Neutralizing Monoclonal Antibody, mAb 10D8, Is an Effective Detoxicant against Abrin-a Both In Vitro and In Vivo
Abstract
1. Introduction
2. Results
2.1. Production and Screening of the Hybridomas
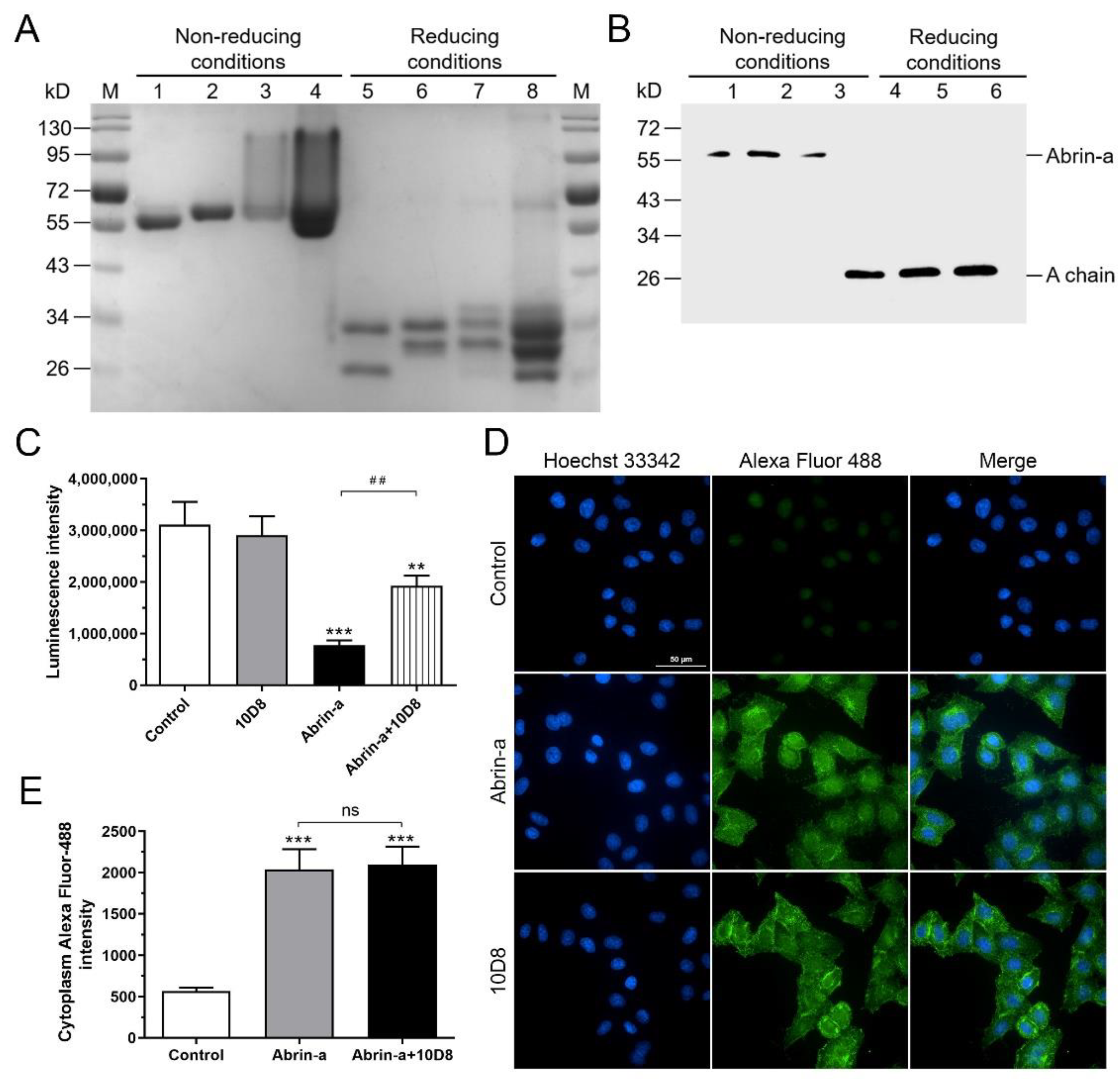
2.2. Identification of the Specificity and Cross-Reactivity of mAbs
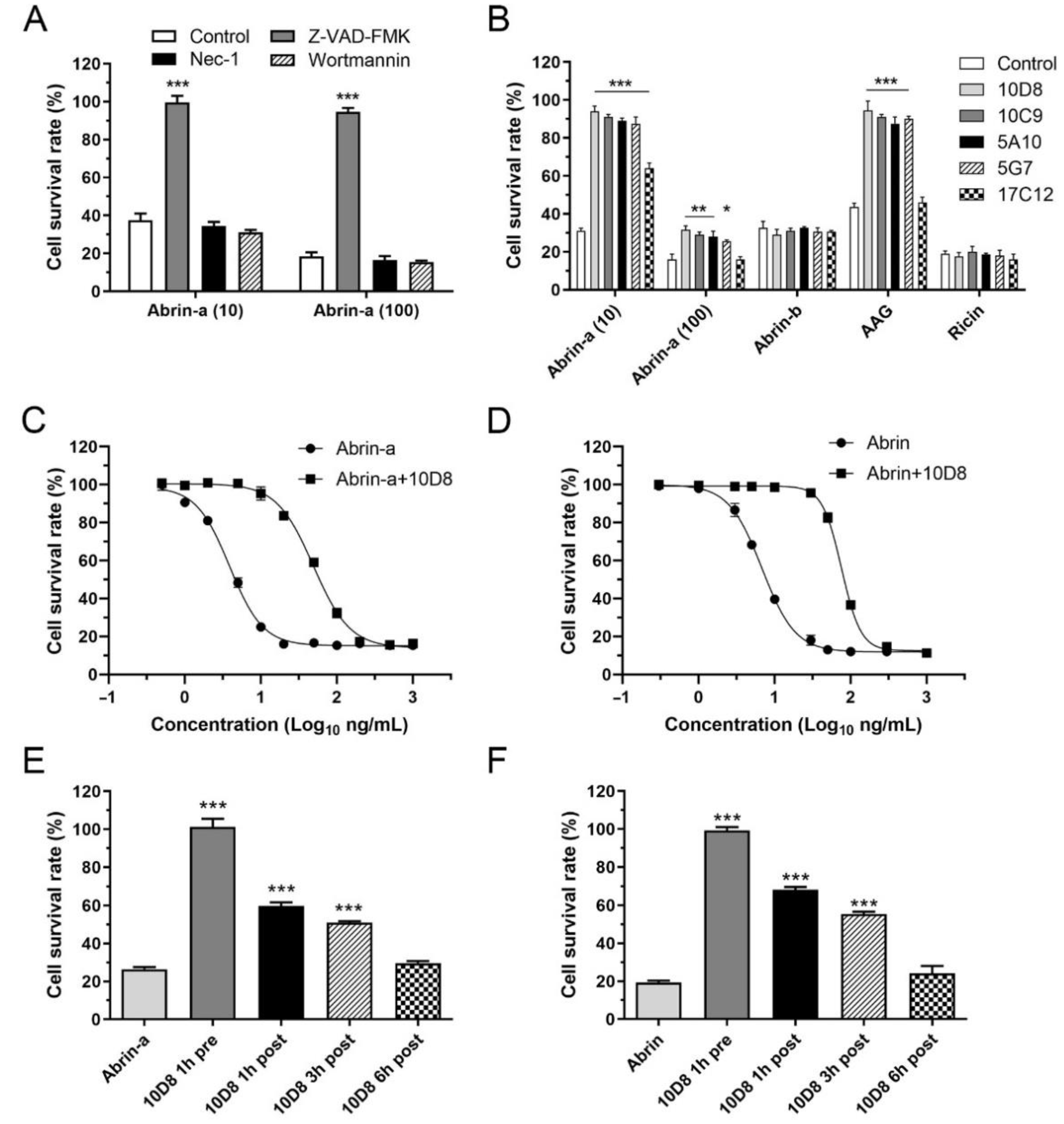
2.3. Abrin-a Induced Apoptosis Is Inhibited by 10D8
2.4. 10D8 Inhibits the Enzymatic Activity of Abrin-a A Chain and Reduces Protein Synthesis Inbibition of Cells
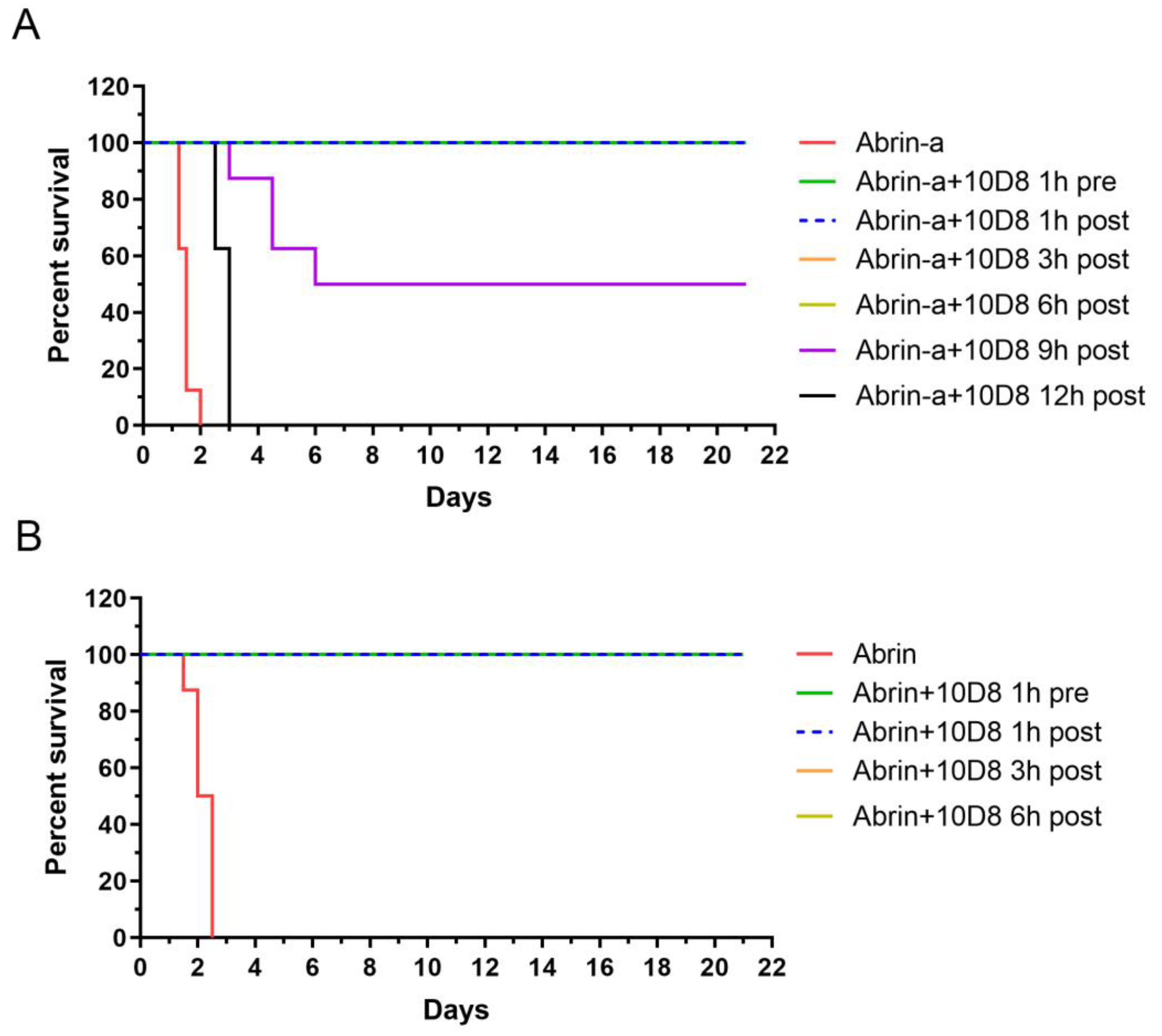
2.5. Prophylactic and Post-Exposure Treatment with 10D8 Protects against Abrin-a-Induced Lethality in Mice
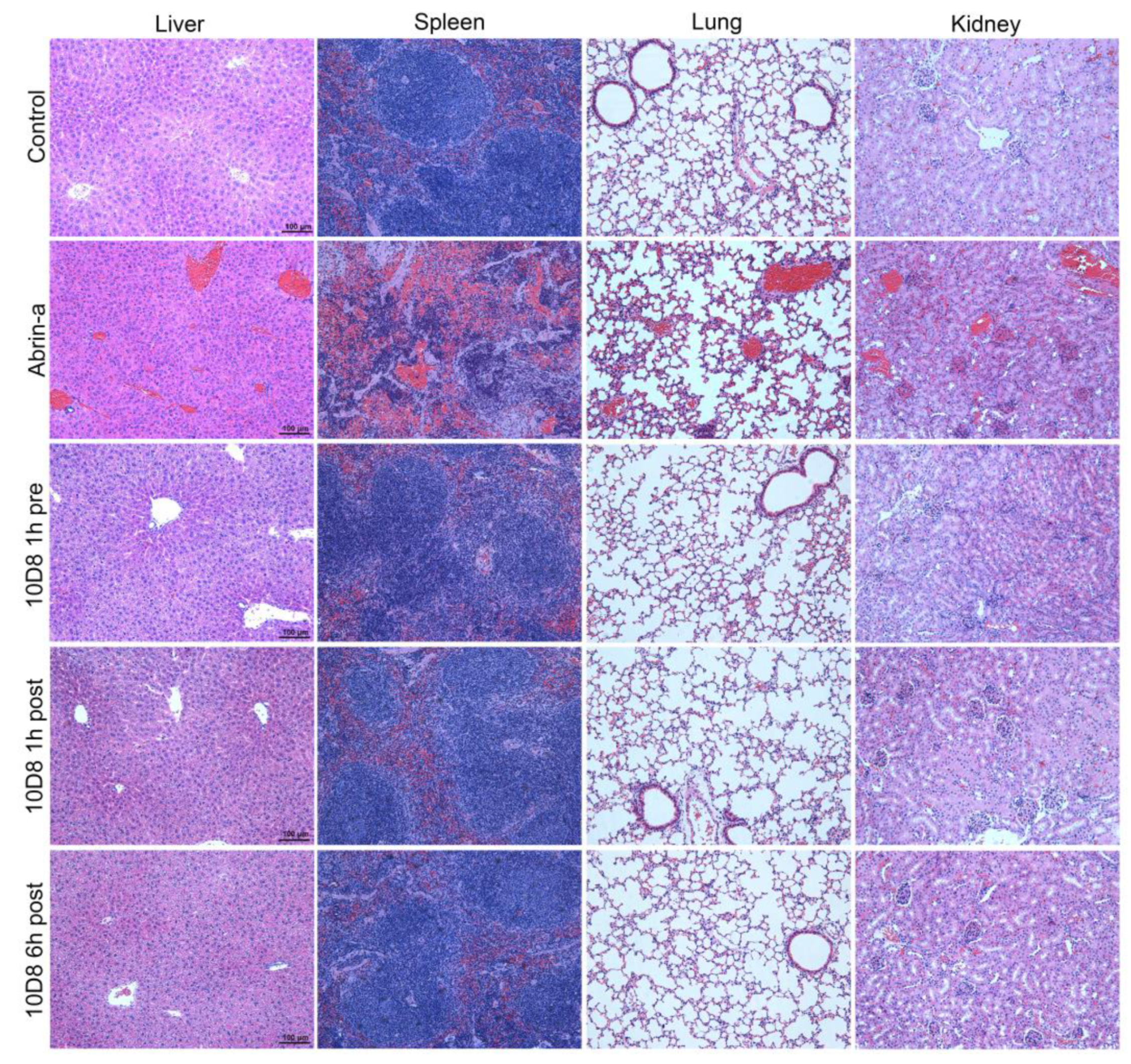
2.6. Treatment with 10D8 Prevents Tissue Damages of Abrin-a Intoxicated Mice
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Ethics Statement
4.3. Antigen Inactivation for Immunization
4.4. Preparation of Anti-Abrin-a mAbs
4.5. Indirect ELISA
4.6. ELISA for Cross-Reactivity Evaluation of mAbs
4.7. Cell Culture
4.8. Cell Cytotoxicity Assay
4.9. Cell-Free Luciferase Assay for Analyzing Protein Synthesis
4.10. Immunofluorescence Assay
4.11. SDS-PAGE
4.12. Western Blot
4.13. Mice Intoxication Model
