New Evidences about the Carcinogenic Effects of Ochratoxin A and Possible Prevention by Target Feed Additives
Abstract
:1. Introduction
2. Common Spontaneous Neoplastic Changes Characteristic for Advanced Age of the Respective Animals/Poultry
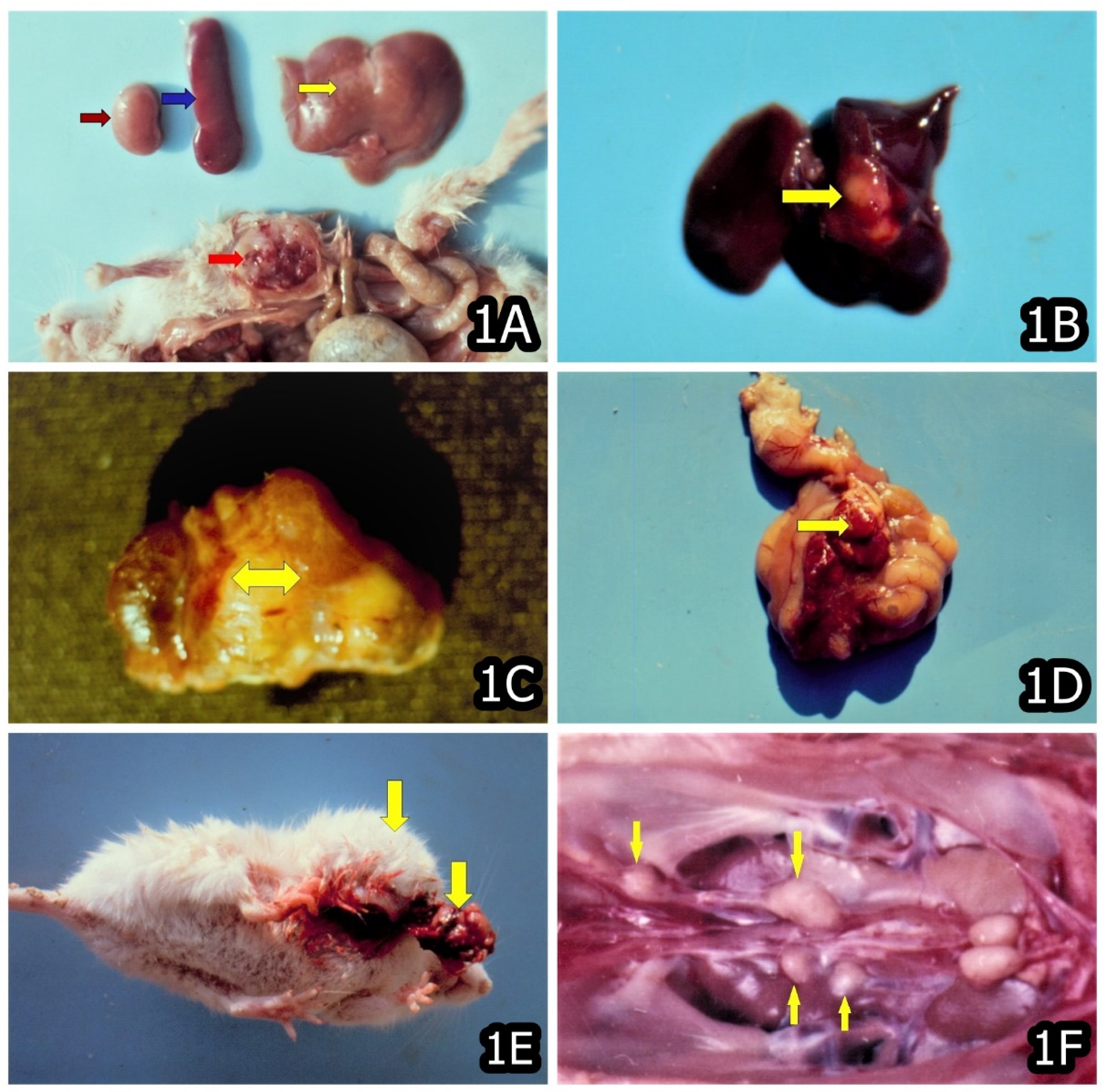
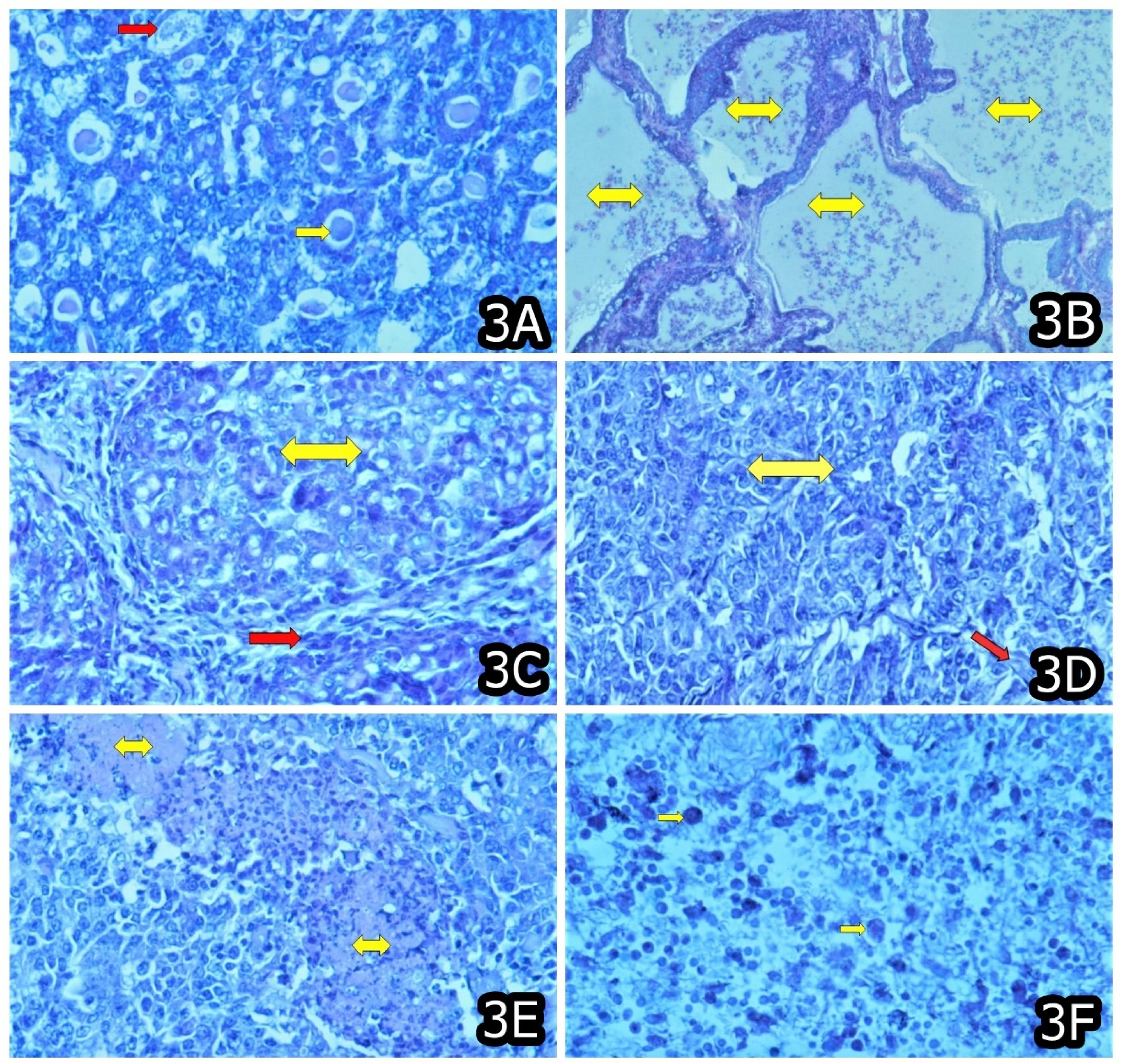
3. Characteristic and Specific Tumor Incidents and Neoplasia in Target Animals/Poultry Exposed to OTA
4. The Possible Preventive Measures of Some Feed Additives against Toxic and Carcinogenic Effects of OTA
5. Concluding Remarks
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin contamination in the EU feed supply chain: A focus on cereal byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Schatzmayr, G.; Streit, E. Global occurrence of mycotoxins in the food and feed chain: Facts and figures. World Mycotox. J. 2013, 6, 213–222. [Google Scholar] [CrossRef]
- IARC. Ochratoxin A. In IARC Monographs on the Evaluation of Carcinogenic Risk to Humans: Some Naturally Occurring Substances; Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; Volume 56, pp. 489–521. [Google Scholar]
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; IARC: Lyon, France, 2002; Volume 82, pp. 1–590. [Google Scholar]
- Kanisawa, M.; Suzuki, S. Induction of renal and hepatic tumors in mice by ochratoxin A, a mycotoxin. Gann 1978, 69, 599–600. [Google Scholar]
- Boorman, G.A. Toxicology and carcinogenesis studies of ochratoxin A (CAS No. 303–47-9) in F344/N rats (gavage studies). Natl. Toxicol. Program Technol. Rep. 1989, 358, 1–142. [Google Scholar]
- Boorman, G.A.; Maronport, R.R.; Eustis, S.L. Rodent carcinogenicity bioassay: Past, present and future. Toxicol. Pathol. 1994, 22, 105–111. [Google Scholar] [CrossRef]
- Castegnaro, M.; Mohr, U.; Pfohl-Leszkowicz, A.; Esteve, J.; Steinmann, J.; Tillmann, T.; Michelon, J.; Bartsch, H. Sex- and strain-specific induction of renal tumors by ochratoxin A in rats correlates with DNA adduction. Int. J. Cancer 1998, 77, 70–75. [Google Scholar] [CrossRef]
- Stoev, S.D.; Hald, B.; Mantle, P. Porcine nephropathy in Bulgaria: A progressive syndrome of complex of uncertain (mycotoxin) etiology. Vet. Rec. 1998, 142, 190–194. [Google Scholar] [CrossRef]
- Stoev, S.D. Studies on carcinogenic and toxic effects of ochratoxin A in chicks, Special issue “Ochratoxins”. Toxins 2010, 2, 649–664. [Google Scholar] [CrossRef] [Green Version]
- Stuart, B.P.; Bedell, D.M. Mycotoxicosis in swine. Veter. Clin. N. Am. 1982, 4, 377–388. [Google Scholar] [CrossRef]
- Krogh, P.; Nesheim, S. Ochratoxin A. In Environmental Carcinogens; Selected Methods of Analysis; Some mycotoxin; IARC Scientific Publications 44: Lyon, France, 1982; Volume 5, pp. 247–253. [Google Scholar]
- Bendele, A.M.; Carlton, M.W.; Krogh, P.; Lillehoj, E.B. Ochratoxin A carcinogenesis in the (C57GL/6J × C3H) F1 mouse. J. Natl. Cancer Inst. 1985, 75, 733–742. [Google Scholar] [PubMed]
