Immunorecognition and Neutralization of Crotalus durissus cumanensis Venom by a Commercial Antivenom Produced in Colombia
Abstract
1. Introduction
2. Results
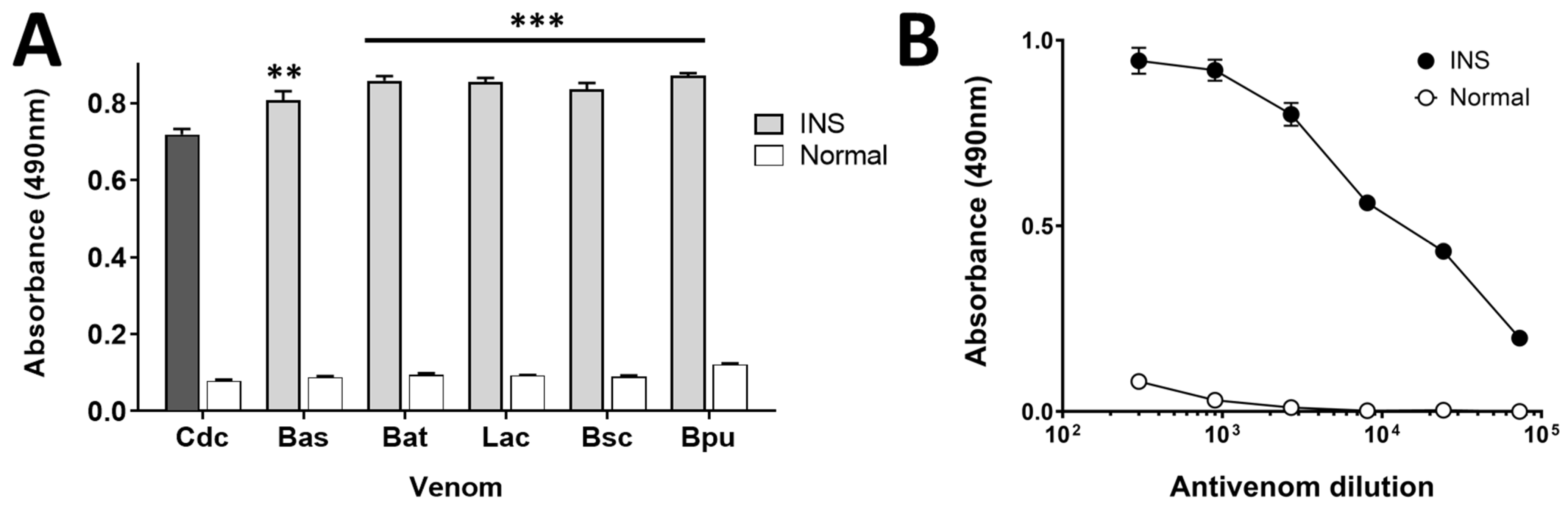
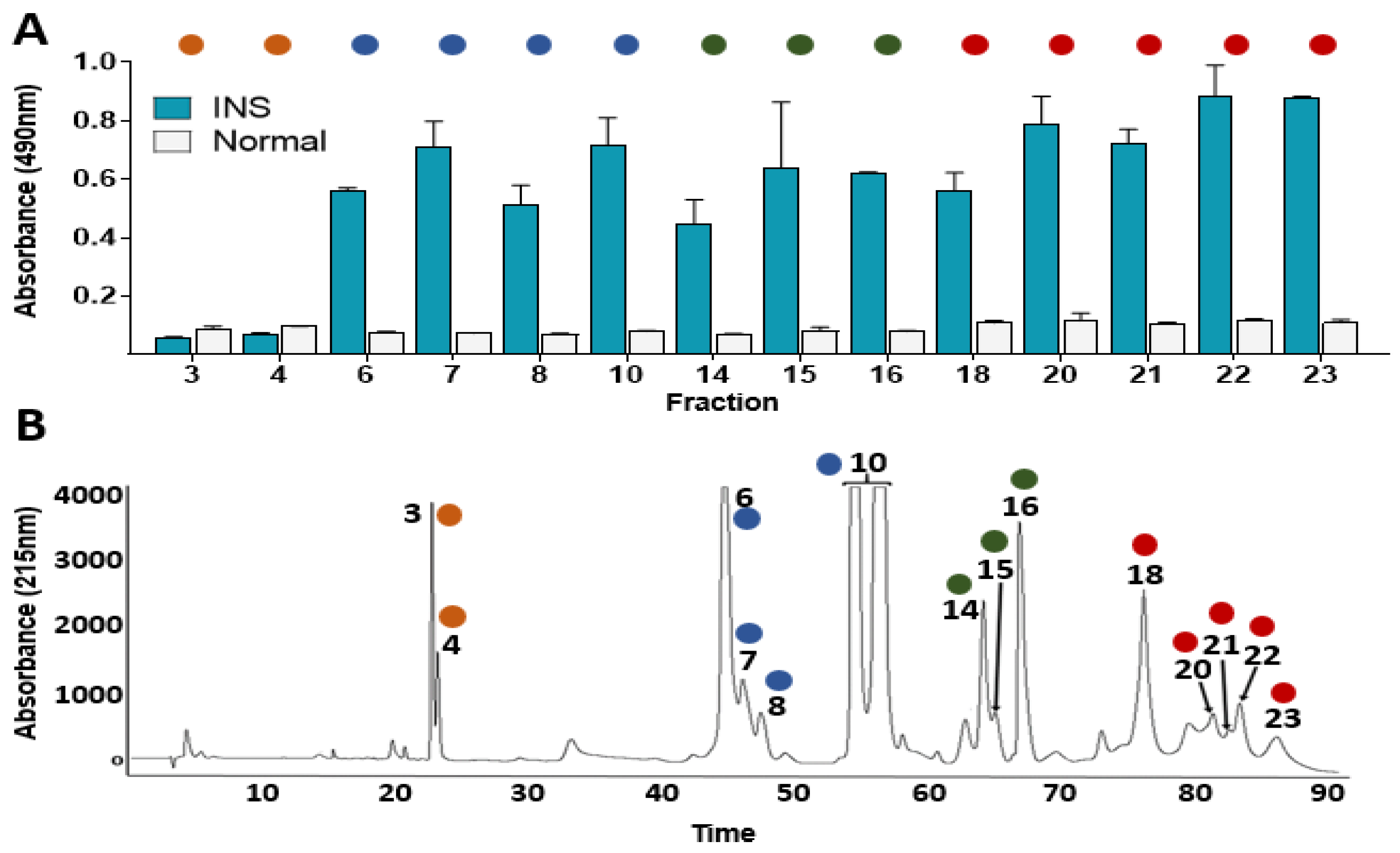
2.1. Immunoreactivity Assessment
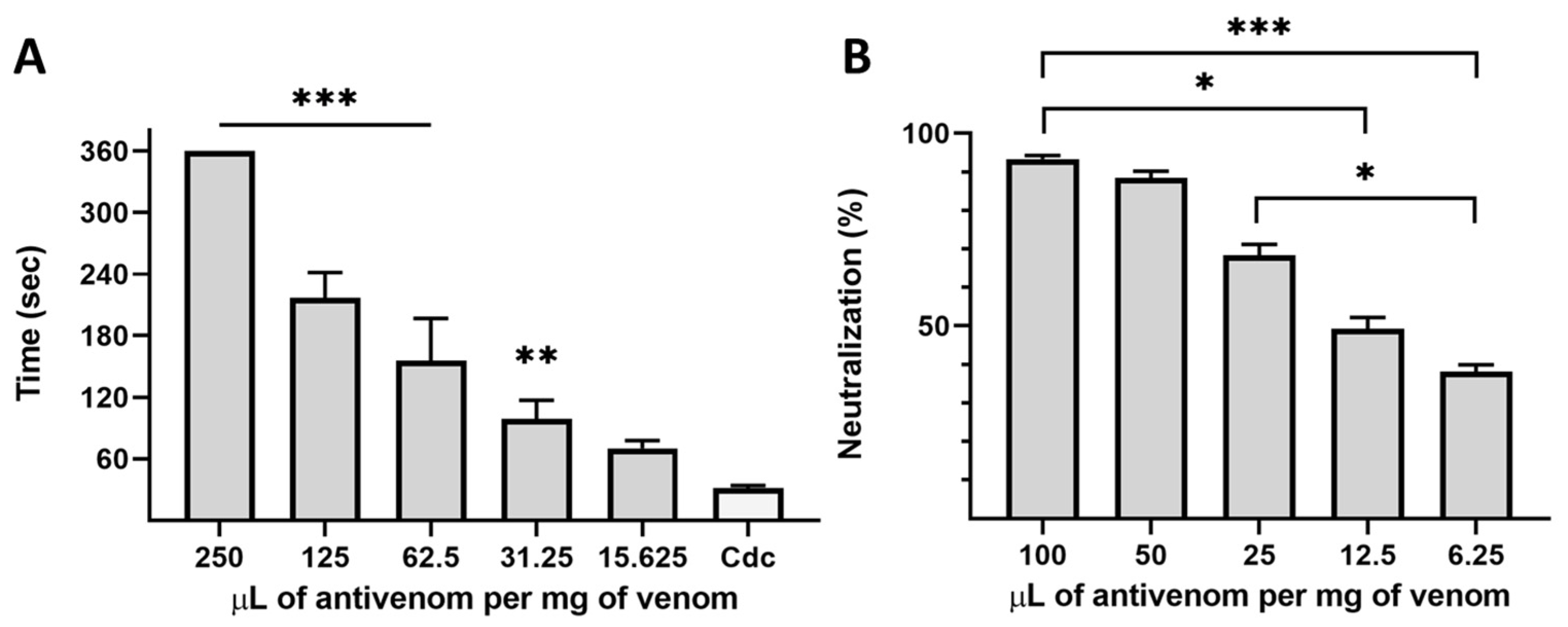
2.2. Biological Activities of Venom and Neutralization Assays
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venoms and Antivenom
5.2. Immunoreactivity Assessments
5.2.1. Antivenomics
5.2.2. Enzyme-Linked Immunosorbent Assay (ELISA)
5.3. Biological Activities of Venom
5.3.1. Coagulant Activity
5.3.2. Indirect Hemolysis
5.3.3. PLA2 Activity
5.3.4. Proteolytic Activity
5.3.5. Neutralization of In-Vitro Assays
5.3.6. Neutralization of Lethality
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Amazonas, D.R.; Portes-Junior, J.A.; Nishiyama-Jr, M.Y.; Nicolau, C.A.; Chalkidis, H.M.; Mourão, R.H.V.; Grazziotin, F.G.; Rokyta, D.R.; Gibbs, H.L.; Valente, R.H.; et al. Molecular mechanisms underlying intraspecific variation in snake venom. J. Proteom. 2018, 181, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Sanz, L.; Cid, P.; de la Torre, P.; Flores-Díaz, M.; Dos Santos, M.C.; Borges, A.; Bremo, A.; Angulo, Y.; Lomonte, B.; et al. Snake Venomics of the Central American Rattlesnake Crotalus simus and the South American Crotalus durissus Complex Points to Neurotoxicity as an Adaptive Paedomorphic Trend along Crotalus Dispersal in South America. J. Proteome Res. 2010, 9, 528–544. [Google Scholar] [CrossRef] [PubMed]
- Daltry, J.C.; Wüster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Menezes, M.C.; Furtado, M.F.; Travaglia-Cardoso, S.R.; Camargo, A.C.; Serrano, S.M.T. Sex-based individual variation of snake venom proteome among eighteen Bothrops jararaca siblings. Toxicon 2006, 47, 304–312. [Google Scholar] [CrossRef]
- Saldarriaga, M.M.; Otero, R.; Núñez, V.; Toro, M.F.; Díaz, A.; Gutiérrez, J.M. Ontogenetic variability of Bothrops atrox and Bothrops asper snake venoms from Colombia. Toxicon 2003, 42, 405–411. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef]
- Chippaux, J.P. Snake-bites: Appraisal of the global situation. Bull. World Health Organ. 1998, 76, 515–524. [Google Scholar]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 38. [Google Scholar] [CrossRef]
- Kasturiratne, A.; Wickremasinghe, A.R.; De Silva, N.; Gunawardena, N.K.; Pathmeswaran, A.; Premaratna, R.; Savioli, L.; Lalloo, D.G.; De Silva, H.J. The Global Burden of Snakebite: A Literature Analysis and Modelling Based on Regional Estimates of Envenoming and Deaths. PLoS Med. 2008, 5, e218. [Google Scholar] [CrossRef]
