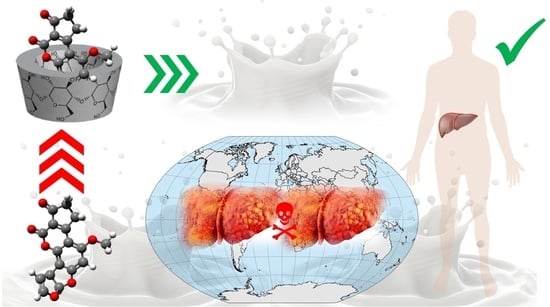
Decrease in Aflatoxin M1 Concentration in Milk during Cholesterol Removal by Application of β-Cyclodextrin
Abstract
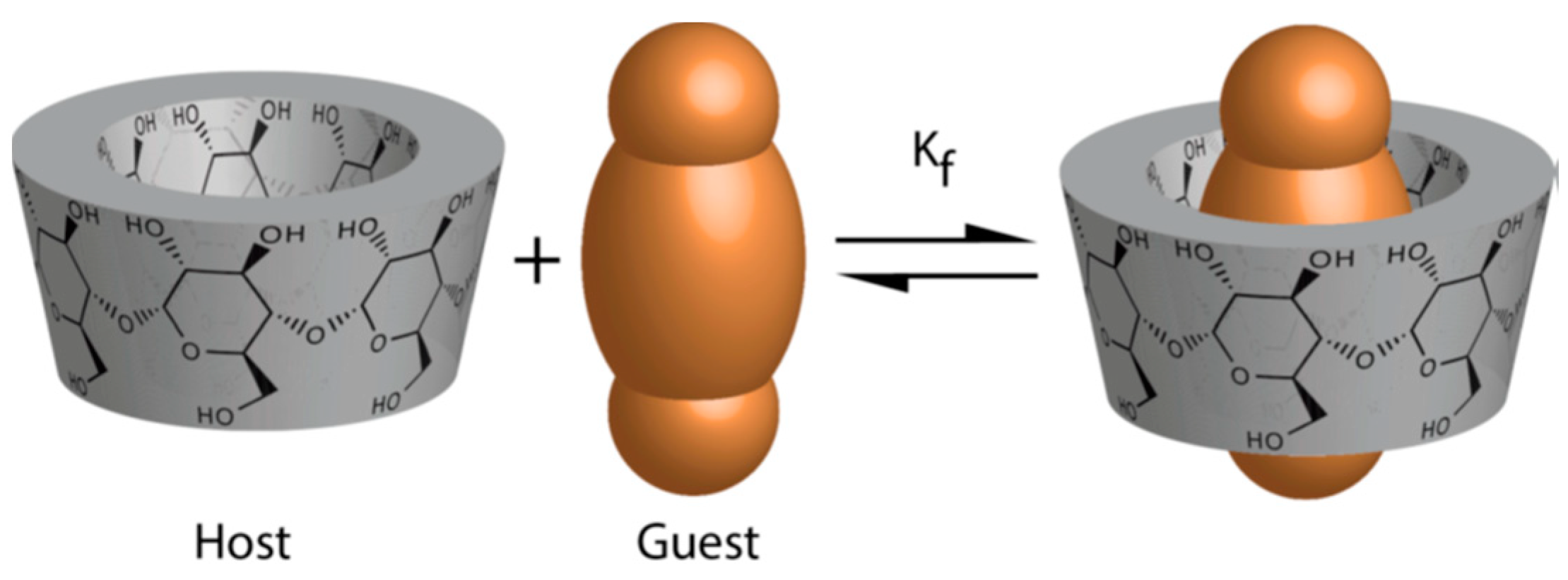
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Samples
4.2. Chemicals
4.3. Instruments
4.4. Experiments
4.5. Treatment of Milk for Removal of CHO and AFM1
4.6. Preparation of Milk for CHO Analysis
4.7. HPLC Determination of CHO Concentration
4.8. Preparation of Milk for AFM1 Analysis
4.9. HPLC Determination of AFM1 Concentration
4.10. Validation of Analytical Procedures
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nut. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Shirani, K.; Zanjani, B.R.; Mahmoudi, M.; Jafarian, A.H.; Hassani, F.V.; Giesy, J.P.; Karimi, G. Immunotoxicity of aflatoxin M1: As a potent suppressor of innate and acquired immune systems in a subacute study. J. Sci. Food Agric. 2018, 98, 5884–5892. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.A.; Hendrich, S.; Landgren, C.; Bryant, C.M. Food mycotoxins: An update. J. Food Sci. 2006, 71, R51–R65. [Google Scholar] [CrossRef]
- Shibahara, T.; Ogawa, H.I.; Ryo, H.; Fujikawa, K. DNA-damaging potency and genotoxicity of aflatoxin M1 in somatic cells in vivo of Drosophila melanogaster. Mutagenesis 1995, 10, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Turna, N.S.; Wu, F. Aflatoxin M1 in milk: A global occurrence, intake, & exposure assessment. Trends Food Sci. Technol. 2021, 110, 183–192. [Google Scholar] [CrossRef]
- Commission Regulation (EC). No 165/2010 amending Regulation 1881/2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2010, 50, 8–12. [Google Scholar]
- Fox, P.F.; Guinee, T.P.; Cogan, T.M.; McSweeney, P.L. Fundamentals of Cheese; Springer: Berlin/Heidelberg, Germany, 2017; Available online: https://link.springer.com/content/pdf/10.1007/978-1-4899-7681-9.pdf (accessed on 20 April 2022).
- FAO. Gateway to Dairy Production and Products. Milk and Milk Products. 2017. Available online: www.fao.org/dairy-production-products/products/en/ (accessed on 20 April 2022).
- OECD-FAO. Agricultural Outlook 2021–2030. 2021. Available online: www.fao.org/documents/card/en/c/cb5332en (accessed on 20 April 2022).