- Stoev, S.D. Balkan Endemic Nephropathy—Still continuing enigma, risk assessment and underestimated hazard of joint mycotoxin exposure of animals or humans. Chem.-Biol. Interact. 2017, 261, 63–79. [Google Scholar] [CrossRef] [PubMed]
- Nordic Working Group on Food Toxicology and Risk Evaluation (NNT). Nordiske Seminar-og Arbeidsrapporter (1991:545). Health Evaluation of Ochratoxin A in Food Products; Nordic Council of Ministers: Copenhagen, Denmark, 1991; pp. 1–29. [Google Scholar]
- Mantle, P.; Kulinskaya, E.; Nestler, S. Renal tumourigenesis in male rats in response to chronic dietary ochratoxin A. Food Addit. Contam. 2005, 22 (Suppl. 1), 58–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gazinska, P.; Herman, D.; Gillett, C.; Pinder, S.E.; Mantle, P.G. Comparative Immunohistochemical Analysis of Ochratoxin A Tumourigenesis in Rats and Urinary Tract Carcinoma in Humans; Mechanistic Significance of p-S6 Ribosomal Protein Expression. Toxins 2012, 4, 643–662. [Google Scholar] [CrossRef] [PubMed]
- Mantle, P. Rat Kidney Cancers Determined by Dietary Ochratoxin A in the First Year of Life. J. Kidney Cancer VHL 2016, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Herman, D.; Mantle, P. Immunohistochemical Analysis of Rat Renal Tumours Caused by Ochratoxin A. Toxins 2017, 9, 384. [Google Scholar] [CrossRef] [Green Version]
- Stoev, S.D. Long term preliminary studies on toxic and carcinogenic effect of individual or simultaneous exposure to ochratoxin A and penicillic acid in mice. Toxicon 2020, 84, 192–201. [Google Scholar] [CrossRef]
- Stoev, S.D. Follow up long term preliminary studies on carcinogenic and toxic effects of ochratoxin A in rats and the putative protection of phenylalanine. Toxicon 2021, 190, 41–49. [Google Scholar] [CrossRef]
- Galtier, P. Contribution of pharmacokinetic studies to mycotoxicology—Ochratoxin A. Vet. Sci. Commun. 1978, 25, 349–358. [Google Scholar] [CrossRef]
- Pitout, M.J. The hydrolysis of ochratoxin A by some proteolytic enzymes. Biochem. Pharmacol. 1969, 18, 485–491. [Google Scholar] [CrossRef]
- Parker, R.W.; Phillips, T.D.; Kubena, L.E.; Russell, L.H.; Heidelbaugh, N.D. Inhibition of pancreatic carboxypeptidase A: A possible mechanism of interaction between penicillic acid and ochratoxin A. J. Environ. Sci. Health B 1982, 17, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D.; Vitanov, S.; Anguelov, G.; Petkova-Bocharova, T.; Creppy, E.E. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and penicillic acid. Vet. Res. Commun. 2001, 25, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Stoev, S.D.; Stefanov, M.; Denev, S.; Radic, B.; Domijan, A.-M.; Peraica, M. Experimental mycotoxicosis in chickens induced by ochratoxin A and penicillic acid and intervention by natural plant extracts. Vet. Res. Commun. 2004, 28, 727–746. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Phillips, T.D.; Witzel, D.A.; Heidelbauch, N.D. Toxicity of Ochratoxin A and Penicillic Acid to chicks. Bull. Environ. Contam. Toxicol. 1984, 32, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Micco, C.; Miraglia, M.; Onori, R.; Libanori, A.; Brera, C.; Mantovani, A.; Macri, C. Effect of combined exposure to ochratoxin A and penicillic acid on residues and toxicity in broilers. Ravista Della Soc. Ital. Sci. Dell’allimentazione 1991, 20, 101–108. [Google Scholar]
- Stoev, S.; Anguelov, G.; Pavlov, D.; Pirovski, L. Some antidotes and paraclinical investigations in experimental intoxication with ochratoxin A and penicillic acid in chicks. Vet. Arhiv. 1999, 69, 179–189. [Google Scholar]
- Stoev, S.D.; Anguelov, G.; Ivanov, I.; Pavlov, D. Influence of ochratoxin A and an extract of artichoke on the vaccinal immunity and health in broiler chicks. Exp. Toxicol. Pathol. 2000, 52, 43–55. [Google Scholar] [CrossRef]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M.; et al. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2012, 64, 733–741. [Google Scholar] [CrossRef]
- Stoev, S.D.; Dutton, M.; Njobeh, P.; Mosonik, J.; Steenkamp, P. Mycotoxic nephropathy in Bulgarian pigs and chickens: Complex aetiology and similarity to Balkan Enedemic Nephropathy. Food Addit. Contam. Part A 2010, 27, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Stoev, S.D.; Denev, S.; Dutton, M.; Njobeh, P.; Mosonik, J.; Steenkamp, P.; Petkov, I. Complex etiology and pathology of mycotoxic nephropathy in South African pigs. Mycotox. Res. 2010, 26, 31–46. [Google Scholar] [CrossRef]
- Stoev, S.D. Complex Etiology, Prophylaxis and Hygiene Control in Mycotoxic Nephropathies in Farm Animals and Humans, Special Issue “Mycotoxins: Mechanisms of Toxicological Activity—Treatment and Prevention”, Section “Molecular Pathology”. Int. J. Mol. Sci. 2008, 9, 578–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansing, G.A.; Lillehof, E.B.; Detroy, R.W.; Muller, M.A. Synergistic toxic effects of citrinin, ochratoxin A and penicillic acid in mice. Toxicon 1976, 4, 213–220. [Google Scholar] [CrossRef]
- Shepherd, E.C.; Philips, T.D.; Joiner, G.N.; Kubena, L.F.; Heidelbaugh, N.D. Ochratoxin A and penicillic acid interaction in mice. J. Environ. Sci. Health B 1981, 16, 557–573. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.K.; Hayes, A.W. Effect of penicillic acid on biliary excretion of indocyanine green in the mouse and rat. J. Toxicol. Environ. Health 1981, 7, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Hamilton, P.B.; Ciegler, A. Evaluation of penicillic acid for toxicity in broiler chickens. Poult. Sci. 1980, 59, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Umeda, M.; Yamamoto, T.; Saito, M. DNA-strand breakage of HeLa cells induced by several mycotoxins. Jpn. J. Exp. Med. 1972, 42, 527–539. [Google Scholar]