- Harrison, R.A.; Hargreaves, A.; Wagstaff, S.; Faragher, B.; Lalloo, D. Snake Envenoming: A Disease of Poverty. PLoS Negl. Trop. Dis. 2009, 3, e569. [Google Scholar] [CrossRef] [PubMed]
- Otero, R. Snake Bites in Colombia. In Clinical Toxinology in Australia, Europe, and Americas; Vogel, C.-W., Seifert, S.A., Tambourgi, D.V., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 3–50. [Google Scholar]
- Quintana-Castillo, J.C.; Vargas, L.J.; Segura, C.; Estrada-Gómez, S.; Bueno-Sánchez, J.C.; Alarcón, J.C. Characterization of the Venom of C. d. cumanesis of Colombia: Proteomic Analysis and Antivenomic Study. Toxins 2018, 10, 85. [Google Scholar] [CrossRef] [PubMed]
- Otero, R.; Osorio, R.G.; Valderrama, R.; Giraldo, A.C. Efectos farmacologicos y enzimaticos de los venenose de Antioquia y Chocó (Colombia). Toxicon 1992, 30, 611–620. [Google Scholar] [CrossRef]
- Habermann, E.; Breithaupt, H. The crotoxin complex—an example of biochemical and pharmacological protein complementation. Toxicon 1978, 16, 19–30. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Núñez, V.; Huancahuire-Vega, S.; Marangoni, S.; Ponce-Soto, L.A. Biochemical and biological characterization of a PLA2 from crotoxin complex of Crotalus durissus cumanensis. Toxicon 2009, 53, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.J.; Faiz, M.A.; Abela-Ridder, B.; Ainsworth, S.; Bulfone, T.C.; Nickerson, A.D.; Habib, A.G.; Junghanss, T.; Fan, H.W.; Turner, M.; et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLoS Negl. Trop. Dis. 2019, 13, e0007059. [Google Scholar] [CrossRef]
- Gómez-Cardona, J.P.; Gómez-Cabal, C.; Gómez-Cabal, M.L. Sueros antiofídicos en Colombia: Análisis de la producción, abastecimiento y recomendaciones para el mejoramiento de la red de producción. Biosalud 2017, 16, 96–117. [Google Scholar]
- Isbister, G.K. Antivenom efficacy or effectiveness: The Australian experience. Toxicology 2010, 268, 148–154. [Google Scholar] [CrossRef]
- Chippaux, J.-P. Estimating the Global Burden of Snakebite Can Help to Improve Management. PLoS Med. 2008, 5, e221. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; León, G.; Rojas, G.; Lomonte, B.; Rucavado, A.; Chaves, F. Neutralization of local tissue damage induced by Bothrops asper (terciopelo) snake venom. Toxicon 1998, 36, 1529–1538. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Theakston, R.D.G.; Warrell, D.A. Confronting the Neglected Problem of Snake Bite Envenoming: The Need for a Global Partnership. PLoS Med. 2006, 3, e150. [Google Scholar] [CrossRef] [PubMed]