- Nazhand, A.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A. Characteristics, occurrence, detection and detoxification of aflatoxins in foods and feeds. Foods 2020, 9, 644. [Google Scholar] [CrossRef]
- Mollayusefian, I.; Ranaei, V.; Pilevar, Z.; Cabral-Pinto, M.M.; Rostami, A.; Nematolahi, A.; Khedher, K.M.; Thai, V.N.; Fakhri, Y.; Khaneghah, A.M. The concentration of aflatoxin M1 in raw and pasteurized milk: A worldwide systematic review and meta-analysis. Trends Food Sci. Technol. 2021, 115, 22–30. [Google Scholar] [CrossRef]
- Roila, R.; Branciari, R.; Verdini, E.; Ranucci, D.; Valiani, A.; Pelliccia, A.; Fioroni, L.; Pecorelli, I. A Study of the Occurrence of Aflatoxin M1 in Milk Supply Chain over a Seven-Year Period (2014–2020): Human Exposure Assessment and Risk Characterization in the Population of Central Italy. Foods 2021, 10, 1529. [Google Scholar] [CrossRef]
- Sharafi, K.; Matin, B.K.; Omer, A.K.; Mansouri, B.; Soleimani, H.; Fattahi, N.; Sharafi, H.; Kiani, A. A worldwide systematic literature review for aflatoxin M1 in infant formula milk: Human health risk assessment by Monte Carlo simulation. Food Control 2021, 134, 108681. [Google Scholar] [CrossRef]
- Sumon, A.H.; Islam, F.; Mohanto, N.C.; Kathak, R.R.; Molla, N.H.; Rana, S.; Degen, G.H.; Ali, N. The Presence of Aflatoxin M1 in Milk and Milk Products in Bangladesh. Toxins 2021, 13, 440. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.; Dalla Rosa, A.; Gonzales, S.L.; Feltes, M.M.C.; Badiale-Furlong, E.; Dors, G.C. Incidence of aflatoxin M1 in fresh milk from small farms. Food Sci. Technol. 2017, 37, 11–15. [Google Scholar] [CrossRef] [Green Version]
- Xiong, J.; Xiong, L.; Zhou, H.; Liu, Y.; Wu, L. Occurrence of aflatoxin B1 in dairy cow feedstuff and aflatoxin M1 in UHT and pasteurized milk in central China. Food Control 2018, 92, 386–390. [Google Scholar] [CrossRef]
- Bellio, A.; Bianchi, D.M.; Gramaglia, M.; Loria, A.; Nucera, D.; Gallina, S.; Gili, M.; Decastelli, L. Aflatoxin M1 in cow’s milk: Method validation for milk sampled in northern Italy. Toxins 2016, 8, 57. [Google Scholar] [CrossRef]
- Pandey, A.K.; Shakya, S.; Patyal, A.; Ali, S.L.; Bhonsle, D.; Chandrakar, C.; Hattimare, D. Detection of aflatoxin M1 in bovine milk from different agro-climatic zones of Chhattisgarh, India, using HPLC-FLD and assessment of human health risks. Mycotoxin Res. 2021, 37, 265–273. [Google Scholar] [CrossRef]