- Dierickx, P.J.; De Beer, J.O. Interaction of the mycotoxin penicillic acid with glutathione and rat liver glutathione S-transferases. Mycopathologia 1984, 86, 137–141. [Google Scholar] [CrossRef]
- Haazele, F.M.; Guenter, W.; Marquardt, R.R.; Frohlich, A.A. Benefical effects of dietary ascorbic acid supplement on hens subjected to ochratoxin A toxicosis under normal and high ambient temperatures. Can. J. Anim. Sci. 1993, 73, 149–157. [Google Scholar] [CrossRef]
- Stoev, S.D.; Djuvinov, D.; Mirtcheva, T.; Pavlov, D.; Mantle, P. Studies on some feed additives giving partial protection against ochratoxin A toxicity in chicks. Toxicol. Lett. 2002, 135, 33–50. [Google Scholar] [CrossRef]
- Stoev, S.D.; Njobeh, P.; Zarkov, I.; Mircheva, T.; Zapryanova, T.; Denev, S.; Dimitrova, B. Selected herbal feed additives showing protective effects against ochratoxin A toxicosis in broiler chicks. World Mycotox. J. 2019, 12, 257–268. [Google Scholar] [CrossRef]
- Stoev, S.D.; Dimitrov, K.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Valchev, I.; Denev, S.; Chobanova, S.; Stefanov, M.; Arora, R. Some Indian herbs having protective effects against deleterious effects of ochratoxin A in broiler chicks. World Mycotox. J. 2021, 14, 525–538. [Google Scholar] [CrossRef]
- Stoev, S.D.; Mircheva, T.; Denev, S.; Chobanova, S.; Ivanov, V. The Protective Effect of Silymarin against Ochratoxin A Induced Histopathological and Biochemical Changes in Chicks. J. Adv. Vet. Res. 2021, 11, 1–8. [Google Scholar]
- Stoev, S.D. Studies on some feed additives and materials giving partial protection against the suppressive effect of ochratoxin A on egg production of laying hens. Res. Vet Sci. 2010, 88, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Bunge, I.; Dirheimer, G.; Roschenthaler, R. In vivo and in vitro inhibition of protein synthesis in Bacillus stearothermophilus by ochratoxin A. Biochem. Biophys. Res. Commun. 1978, 83, 398–405. [Google Scholar] [CrossRef]
- Bailey, C.A.; Gibson, R.M.; Kubena, L.F.; Huff, W.E.; Harvey, R.B. Impact of L-phenylalanine supplementation on the performance of three-week-old broilers fed diets containing ochratoxin A. 2. Effect on hematology and clinical chemistry. Poult. Sci. 1990, 69, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.M.; Bailey, C.A.; Kubena, L.F.; Huff, W.E.; Harvey, R.B. Impact of L-phenylalanine supplementation on the performance of three-week-old broilers fed diets containing ochratoxin A. 1. Effect on body weight, feed conversion, relative organ weight, and mortality. Poult. Sci. 1990, 69, 414–419. [Google Scholar] [CrossRef]
- Creppy, E.E.; Lugnier, A.A.J.; Fasiolo, F.; Heller, K.; Roschenthaler, R.; Dirheimer, G. In vitro inhibition of yeast phenylalanyl-tRNA synthetase by ochratoxin A. Chem. Biol. Interact. 1979, 24, 257–261. [Google Scholar] [CrossRef]