- Lovecchio, F.; Klemens, J.; Roundy, E.B.; Klemens, A. Serum Sickness Following Administration of Antivenin (Crotalidae) Polyvalent in 181 Cases of Presumed Rattlesnake Envenomation. Wilderness Environ. Med. 2003, 14, 220–221. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Burnouf, T.; Harrison, R.A.; Calvete, J.J.; Kuch, U.; Warrell, D.A.; Williams, D.J. A multicomponent strategy to improve the availability of antivenom for treating snakebite envenoming. Bull. World Health Organ. 2014, 92, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, J.M.; Solano, G.; Pla, D.; Herrera, M.; Segura, Á; Villalta, M.; Vargas, M.; Sanz, L.; Lomonte, B.; Calvete, J.; et al. Assessing the preclinical efficacy of antivenoms: From the lethality neutralization assay to antivenomics. Toxicon 2013, 69, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectrom. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Escolano, J.; Fernández, J.; Sanz, L.; Angulo, Y.; Gutiérrez, J.M.; Calvete, J.J. Snake Venomics and Antivenomics of the Arboreal Neotropical Pitvipers Bothriechis lateralis and Bothriechis schlegelii. J. Proteome Res. 2008, 7, 2445–2457. [Google Scholar] [CrossRef]
- Calvete, J.J.; Rodríguez, Y.; Quesada-Bernat, S.; Pla, D. Toxin-resolved antivenomics-guided assessment of the immunorecognition landscape of antivenoms. Toxicon 2018, 148, 107–122. [Google Scholar] [CrossRef]
- Laing, G.D.; Yarleque, A.; Marcelo, A.; Rodriguez, E.; Warrell, D.A.; Theakston, R.D.G. Preclinical testing of three south American antivenoms against the venoms of five medically-important Peruvian snake venoms. Toxicon 2004, 44, 103–106. [Google Scholar] [CrossRef]
- Otero, R.; Gutiérrez, J.; Mesa, B.M.; Duque, E.; Rodríguez, O.; Arango, L.J.; Gómez, F.; Toro, A.; Cano, F.; María Rodríguez, L.; et al. Complications of Bothrops, Porthidium, and Bothriechis snakebites in Colombia. A clinical and epidemiological study of 39 cases attended in a university hospital. Toxicon 2002, 40, 1107–1114. [Google Scholar] [CrossRef]
- Otero, R.; Núñez, V.; Barona, J.; Díaz, A.; Saldarriaga, M. Características bioquímicas y capacidad neutralizante de cuatro antivenenos polivalentes frente a los efectos farmacológicos y enzimáticos del veneno de Bothrops asper y Porthidium nasutum de Antioquia y Chocó. Iatreia 2002, 15, 5–15. [Google Scholar]
- Otero, R.; Nunez, V.; Osorio, R.G.; Gutierrez, J.M.; Giraldo, C.A.; Posada, L.E. Ability of six Latin American antivenoms to neutralize the venom of Mapana equis (Bothrops atrox) from Antioquia and Chocó (Colombia). Toxicon 1995, 33, 809–815. [Google Scholar] [CrossRef]