- Carvajal, M.; Bolaños, A.; Rojo, F.; Mendez, I. Aflatoxin M1 in pasteurized and ultrapasteurized milk with different fat content in Mexico. J. Food Prot. 2003, 66, 1885–1892. [Google Scholar] [CrossRef]
- Akinyemi, M.O.; Braun, D.; Windisch, P.; Warth, B.; Ezekiel, C.N. Assessment of multiple mycotoxins in raw milk of three different animal species in Nigeria. Food Control 2022, 131, 108258. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R. Assessment of aflatoxin M1 in milk and milk products from Punjab, Pakistan. Food Control 2013, 30, 235–239. [Google Scholar] [CrossRef]
- Kos, J.; Lević, J.; Duragić, O.; Kokić, B.; Miladinović, I. Occurrence and estimation of aflatoxin M1 exposure in milk in Serbia. Food Control 2014, 38, 41–46. [Google Scholar] [CrossRef]
- Lee, J.E.; Kwak, B.M.; Ahn, J.H.; Jeon, T.H. Occurrence of aflatoxin M1 in raw milk in South Korea using an immunoaffinity column and liquid chromatography. Food Control 2009, 20, 136–138. [Google Scholar] [CrossRef]
- Elzupir, A.O.; Elhussein, A.M. Determination of aflatoxin M1 in dairy cattle milk in Khartoum State, Sudan. Food Control 2010, 21, 945–946. [Google Scholar] [CrossRef]
- Pankaj, S.K.; Shi, H.; Keener, K.M. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Technol. 2018, 71, 73–83. [Google Scholar] [CrossRef]
- Marshall, H.; Meneely, J.P.; Quinn, B.; Zhao, Y.; Bourke, P.; Gilmore, B.F.; Zhang, G.; Elliott, C.T. Novel decontamination approaches and their potential application for post-harvest aflatoxin control. Trends Food Sci. Technol. 2020, 106, 489–496. [Google Scholar] [CrossRef]
- Gavahian, M.; Pallares, N.; Al Khawli, F.; Ferrer, E.; Barba, F.J. Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods. Trends Food Sci. Technol. 2020, 106, 209–218. [Google Scholar] [CrossRef]
- Matencio, A.; Navarro-Orcajada, S.; García-Carmona, F.; López-Nicolás, J.M. Applications of cyclodextrins in food science: A review. Trends Food Sci. Technol. 2020, 104, 132–143. [Google Scholar] [CrossRef]
- Kolarič, L.; Šimko, P. Effect of processing conditions on measure of cholesterol removal from milk and cream. Monatsh. Chem. [CrossRef]
- Astray, G.; Gonzales-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cholesterol;https://pubchem.ncbi.nlm.nih.gov/compound/Aflatoxin-M1; (accessed on 20 April 2022).