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stirpe, F.; Barbieri, L.; Battelli, M.G.; Soria, M.; Lappi, D.A. Ribosome-inactivating proteins from plants: Present status and future prospects. Biotechnology 1992, 10, 405–412. [Google Scholar] [CrossRef]
- Narayanan, S.; Surendranath, K.; Bora, N.; Surolia, A.; Karande, A.A. Ribosome inactivating proteins and apoptosis. FEBS Lett. 2005, 579, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Janik, E.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yu, H.; Wang, Y.; Chang, Y.; Wan, J.; Xu, N.; Wang, J.; Liu, W. Integration of transcriptomics, proteomics and metabolomics data to reveal the biological mechanisms of abrin injury in human lung epithelial cells. Toxicol. Lett. 2019, 312, 1–10. [Google Scholar] [CrossRef]
- Polito, L.; Bortolotti, M.; Battelli, M.G.; Calafato, G.; Bolognesi, A. Ricin: An Ancient Story for a Timeless Plant Toxin. Toxins 2019, 11, 324. [Google Scholar] [CrossRef]
- Franke, H.; Scholl, R.; Aigner, A. Ricin and Ricinus communis in pharmacology and toxicology-from ancient use and “Papyrus Ebers” to modern perspectives and “poisonous plant of the year 2018”. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1181–1208. [Google Scholar] [CrossRef] [PubMed]