- Segura, A.; Castillo, M.C.; Núñez, V.; Yarlequé, A.; Gonçalves, L.R.; Villalta, M.; Bonilla, C.; Herrera, M.; Vargas, M.; Fernández, M.; et al. Preclinical assessment of the neutralizing capacity of antivenoms produced in six Latin American countries against medically-relevant Bothrops snake venoms. Toxicon 2010, 56, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.G.; Laing, G.D.; Fielding, C.M.; Lascano, A.F.; Touzet, J.M.; Vallejo, F.; Guderian, R.H.; Nelson, S.J.; Wüster, W.; Richards, A.M.; et al. Treatment of snake bites by Bothrops species and Lachesis muta in Ecuador: Laboratory screening of candidate antivenoms. Trans. R. Soc. Trop. Med. Hyg. 1995, 89, 550–554. [Google Scholar] [CrossRef]
- Ramirez, J.; Renjifo, J.M.; Forero, M.C. Estabilidad de la actividad neutralizante del antiveneno ofídico conservado a 4 °C y a 16 °C contra el veneno de Cascabel (Crotalus durissus cumanensis) de Colombia. Biomédica 1995, 15, 215–219. [Google Scholar] [CrossRef][Green Version]
- Leon, G.; Sanchez, L.; Hernandez, A.; Villalta, M.; Herrera, M.; Segura, A.; Estrada, R.; Gutierrez, J.M. Immune response towards snake venoms. Inflamm. Allergy-Drug Targets 2011, 10, 381–398. [Google Scholar] [CrossRef]
- Boldrini-França, J.; Corrêa-Netto, C.; Silva, M.M.; Rodrigues, R.S.; De La Torre, P.; Pérez, A.; Soares, A.M.; Zingali, R.B.; Nogueira, R.A.; Rodrigues, V.M.; et al. Snake venomics and antivenomics of Crotalus durissus subspecies from Brazil: Assessment of geographic variation and its implication on snakebite management. J. Proteom. 2010, 73, 1758–1776. [Google Scholar] [CrossRef]
- Furtado, M.D.F.D.; Cardoso, S.T.; Soares, O.E.; Pereira, A.P.; Fernandes, D.S.; Tambourgi, D.V.; Sant’Anna, O.A. Antigenic cross-reactivity and immunogenicity of Bothrops venoms from snakes of the Amazon region. Toxicon 2010, 55, 881–887. [Google Scholar] [CrossRef]
- Muniz, E.G.; Maria, W.S.; Estevão-Costa, M.I.; Buhrnheim, P.; Chávez-Olórtegui, C. Neutralizing potency of horse antibothropic Brazilian antivenom against Bothrops snake venoms from the Amazonian rain forest. Toxicon 2000, 38, 1859–1863. [Google Scholar] [CrossRef]
- Judge, R.K.; Henry, P.J.; Mirtschin, P.; Jelinek, G.; Wilce, J.A. Toxins not neutralized by brown snake antivenom. Toxicol. Appl. Pharmacol. 2006, 213, 117–125. [Google Scholar] [CrossRef]
- Laustsen, A.H.; Lomonte, B.; Lohse, B.; Fernández, J.; Gutiérrez, J.M. Unveiling the nature of black mamba (Dendroaspis polylepis) venom through venomics and antivenom immunoprofiling: Identification of key toxin targets for antivenom development. J. Proteom. 2015, 119, 126–142. [Google Scholar] [CrossRef]
- Schaeffer, R.C., Jr.; Randall, H.; Resk, J.; Carlson, R.W. Enzyme-linked immunosorbant assay (ELISA) of size-selected crotalid venom antigens by Wyeth’s polyvalent antivenom. Toxicon 1988, 26, 67–76. [Google Scholar] [CrossRef]