- Crini, G. A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Cramer, F.; Saenger, W.; Spatz, H.C. Inclusion compounds. XIX. The formation of inclusion compounds of α-cyclodextrin in aqueous solutions. Thermodynamics and kinetics. J. Am. Chem. Soc. 1967, 89, 14–20. [Google Scholar] [CrossRef]
- Kolarič, L.; Šimko, P. Application of β-cyclodextrin in the production of low-cholesterol milk and dairy products. Trends Food Sci. Technol. 2022, 119, 13–22. [Google Scholar] [CrossRef]
- Kolarič, L.; Kántorová, P.; Šimko, P. β-Cyclodextrin as the Key Issue in Production of Acceptable Low-Cholesterol Dairy Products. Molecules 2022, 27, 2919. [Google Scholar] [CrossRef] [PubMed]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Gathumbi, J.K. A review of the impact of mycotoxins on dairy cattle health: Challenges for food safety and dairy production in sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naeimipour, F.; Aghajani, J.; Kojuri, S.A.; Ayoubi, S. Useful approaches for reducing aflatoxin M1 content in milk and dairy products. Biomed. Biotechnol. Res. J. 2018, 2, 94–99. [Google Scholar] [CrossRef]
- Hassanpour, M.; Rezaie, M.R.; Baghizadeh, A. Practical analysis of aflatoxin M1 reduction in pasteurized milk using low dose gamma irradiation. J. Environ. Health Sci. Eng. 2019, 17, 863–872. [Google Scholar] [CrossRef]
- Chaudhary, H.J.; Patel, A.R. Removal of aflatoxin M1 from milk and aqueous medium by indigenously isolated strains of W. confuse H1 and L. plantarum S2. Food Biosci. 2022, 45, 101468. [Google Scholar] [CrossRef]
- Kuharić, Ž.; Jakopović, Ž.; Čanak, I.; Frece, J.; Bošnir, J.; Pavlek, Ž.; Ivešić, M.; Markov, K. Removing aflatoxin M1 from milk with native lactic acid bacteria, centrifugation, and filtration. Arh. Hig. Rada Toksikol. 2018, 69, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Assaf, J.C.; Nahle, S.; Chokr, A.; Louka, N.; Atoui, A.; El Khoury, A. Assorted methods for decontamination of aflatoxin M1 in milk using microbial adsorbents. Toxins 2019, 11, 304. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.; Flint, S.; Palmer, J. Control of aflatoxin M1 in milk by novel methods: A review. Food Chem. 2020, 311, 125984. [Google Scholar] [CrossRef]
- Semanová, J.; Skláršová, B.; Šimon, P.; Šimko, P. Elimination of polycyclic aromatic hydrocarbons from smoked sausages by migration into polyethylene packaging. Food Chem. 2016, 201, 1–6. [Google Scholar] [CrossRef]
- Kolarič, L.; Šimko, P. Determination of cholesterol content in butter by HPLC: Up-to-date optimization, and in-house validation using reference materials. Foods 2020, 9, 1378. [Google Scholar] [CrossRef] [PubMed]
- Kolarič, L.; Šimko, P. Simultaneous determination of cholesterol, stigmasterol, and β-sitosterol contents in milk and dairy products. J. Food Process. Preserv. 2022, 46, e16146. [Google Scholar] [CrossRef]
- AOAC Official Method 2000.08-2004. In Aflatoxin M1 in Liquid Milk. Immunoaffinity Column by Liquid Chromatography; AOAC International: Rockville, MD, USA, 2004; pp. 1–3.