- Creppy, E.E.; Baudrimont, I.; Betbeder, A.M. Prevention of nephrotoxicity of ochratoxin A, a food contaminant. Toxicol. Lett. 1995, 83, 869–877. [Google Scholar] [CrossRef]
- Eiben, R.; Bomhard, E.M. Trends in mortality, body weights and tumor incidences of Wistar rats over 20 years. Exp. Toxicol. Pathol. 1999, 51, 523–536. [Google Scholar] [CrossRef]
- Greim, H.; Gelbke, H.-P.; Reuter, U.; Thielmann, H.W.; Edler, L. Evaluation of historical control data in carcinogenicity studies. Hum. Exp. Toxicol. 2003, 22, 541–549. [Google Scholar] [CrossRef]
- Montesano, R.; Bartsch, H.; Vanio, H.; Wilbourn, H.J.; Yamasaki, H. Long-Term and Short-Term Assays for Carcinogenicity: A Critical Appraisal; IARC Scientific Publications No 83; International Agency for Research on Cancer: Lyon, France, 1986. [Google Scholar]
- Bomhard, E.; Rinke, M. Frequency of spontaneous tumours in Wistar rats in 2-year studies. Exp. Toxicol. Pathol. 1994, 46, 17–29. [Google Scholar] [CrossRef]
- Walsh, K.M.; Poteracki, J. Spontaneous neoplasms in control Wistar rats. Fundam. Appl. Toxicol. 1994, 22, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Poteracki, J.; Walsh, K.M. Spontaneous neoplasms in control Wistar rats: A comparison of reviews. Toxicol. Sci. 1998, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bomhard, E. Frequency of spontaneous tumors in Wistar rats in 30-months studies. Exp. Toxicol. Pathol. 1992, 44, 381–392. [Google Scholar] [CrossRef]
- Ramos, M.F.; Baker, J.; Atzpodien, E.-A.; Bach, U.; Brassard, J.; Cartwright, J.; Farman, C.; Fishman, C.; Jacobsen, M.; Junker-Walker, U.; et al. Nonproliferative and Proliferative Lesions of the Rat and Mouse Special Sense Organs (Ocular [eye and glands], Olfactory and Otic). J. Toxicol. Pathol. 2018, 31 (Suppl. 3), 97S–214S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, R.; Tsaih, S.-W.; Petkova, S.B.; Evsikova, C.M.; Xing, S.; Marion, M.A.; Bogue, M.A.; Mills, K.D.; Peters, L.L.; Bult, C.J.; et al. Aging in inbred strains of mice: Study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell 2009, 8, 277–287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anisimov, V.N.; Zabezhinski, M.A.; Rossolini, G.; Zaia, A.; Piantanelli, A.; Basso, A.; Piantanelli, L. Long-live euthymic BALB/c-nu mice. II: Spontaneous tumors and other pathologies. Mech. Ageing Dev. 2001, 122, 477–489. [Google Scholar] [CrossRef]
- Mikaelian, I.; Ichiki, T.; Ward, J.M.; Sundberg, J.P. Diversity of Spontaneous Neoplasms in Commonly Used Inbred Strains and Stocks of Laboratory Mice. In The Laboratory Mouse; Hedrich, H.J., Ed.; Elsevier: London, UK, 2004; pp. 345–354. [Google Scholar]
- Booth, C.J.; Sundberg, J.P. Spontaneous neoplasms in a large breeding colony of BALB/cJ and BALB /cByJ mice. In Pathobiology of the Aging Mouse; Mohr, U., Dungworth, D.L., Capen, C.C., Carlton, W.W., Sundberg, J.P., Ward, J.M., Eds.; ILSI Press: Washington, DC, USA, 1996; Volume 1, pp. 51–65. [Google Scholar]