- Calvete, J.J.; Cid, P.; Sanz, L.; Segura, A.; Villalta, M.; Herrera, M.; León, G.; Harrison, R.; Durfa, N.; Nasidi, A.; et al. Antivenomic Assessment of the Immunological Reactivity of EchiTAb-Plus-ICP, an Antivenom for the Treatment of Snakebite Envenoming in Sub-Saharan Africa. Am. J. Trop. Med. Hyg. 2010, 82, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Pereañez, J.A.; Gómez, I.D.; Patiño, A.C. Relationship between the structure and the enzymatic activity of crotoxin complex and its phospholipase A2 subunit: An in silico approach. J. Mol. Gr. Model. 2012, 35, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Faure, G.; Saul, F. Crystallographic characterization of functional sites of crotoxin and ammodytoxin, potent β-neurotoxins from Viperidae venom. Toxicon 2012, 60, 531–538. [Google Scholar] [CrossRef]
- Hawgood, B.J.; Smith, J.W. The mode of action at the mouse neuromuscular junction of the phospholipase A-crotapotin complex isolated from venom of the South American rattlesnake. Br. J. Pharmacol. 1977, 61, 597–606. [Google Scholar] [CrossRef]
- Hendon, R.A.; Fraenkel-Conrat, H. Biological Roles of the Two Components of Crotoxin. Proc. Natl. Acad. Sci. USA 1971, 68, 1560–1563. [Google Scholar] [CrossRef]
- Tonello, F.; Rigoni, M. Cellular Mechanisms of Action of Snake Phospholipase A2 Toxins. In Snake Venoms; Inagaki, H., Vogel, C.-W., Mukherjee, A.K., Rahmy, T.R., Gopalakrishnakone, P., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 49–65. [Google Scholar]
- Gutiérrez, J.M.; Ponce-Soto, L.A.; Marangoni, S.; Lomonte, B. Systemic and local myotoxicity induced by snake venom group II phospholipases A2: Comparison between crotoxin, crotoxin B and a Lys49 PLA2 homologue. Toxicon 2008, 51, 80–92. [Google Scholar] [CrossRef]
- Martins, A.M.; Toyama, M.H.; Havt, A.; Novello, J.C.; Marangoni, S.; Fonteles, M.C.; Monteiro, H.S. Determination of Crotalus durissus cascavella venom components that induce renal toxicity in isolated rat kidneys. Toxicon 2002, 40, 1165–1171. [Google Scholar] [CrossRef]
- Salvini, T.F.; Amaral, A.; Miyabara, E.; Turri, J.A.O.; Danella, P.M.; de Araújo, H.S.S. Systemic skeletal muscle necrosis induced by crotoxin. Toxicon 2001, 39, 1141–1149. [Google Scholar] [CrossRef]
- Sintiprungrat, K.; Chaisuriya, P.; Watcharatanyatip, K.; Ratanabanangkoon, K. Immunoaffinity chromatography in antivenomics studies: Various parameters that can affect the results. Toxicon 2016, 119, 129–139. [Google Scholar] [CrossRef]
- Santos, A.R.; Mota, I. Effect of heating on the toxic, immunogenic and immunosuppressive activities of Crotalus durissus terrificus venom. Toxicon 2000, 38, 1451–1457. [Google Scholar] [CrossRef]
- Cardoso, D.F.; Mota, I. Effect of Crotalus venom on the humoral and cellular immune response. Toxicon 1997, 35, 607–612. [Google Scholar] [CrossRef]