- Commission Regulation (EC). No 401/2006 of 23 February 2006 laying down the methods of sampling and analysis for the official control of the levels of mycotoxins in foodstuffs. Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
| Sample | No. of Samples/No. of Positive Samples | Concentration Range of AFM1 (µg/kg) | Country | Source |
|---|---|---|---|---|
| Raw milk Pasteurized milk UHT milk | 105/75 15/15 15/15 | 0.005–0.198 0.017–0.187 0.012–0.146 | Bangladesh | Sumon et al. [15] |
| Fresh milk | 52/21 | 0.01–3.385 | Brazil | Goncalves et al. [16] |
| Pasteurized and UHT milk | 242/178 | 0.001–0.352 | China | Xiong et al. [17] |
| Raw milk | 1668/36 | 0.01–0.208 | Italy | Bellio et al. [18] |
| Bovine milk Buffalo milk | 375/154 170/70 | 0.01–9.18 0.01–6.41 | India | Pandey et al. [19] |
| Raw milk | 290/145 | Nd *–8.35 | Mexico | Carvajal et al. [20] |
| Bovine milk Goat milk | 29/29 87/41 | up to 0.081 up to 3.108 | Nigeria | Akinyemi et al. [21] |
| Fresh milk | 107/76 | 0.004–0.845 | Pakistan | Iqbal et al. [22] |
| Raw milk | 150/150 | 0.01–1.2 | Serbia | Kos et al. [23] |
| Raw milk | 100/45 | 0.02–0.08 | South Korea | Lee et al. [24] |
| Fresh milk | 44/42 | 0.22–6.90 | Sudan | Elzupir et al. [25] |
| A | B | C | D | |
|---|---|---|---|---|
| Sample No. | Initial Concentration of Cholesterol (mg/kg) a | Concentration of Cholesterol after Removal (mg/kg) a | Measure of Cholesterol Removal (%) | Distribution Coefficient δCHO |
| 1 | 129.04 ± 2.13 | 10.36 ± 2.11 + | 92.0 | 11.46 |
| 2 | 135.78 ± 6.01 | 6.47 ± 1.59 + | 95.2 | 19.99 |
| 3 | 150.39 ± 0.64 | 5.25 ± 0.03 + | 96.5 | 27.65 |
| 4 | 113.32 ± 6.30 | 8.92 ± 0.02 + | 92.1 | 11.70 |
| 5 | 123.01 ± 2.21 | 1.43 ± 0.63 + | 98.8 | 85.02 |
| 6 | 103.92 ± 0.43 | 9.47 ± 0.21 + | 90.9 | 9.97 |
| 7 | 122.33 ± 1.45 | 23.49 ± 1.50 + | 80.8 | 4.21 |
| Average | 125.40 ± 2.74 | 9.34 ± 0.87 + | 92.3 | 24.28 |
| A* | B* | C* | D* | |
|---|---|---|---|---|
| Sample No. | Concentration of AFM1 after Spiking (µg/kg) | Concentration of AFM1 after Removal (µg/kg) a | Measure of AFM1 Removal (%) | Distribution Coefficient δAFM1 |
| 1 | 0.20 | 0.13 ± 0.06 + | 35 | 0.54 |
| 2 | 0.40 | 0.25 ± 0.02 + | 38 | 0.60 |
| 3 | 0.60 | 0.36 ± 0.02 + | 40 | 0.67 |
| 4 | 0.80 | 0.47 ± 0.03 + | 41 | 0.70 |
| 5 | 1.00 | 0.55 ± 0.04 + | 45 | 0.82 |
| 6 | 1.20 | 0.80 ± 0.04 + | 33 | 0.50 |
| 7 | 2.00 | 1.16 ± 0.06 + | 42 | 0.72 |
| Average | 0.89 | 0.53 ± 0.04 + | 39.1 | 0.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimko, P.; Kolarič, L. Decrease in Aflatoxin M1 Concentration in Milk during Cholesterol Removal by Application of β-Cyclodextrin. Toxins 2022, 14, 379. https://doi.org/10.3390/toxins14060379
Šimko P, Kolarič L. Decrease in Aflatoxin M1 Concentration in Milk during Cholesterol Removal by Application of β-Cyclodextrin. Toxins. 2022; 14(6):379. https://doi.org/10.3390/toxins14060379
Chicago/Turabian StyleŠimko, Peter, and Lukáš Kolarič. 2022. "Decrease in Aflatoxin M1 Concentration in Milk during Cholesterol Removal by Application of β-Cyclodextrin" Toxins 14, no. 6: 379. https://doi.org/10.3390/toxins14060379
APA StyleŠimko, P., & Kolarič, L. (2022). Decrease in Aflatoxin M1 Concentration in Milk during Cholesterol Removal by Application of β-Cyclodextrin. Toxins, 14(6), 379. https://doi.org/10.3390/toxins14060379