- Tapia, M.O.; Seawright, A.A. Experimental ochratoxicosis in pigs. Aust. Vet. J. 1984, 61, 219–222. [Google Scholar] [CrossRef]
- Suzuki, S.; Satoh, T.; Yamazaki, M. The pharmacokinetics of ochratoxin A in rats. Jpn. J. Pharmacol. 1977, 27, 735–744. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.; Chakor, K.; Creppy, E.E.; Kane, A.; Roschenthaler, R.; Dirheimer, G. Evidence for an enterohepatic circulation of ochratoxin A in mice. Toxicology 1988, 48, 293–308. [Google Scholar] [CrossRef]
- Fuchs, R. Distribution and Fate of Ochratoxin A in Experimental Animals. Doctoral Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 1988. [Google Scholar]
- Kumar, S.N.; Bastia, B.; Telang, A.; Singh, K.P.; Singh, R.; Jain, A.K. Combined toxicity of endosulfan and ochratoxin-A in rats: Histopathological changes. J. Histol. Histopathol. 2015, 2, 22. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.L.; Odell, E.W.; Mantle, P.G. DNA ploidy distribution in renal tumours induced in male rats by dietary ochratoxin A. Exp. Toxicol. Pathol. 2007, 59, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Munro, I.C.; Moodie, C.A.; Kuiper-Goodman, T.; Scott, P.M.; Grice, H.C. Toxicologic changes in rats fed graded dietary levels of ochratoxin A. Toxicol. Appl. Pharmacol. 1974, 28, 180–188. [Google Scholar] [CrossRef]
- Maaroufi, K.; Zakhama, A.; Baudrimont, I.; Achour, A.; Abid, S.; Ellouz, F.; Dhouib, S.; Creppy, E.E.; Bacha, H. Karyomegaly of tubular cells as early stage marker of the nephrotoxicity induced by ochratoxin A in rats. Hum. Exp. Toxicol. 1999, 18, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T.; Scott, P.M. Risk assessment of the mycotoxin ochratoxin A. Biomed. Environ. Sci. 1989, 2, 179–248. [Google Scholar]
- Pfohl-Leszkowicz, A.; Pinelli, E.; Bartsch, H.; Mohr, U.; Castegnaro, M. Sex and strain differences in ochratoxin A metabolism and DNA adduction in two strains of rats. Mol. Carcinogen. 1998, 23, 76–83. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Pinelli, E.; Mohr, U.; Bartsch, H.; Castegnaro, M. Strain and sex specific genotoxic and carcinogenic response of OTA in rats is in part controled by CYP-mediated metabolic reactions. Revue Med. Vet. 1998, 149, 659. [Google Scholar]
- Ljubojevic, M.; Herak-Kramberger, C.M.; Hagos, Y.; Bahn, A.; Endou, H.; Burckhardt, G.; Sabolic, I. Rat renal cortical OAT1 and OAT3 exhibit gender differences determined by both androgen stimulation and estrogen inhibition. Am. J. Physiol. Renal. Physiol. 2004, 287, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Pfohl-Leszkowicz, A.; Manderville, R. Review on Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Manderville, R.; Pfohl-leszkowicz, A. Bioactivation and DNA Adduction as a Rationale for Ochratoxin A Carcinogenesis. World Mycotox. J. 2008, 1, 357–367. [Google Scholar] [CrossRef]