- Kaiser, I.I.; Middlebrook, J.L.; Crumrine, M.H.; Stevenson, W.W. Cross-reactivity and neutralization by rabbit antisera raised against crotoxin, its subunits and two related toxins. Toxicon 1986, 24, 669–678. [Google Scholar] [CrossRef]
- Rangel-Santos, A.; Lima, C.; Lopes-Ferreira, M.; Cardoso, D.F. Immunosuppresive role of principal toxin (crotoxin) of Crotalus durissus terrificus venom. Toxicon 2004, 44, 609–616. [Google Scholar] [CrossRef]
- Kini, R.M.; Sidhu, S.S.; Laustsen, A.H. Biosynthetic Oligoclonal Antivenom (BOA) for Snakebite and Next-Generation Treatments for Snakebite Victims. Toxins 2018, 10, 534. [Google Scholar] [CrossRef]
- Gómez-Betancur, I.; Gogineni, V.; Salazar-Ospina, A.; León, F. Perspective on the Therapeutics of Anti-Snake Venom. Molecules 2019, 24, 3276. [Google Scholar] [CrossRef]
- Núñez, V.; Otero, R.; Barona, J.; Fonnegra, R.; Jiménez, S.L.; Osorio, R.; Quintana-Castillo, J.C.; Díaz, A. Inhibition of the Toxic Effects of Lachesis muta, Crotalus durissus cumanensis and Micrurus mipartitus Snake Venoms by Plant Extracts. Pharm. Biol. 2004, 42, 49–54. [Google Scholar] [CrossRef]
- de la Rosa, G.; Corrales-Garcia, L.L.; Rodriguez-Ruiz, X.; Vera, E.L.; Corzo, G. Short-chain consensus alpha-neurotoxin: A synthetic 60-mer peptide with generic traits and enhanced immunogenic properties. Amino Acids 2018, 50, 885–895. [Google Scholar] [CrossRef]
- Guerrero-Garzón, J.F.; Valle, M.B.; Restano-Cassulini, R.; Zamudio, F.; Corzo, G.; Alagón, A.; Olvera-Rodríguez, A. Cloning and sequencing of three-finger toxins from the venom glands of four Micrurus species from Mexico and heterologous expression of an alpha-neurotoxin from Micrurus diastema. Biochimie 2018, 147, 114–121. [Google Scholar] [CrossRef]
- Ramos, H.R.; Junqueira-De-Azevedo, I.; Novo, J.B.; Castro, K.; Guerra-Duarte, C.; de Ávila, R.A.M.; Olórtegui, C.D.C.; Ho, P.L. A Heterologous Multiepitope DNA Prime/Recombinant Protein Boost Immunisation Strategy for the Development of an Antiserum against Micrurus corallinus (Coral Snake) Venom. PLoS Negl. Trop. Dis. 2016, 10, e0004484. [Google Scholar] [CrossRef]
- Deka, A.; Reza, A.; Hoque, K.M.F.; Deka, K.; Saha, S.; Doley, R. Comparative analysis of Naja kaouthia venom from North-East India and Bangladesh and its cross reactivity with Indian polyvalent antivenoms. Toxicon 2019, 164, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Gay, C.; Sanz, L.; Calvete, J.J.; Pla, D. Snake Venomics and Antivenomics of Bothrops diporus, a Medically Important Pitviper in Northeastern Argentina. Toxins 2015, 8, 9. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage Caused by Snake Venom Metalloproteinases: A Journey of Discovery and Understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Flores, M.P.; Faria, F.; de Andrade, S.A.; Chudzinski-Tavassi, A.M. Snake Venom Components Affecting the Coagulation System. In Snake Venoms; Gopalakrishnakone, P., Inagaki, H., Mukherjee, A.K., Rahmy, T.R., Vogel, C.-W., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 1–20. [Google Scholar]