- Rasooly, R.; Do, P.; Hernlem, B. CCD Based Detector for Detection of Abrin Toxin Activity. Toxins 2020, 12, 120. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Patfield, S.; Cheng, L.W.; Stanker, L.H.; Rasooly, R.; McKeon, T.A.; Zhang, Y.; Brandon, D.L. Detection of Abrin Holotoxin Using Novel Monoclonal Antibodies. Toxins 2017, 9, 386. [Google Scholar] [CrossRef]
- Phatak, P.; Nagar, D.P.; Saxena, N. Dose dependent acute toxicity of abrin in Balb/c mice after intraperitoneal administration. Toxicon 2019, 167, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Worbs, S.; Kampa, B.; Skiba, M.; Hansbauer, E.M.; Stern, D.; Volland, H.; Becher, F.; Simon, S.; Dorner, M.B.; Dorner, B.G. Differentiation, Quantification and Identification of Abrin and Abrus precatorius Agglutinin. Toxins 2021, 13, 284. [Google Scholar] [CrossRef]
- Lin, J.Y.; Lee, T.C.; Hu, S.T.; Tung, T.C. Isolation of four isotoxic proteins and one agglutinin from jequiriti bean (Abrus precatorius). Toxicon 1981, 19, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Y.; Lee, T.C.; Tung, T.C. Inhibitory effects of four isoabrins on the growth of sarcoma 180 cells. Cancer Res. 1982, 42, 276–279. [Google Scholar]
- Yang, W.; Li, X.B.; Liu, G.W.; Zhang, B.B.; Zhang, Y.; Kong, T.; Tang, J.J.; Li, D.N.; Wang, Z. A colloidal gold probe-based silver enhancement immunochromatographic assay for the rapid detection of abrin-a. Biosens. Bioelectron. 2011, 26, 3710–3713. [Google Scholar] [CrossRef]