- Stoev, S.D.; Goundasheva, D.; Mirtcheva, T.; Mantle, P.G. Susceptibility to secondary bacterial infections in growing pigs as an early response in ochratoxicosis. Exp. Toxicol. Pathol. 2000, 52, 287–296. [Google Scholar] [CrossRef]
- Luster, M.I.; Germolec, D.R.; Burleson, G.R.; Jameson, C.W.; Ackermann, M.F.; Lamm, K.R.; Hayes, H.T. Selectiv immunosuppression in mice of natural killer cell activity by ochratoxin A. Cancer Res. 1987, 7, 2259–2263. [Google Scholar]
- Chernozemsky, I.N.; Stoyanov, I.S.; Petkova–Bocharova, T.K.; Nikolov, I.G.; Draganov, I.V.; Stoichev, I.; Tanchev, Y.; Naidenov, D.; Kalcheva, N.D. Geographic correlation between the occurrence of endemic nephropathy and urinary tract tumours in Vratza district, Bulgaria. J. Cancer 1977, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, S.; Jankovic, S.; Jeremovic, J. Incidence of tumours of urinary organs in focus of Balkan endemic nephropathy. Kidney Int. 1991, 40 (Suppl. 34), 75–77. [Google Scholar]
- Pfohl-Leszkowicz, A.; Grosse, Y.; Castegnaro, M.; Petkova-Bocharova, T.; Nicolov, I.G.; Chernozemsky, I.N.; Bartsch, H.; Betbeder, A.M.; Creppy, E.E.; Dirheimer, G. Ochratoxin A related DNA adducts in urinary tract tumours of Bulgarian subjects. IARC Sci. Publ. 1993, 124, 141–148. [Google Scholar]
- Pfohl-Leszkowicz, A.; Tozlovanu, M.; Manderville, R.; Peraica, M.; Castegnaro, M.; Stefanovic, V. New molecular and field evidences for the implication of mycotoxins but not aristolochic acid in Human Nephropathy and Urinary tract tumor. Mol. Nutr. Food Res. 2007, 51, 131–146. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A. Ochratoxin A and aristolochic acid in the Nephropathies and associated Urothelial tract Tumours development. Arh. Hig. Rada Toksikol. 2009, 60, 465–483. [Google Scholar] [CrossRef] [Green Version]
- Creppy, E.E.; Roschenthaler, R.; Dirheimer, G. Inhibition of protein synthesis in mice by ochratoxin A and its prevention by phenylalanine. Food Chem. Toxicol. 1984, 22, 883–886. [Google Scholar] [CrossRef]
- Harvey, R.B.; Elissalde, M.H.; Kubena, L.F.; Weaver, E.A.; Corrie, D.E.; Clement, B.A. Immunotoxicity of ochratoxin A to growing gilts. Am. J. Vet. Res. 1992, 53, 1966–1970. [Google Scholar]
- Haubeck, H.D.; Lorkowski, G.; Kolsh, E.; Roschenthaler, R. Immunosuppression by ochratoxin A and its preventation by phenylalanine. Appl. Environ. Microbiol. 1981, 41, 1040–1042. [Google Scholar] [CrossRef] [Green Version]
- Lea, T.; Steien, K.; Stormer, F.C. Mechanism of ochratoxin A induced immunosuppression. Mycopathologia 1989, 107, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, T.; Thuvander, A.; Hult, K. Ochratoxin A as a suppressor of mitogen induced blastogenesis of porcine blood lymphocytes. Acta Vet. Scand. 1988, 29, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Kozaczynski, W. Experimental ochratoxicosis A in chickens. Immunological study. Bull. Vet. Inst. Pulawy 1994, 38, 1–8. [Google Scholar]