- Patiño, A.C.; Pereañez, J.A.; Gutiérrez, J.M.; Rucavado, A. Biochemical and biological characterization of two serine proteinases from Colombian Crotalus durissus cumanensis snake venom. Toxicon 2013, 63, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S. Venom Composition in Rattlesnakes: Trends and Biological Significance. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 495–510. [Google Scholar]
- Aguilar, I.; Guerrero, B.; Salazar, A.M.; Girón, M.E.; Pérez, J.C.; Sánchez, E.E.; Rodríguez-Acosta, A. Individual venom variability in the South American rattlesnake Crotalus durissus cumanensis. Toxicon 2007, 50, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, O.; Scannone, H.R.; Parra, N.D. Enzymatic activities and other characteristics of Crotalus durissus cumanensis venom. Toxicon 1974, 12, 297–302. [Google Scholar] [CrossRef]
- Pirela De las Salas, R.C.; López-Jonsthon, J.C.; Hernández Rangel, J.L. Toxinological Characterization of the Whole Venom of the Rattlesnake Crotalus durissus cumanensis (Viperidae) from Porshoure, Venezuelan Guajira. Rev. Cient. 2006, 16, 232–238. [Google Scholar]
- Saravia, P.; Rojas, E.; Arce, V.; Guevara, C.; López, J.C.; Chaves, E.; Velásquez, R.; Rojas, G.; Gutiérrez, J.M. Geographic and ontogenic variability in the venom of the neotropical rattlesnake Crotalus durissus: Pathophysiological and therapeutic implications. Rev. Biol. Trop. 2002, 50, 337–346. [Google Scholar]
- Pineda, M.; Quiroga, N.; Fernández, I.; Scannone, H.; Vargas, A. Toxinological and biochemical characterization of Crotalus durissus cumanensis venom from Falcon State, Venezuela. Rev. Fac. Farm. 2013, 76, 76–83. [Google Scholar]
- Céspedes, N.; Castro, F.; Jiménez, E.; Montealegre, L.; Castellanos, A.; Cañas, C.; Arévalo-Herrera, M.; Herrera, S. Biochemical comparison of venoms from young Colombian Crotalus durissus cumanensis and their parents. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 268–284. [Google Scholar] [CrossRef]
- Pla, D.; Gutiérrez, J.M.; Calvete, J.J. Second generation snake antivenomics: Comparing immunoaffinity and immunodepletion protocols. Toxicon 2012, 60, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Pla, D.; Rodríguez, Y.; Calvete, J.J. Third Generation Antivenomics: Pushing the Limits of the In Vitro Preclinical Assessment of Antivenoms. Toxins 2017, 9, 158. [Google Scholar] [CrossRef]
