [Google Scholar] [CrossRef]
- Podsiadlowski, L.; Matha, V.; Vilcinskas, A. Detection of a P-glycoprotein related pump in chironomus larvae and its inhibition by verapamil and cyclosporin A. Comp. Biochem. Physiol. 1998, 121, 443–450. [Google Scholar] [CrossRef]
- Weiser, J.; Matha, V. The insecticidal activity of cyclosporines on mosquito larvae. J. Invertebr. Pathol. 1988, 51, 92–93. [Google Scholar] [CrossRef]
- Weiser, J.; Matha, V.; Zizka, Z.; Jegorov, A. Pathology of cyclosporin A in mosquito larvae. Cytobios 1989, 59, 143–150. [Google Scholar] [PubMed]
- Matha, V.; Jegorov, A.; Weiser, J.; Pillai, J.S. The mosquitocidal activity of conidia of Tolypocladium tundrense and Tolypocladium terricola. Cytobios 1992, 69, 163–170. [Google Scholar]
- Moussaif, M.; Jacques, P.; Schaarwachter, P.; Budzikiewicz, H.; Thonart, P. Cyclosporin C is the main antifungal compound produced by Acremonium luzulae. Appl. Environ. Mirobiol. 1997, 63, 1739–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanakala, S.; Ghanim, M. Implication of the Whitefly Bemisia tabaci cyclophilin B protein in the transmission of tomato yellow leaf curl virus. Front. Plant Sci. 2016, 7, 1702. [Google Scholar] [CrossRef] [Green Version]
- Soleymani, T.; Vassantachart, J.M.; Wu, J.J. Comparison of guidelines for the use of cyclosporine for psoriasis: A critical appraisal and comprehensive review. J. Drugs Dermatol. 2016, 15, 293–301. [Google Scholar]
- Buscher, A.K.; Beck, B.B.; Melk, A.; Hoefele, J.; Kranz, B.; Bamborschke, D.; Baig, S.; Lang-Sperandio, B.; Jungraithmayr, T.; Weber, L.T.; et al. Rapid response to cyclosporin a and favorable renal outcome in nongenetic versus genetic steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 2016, 11, 245–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chighizola, C.B.; Ong, V.H.; Meroni, P.L. The Use of Cyclosporine a in rheumatology: A 2016 comprehensive review. Clin. Rev. Allergy Immunol. 2017, 52, 401–423. [Google Scholar] [CrossRef] [Green Version]
- Kahan, B.D. Cyclosporine. N. Engl. J. Med. 1989, 321, 1725–1738. [Google Scholar]
- Calne, R.Y.; Thiru, S.; McMaster, P.; Craddock, G.N.; White, D.J.G.; Evans, D.B. Cyclosporin A in patients receiving renal allografts from cadaver donors. Lancet 1978, 312, 1323–1327. [Google Scholar] [CrossRef]
- Flores, C.; Fouquet, G.; Moura, I.C.; Maciel, T.T.; Hermine, O. Lessons to learn from low-dose cyclosporin-A: A new Approach for unexpected clinical applications. Front. Immunol. 2019, 10, 588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ezekiel, C.N.; Kraak, B.; Sandoval-Denis, M.; Sulyok, M.; Oyedele, O.A.; Ayeni, K.I.; Makinde, O.M.; Akinyemi, O.M.; Krska, R.; Crous, P.W.; et al. Diversity and toxigenicity of fungi and description of Fusarium madaense sp. nov. from cereals, legumes and soils in north-central Nigeria. MycoKeys 2020, 67, 95–124. [Google Scholar] [CrossRef] [PubMed]
- Avery, S.V.; Singleton, I.; Magan, N.; Goldman, G.H. The fungal threat to global food security. Fungal Biol. 2019, 123, 555–557. [Google Scholar] [CrossRef]
- Zalucki, M.P.; Shabbir, A.; Silva, R.; Adamson, D.; Shu-Sheng, L.; Furlong, M.J. Estimating the economic cost of one of the world’s major insect pests, Plutella xylostella (Lepidoptera: Plutellidae): Just how long is a piece of string? J. Econ. Entomol. 2012, 105, 1115–1129. [Google Scholar] [CrossRef]
- Shakeel, M.; Farooq, M.; Nasim, W.; Akram, W.; Khan, F.Z.A.; Jaleel, W. Environment polluting conventional chemical control compared to an environmentally friendly IPM approach for control of diamondback moth, Plutella xylostella (L.), in China: A review. Environ. Sci. Pollut. Res. 2017, 24, 14537–14550. [Google Scholar] [CrossRef] [PubMed]
- Amiri, B.; Ibrahim, L.; Butt, T.M. Antiffedant properties of destruxins and their potential use with the entomogenous fungus Metarhizium anisopliae for improved control of crucifer pests. Biocont. Sci. Technol. 1999, 9, 487–498. [Google Scholar] [CrossRef]
- Huang, S.; Ali, S.; Ren, S.X. The combination of destruxin A with insecticide chlorantraniliprole increases virulence against Plutella xylostella. Pak. J. Zool. 2016, 48, 1849–1855. [Google Scholar]
- Arthurs, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2018, 165, 13–21. [Google Scholar] [CrossRef]
- Bordalo, M.D.; Gravato, C.; Beleza, S.; Campos, D.; Lopes, I.; Pestana, J.L.T. Lethal and sublethal toxicity assessment of Bacillus thuringiensis var. israelensis and Beauveria bassiana based bioinsecticides to the aquatic insect Chironomus riparius. Sci. Total Environ. 2020, 698, 134155. [Google Scholar] [CrossRef]