- Meisner, H.; Chan, S. Ochratoxin A, an inhibitor of mitochondrial transport system. Biochemistry 1974, 13, 2795–2799. [Google Scholar] [CrossRef] [PubMed]
- Gahnian, R.; Mihailov, G.; Pavlov, D.; Panchev, I.; Nikolov, A. Influence of Artichoke (Cynara scolymus L.) leaves extract on development of experimental liver dystrophy in sheep. Vet. Med. 1995, 1 (Suppl. 1), 104–105, (Bulg). [Google Scholar]
- Cairela, M.; Nasta, G.; Vcci, L.; Veipari, B. Cynarine effect on hepatic damages due to food excesses. In Proceedings of the Second International meeting on globe Artichoke, Bari, Italy, 21–24 November 1973; p. 15. [Google Scholar]
- Mantia, G.; Leone, F.; Hopps, V.; Consiglio, D. Cynarine influence on hepatotoxic effects of some chemic antibiotics. In Proceedings of the Second International meeting on globe Artichoke, Bari, Italy, 21–24 November 1973; p. 10. [Google Scholar]
- Denev, S.; Sotirov, L.; Chobanova, S.; Koynarski, T.; Ivanov, V.; Bozakova, N.; Stoev, S. Effect of silymarin and ochratoxin A on humoral natural immunity of broiler chickens. J. Cent. Eur. Agric. 2020, 21, 492–498. [Google Scholar] [CrossRef]
- Stoev, S.D. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit. Rev. Food Sci. 2013, 53, 887–901. [Google Scholar] [CrossRef]
- Stoev, S.D. Foodborne mycotoxicoses, risk assessment and underestimated hazard of masked mycotoxins and joint mycotoxin effects or interaction. Environ. Toxicol. Pharmacol. 2015, 9, 794–809. [Google Scholar] [CrossRef]

| Neoplasia in Various Tissues and Organs in Experimental Laboratory Animals or Poultry | ||
| Mainly attributed to OTA | Possibly attributed to OTA | Not attributed to OTA but to age |
| References [6,9,11,14,21,22] | References [11,21,22] | References [53,54,55,56,57,58] |
| kidneys | lung | endocrine system |
| liver | ureters | reproductive system |
| intestine | subcutaneous tissue | hematopoietic system |
| intestinal mesenterium | muscle | lymphatic system |
| eyes | integumentary system | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stoev, S.D. New Evidences about the Carcinogenic Effects of Ochratoxin A and Possible Prevention by Target Feed Additives. Toxins 2022, 14, 380. https://doi.org/10.3390/toxins14060380
Stoev SD. New Evidences about the Carcinogenic Effects of Ochratoxin A and Possible Prevention by Target Feed Additives. Toxins. 2022; 14(6):380. https://doi.org/10.3390/toxins14060380
Chicago/Turabian StyleStoev, Stoycho D. 2022. "New Evidences about the Carcinogenic Effects of Ochratoxin A and Possible Prevention by Target Feed Additives" Toxins 14, no. 6: 380. https://doi.org/10.3390/toxins14060380
APA StyleStoev, S. D. (2022). New Evidences about the Carcinogenic Effects of Ochratoxin A and Possible Prevention by Target Feed Additives. Toxins, 14(6), 380. https://doi.org/10.3390/toxins14060380