- Lomonte, B.; Tsai, W.-C.; Ureña-Diaz, J.M.; Sanz, L.; Mora-Obando, D.; Sánchez, E.E.; Fry, B.G.; Gutiérrez, J.M.; Gibbs, H.L.; Sovic, M.G.; et al. Venomics of New World pit vipers: Genus-wide comparisons of venom proteomes across Agkistrodon. J. Proteom. 2014, 96, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Theakston, R.D.; Reid, A.H. Development of simple standard assay procedures for the characterization of snake venom. Bull. World Health Organ. 1983, 61, 949–956. [Google Scholar]
- Habermann, E.; Hardt, K.L. A sensitive and specific plate test for the quantitation of phospholipases. Anal. Biochem. 1972, 50, 163–173. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Avila, C.; Rojas, E.; Cerdas, L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon 1988, 26, 411–413. [Google Scholar] [CrossRef]
- Cho, W.; Kézdy, F.J. Chromogenic substrates and assay of phospholipases A2. Methods Enzym. 1991, 197, 75–79. [Google Scholar] [CrossRef]
- Holzer, M.; Mackessy, S. An aqueous endpoint assay of snake venom phospholipase A2. Toxicon 1996, 34, 1149–1155. [Google Scholar] [CrossRef]
- Ponce-Soto, L.A.; Toyama, M.H.; Hyslop, S.; Novello, J.C.; Marangoni, S. Isolation and preliminary enzymatic characterization of a novel PLA2 from Crotalus durissus collilineatus venom. J. Protein Chem. 2002, 21, 131–136. [Google Scholar] [CrossRef]
- Wang, W.-J.; Shih, C.-H.; Huang, T.-F. A novel P-I class metalloproteinase with broad substrate-cleaving activity, agkislysin, from Agkistrodon acutus venom. Biochem. Biophys. Res. Commun. 2004, 324, 224–230. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Peña, A.; Núñez, V.; Pereañez, J.A.; Rey-Suárez, P. Immunorecognition and Neutralization of Crotalus durissus cumanensis Venom by a Commercial Antivenom Produced in Colombia. Toxins 2022, 14, 235. https://doi.org/10.3390/toxins14040235
Acosta-Peña A, Núñez V, Pereañez JA, Rey-Suárez P. Immunorecognition and Neutralization of Crotalus durissus cumanensis Venom by a Commercial Antivenom Produced in Colombia. Toxins. 2022; 14(4):235. https://doi.org/10.3390/toxins14040235
Chicago/Turabian StyleAcosta-Peña, Augusto, Vitelbina Núñez, Jaime Andres Pereañez, and Paola Rey-Suárez. 2022. "Immunorecognition and Neutralization of Crotalus durissus cumanensis Venom by a Commercial Antivenom Produced in Colombia" Toxins 14, no. 4: 235. https://doi.org/10.3390/toxins14040235
APA StyleAcosta-Peña, A., Núñez, V., Pereañez, J. A., & Rey-Suárez, P. (2022). Immunorecognition and Neutralization of Crotalus durissus cumanensis Venom by a Commercial Antivenom Produced in Colombia. Toxins, 14(4), 235. https://doi.org/10.3390/toxins14040235