- Duchet, C.; Franquet, E.; Lagadic, L.; Lagneau, C. Effects of Bacillus thuringiensis israelensis and spinosad on adult emergence of the non-biting midges Polypedilum nubifer (Skuse) and Tanytarsus curticornis Kieffer (Diptera: Chironomidae) in coastal wetlands. Ecotoxicol. Environ. Saf. 2015, 115, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Vereb, G.; Panyi, J.G.; Balazs, M.; Matyus, L.; Matko, J.; Damjanovich, S. Effcet of cyclosporin A on the membrane potential and Ca++ level of human lymphoid cell lines and mouse thymocytes. Biochim. Biophys. Acta 1990, 109, 159–165. [Google Scholar] [CrossRef]
- Griffiths, E.J.; Halestrap, A. Further evidence that cyclosporin A protects mitochondria from calcium overload by inhibiting a matrix peptidyl-prolyl cis-trans isomerase. Biochem. J. 1991, 274, 611–614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Claudianoc, C.; Ranson, H.; Jhonson, R.M.; Biswas, S.; Schuler, M.A.; Berenbaum, M.R.; Feyereisen, R.; Oakeshott, J.G. A deficit of detoxification enzymes: Pesticide sensitivity and environmental response in the honeybee. Insect Mol. Biol. 2006, 15, 615–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, W.C.; Zhang, Q. The effects of Sophora alopecuroids alkaloids on metabolic esterases of the diamondback moth. Acta. Entomol. Sin. 2003, 46, 122–125. [Google Scholar]
- Habig, W.H. Assays for Differentiation of Glutathione S-Transferase. In Method in Enzymology; Willian, B.J., Ed.; Academic Press: New York, NY, USA, 1981; pp. 398–405. [Google Scholar]
- Ding, S.Y.; Li, H.Y.; Li, X.F.; Zhang, Z.Y. Effects of two kinds of transgenic poplar on protective enzymes system in the midgut of larvae of American white moth. J. For. Res. Jpn. 2001, 12, 119–122. [Google Scholar]
- Ali, S.; Huang, Z.; Ren, S.X. Media composition influences on growth, enzyme activity and virulence of the entomopathogen hyphomycete Isaria fumosorosea. Entomol. Experiment. Appl. 2009, 131, 30–38. [Google Scholar] [CrossRef]
- Burhan, A.H.; Mohammad, R.A. Pathogenesis of Paecilomyces lilacinus against the immature stages of Musca domestica L. J. Pharm. Sci. Res. 2019, 11, 1595–1601. [Google Scholar]
- Ali, S.; Huang, Z.; Ren, S.X. Production of cuticle degrading enzymes by Isaria fumosorosea and their evaluation as a biocontrol agent against diamondback moth. J. Pest Sci. 2010, 83, 361–370. [Google Scholar] [CrossRef]
- Wu, J.; Li, J.; Zhang, C.; Yu, X.; Cuthbertson, A.G.S.; Ali, S. Biological impact and enzyme activities of Spodoptera litura (Lepidoptera: Noctuidae) in response to synergistic action of matrine and Beauveria brongniartii. Front. Physiol. 2020, 11, 584405. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Byrne, F.J.; Gorman, K.J.; Cahill, M.; Denholm, I.; Devonshire, A.L. The role of B-type esterases in conferring insecticide resistance in the tobacco whitefly, Bemisia tabaci (Genn). Pest. Manag. Sci. 2000, 56, 867–874. [Google Scholar]
- Ellman, G.L.; Coutney, K.D.; Andre, V.; Featherstone, R.M. A new and rapid calorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Shannon, L.M.; Kay, E.; Lew, J.Y. Peroxidase isozymes from horseradish roots. I. Isolation and physical properties. J. Biol. Chem. 1966, 241, 2166–2172. [Google Scholar] [CrossRef]
- SAS Institute. SAS User’s Guide; Statistics SAS Institute: Cary, NC, USA, 2000. [Google Scholar]


| Life History Parameters | Control | 30 μg/mL | 80 μg/mL |
|---|---|---|---|
| Pupation (%) | 96.66 ± 1.66 a | 58.33 ± 1.40 b | 14.44 ± 0.93 c |
| Adult emergence (%) | 66.66 ± 2.33 a | 23.33 ± 1.66 b | 7.78 ± 1.11 c |
| Pre-oviposition period (h) | 7.25 ± 0.66 c | 9.33 ± 0.50 b | 11.50 ± 0.33 a |
| Fecundity (eggs/female) | 197 ± 4.48 a | 137 ± 2.93 b | 82 ± 2.34 |
| Female longevity (d) | 12 ± 0.33 a | 9 ± 0.66 b | 7 ± 0.66 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Zhang, X.; Bashir, M.H.; Ali, S. Lethal and Sublethal Toxicity Assessment of Cyclosporin C (a Fungal Toxin) against Plutella xylostella (L.). Toxins 2022, 14, 514. https://doi.org/10.3390/toxins14080514
Wu J, Zhang X, Bashir MH, Ali S. Lethal and Sublethal Toxicity Assessment of Cyclosporin C (a Fungal Toxin) against Plutella xylostella (L.). Toxins. 2022; 14(8):514. https://doi.org/10.3390/toxins14080514
Chicago/Turabian StyleWu, Jianhui, Xiaochen Zhang, Muhammad Hamid Bashir, and Shaukat Ali. 2022. "Lethal and Sublethal Toxicity Assessment of Cyclosporin C (a Fungal Toxin) against Plutella xylostella (L.)" Toxins 14, no. 8: 514. https://doi.org/10.3390/toxins14080514
APA StyleWu, J., Zhang, X., Bashir, M. H., & Ali, S. (2022). Lethal and Sublethal Toxicity Assessment of Cyclosporin C (a Fungal Toxin) against Plutella xylostella (L.). Toxins, 14(8), 514. https://doi.org/10.3390/toxins14080514