- Dickers, K.J.; Bradberry, S.M.; Rice, P.; Griffiths, G.D.; Vale, J.A. Abrin poisoning. Toxicol. Rev. 2003, 22, 137–142. [Google Scholar] [CrossRef]
- Gal, Y.; Sapoznikov, A.; Falach, R.; Mazor, O.; Alcalay, R.; Elhanany, E.; Aftalion, M.; Ehrlich, S.; Kronman, C.; Sabo, T. Equal Neutralization Potency of Antibodies Raised against Abrin Subunits. Antibodies 2020, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Surendranath, K.; Karande, A.A. A neutralizing antibody to the a chain of abrin inhibits abrin toxicity both in vitro and in vivo. Clin. Vaccine Immunol. 2008, 15, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S.; Karande, A.A. A monoclonal antibody to an abrin chimera recognizing a unique epitope on abrin A chain confers protection from abrin-induced lethality. Hum. Vaccines Immunother. 2016, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Mechaly, A.; Alcalay, R.; Noy-Porat, T.; Epstein, E.; Gal, Y.; Mazor, O. Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication. Toxins 2018, 10, 80. [Google Scholar] [CrossRef]
- Luo, L.; Yang, J.; Li, Z.; Xu, H.; Guo, L.; Wang, L.; Wang, Y.; Luo, L.; Wang, J.; Zhang, P.; et al. Label-free differentiation and quantification of ricin, abrin from their agglutinin biotoxins by surface plasmon resonance. Talanta 2022, 238, 122860. [Google Scholar] [CrossRef] [PubMed]
- Ohba, H.; Moriwaki, S.; Bakalova, R.; Yasuda, S.; Yamasaki, N. Plant-derived abrin-a induces apoptosis in cultured leukemic cell lines by different mechanisms. Toxicol. Appl. Pharmacol. 2004, 195, 182–193. [Google Scholar] [CrossRef]
- Qu, X.; Qing, L. Abrin induces HeLa cell apoptosis by cytochrome c release and caspase activation. J. Biochem. Mol. Biol. 2004, 37, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Rinner, G.R.; Watkins, S.A.; Shirazi, F.M.; Fernandez, M.C.; Hess, G.; Mihalic, J.; Runcorn, S.; Waddell, V.; Ritter, J.; Reagan-Steiner, S.; et al. Fatal abrin poisoning by injection. Clin. Toxicol. 2021, 59, 169–171. [Google Scholar] [CrossRef] [PubMed]
- Ninan, E.C.; James, E. Acute disseminated encephalomyelitis due to abrus precatorius poisoning-A case report. Saudi Pharm. J. 2019, 27, 521–524. [Google Scholar] [CrossRef]
- Alhamdani, M.; Brown, B.; Narula, P. Abrin poisoning in an 18-month-old child. Am. J. Case Rep. 2015, 16, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Audi, J.; Belson, M.; Patel, M.; Schier, J.; Osterloh, J. Ricin poisoning: A comprehensive review. JAMA 2005, 294, 2342–2351. [Google Scholar] [CrossRef] [PubMed]
- Bagaria, S.; Ponnalagu, D.; Bisht, S.; Karande, A.A. Mechanistic insights into the neutralization of cytotoxic abrin by the monoclonal antibody D6F10. PLoS ONE 2013, 8, e70273. [Google Scholar] [CrossRef]
- Hegde, R.; Maiti, T.K.; Podder, S.K. Purification and characterization of three toxins and two agglutinins from Abrus precatorius seed by using lactamyl-Sepharose affinity chromatography. Anal. Biochem. 1991, 194, 101–109. [Google Scholar] [CrossRef]
- Tang, J.; Xie, J.; Shao, N.; Yan, Y. The DNA aptamers that specifically recognize ricin toxin are selected by two in vitro selection methods. Electrophoresis 2006, 27, 1303–1311. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Luo, L.; Li, Z.; Xu, B.; Guo, L.; Xie, J. Highly sensitive MALDI-MS measurement of active ricin: Insight from more potential deoxynucleobase-hybrid oligonucleotide substrates. Analyst 2021, 146, 2955–2964. [Google Scholar] [CrossRef]
- Che, X.Y.; Qiu, L.W.; Pan, Y.X.; Wen, K.; Hao, W.; Zhang, L.Y.; Wang, Y.D.; Liao, Z.Y.; Hua, X.; Cheng, V.C.; et al. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 2004, 42, 2629–2635. [Google Scholar] [CrossRef][Green Version]
- Beatty, J.D.; Beatty, B.G.; Vlahos, W.G. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J. Immunol. Methods 1987, 100, 173–179. [Google Scholar] [CrossRef]
- Saxena, N.; Bhutia, Y.D.; Kumar, O.; Phatak, P.; Kaul, R.K. Prophylactic efficacy of some chemoprotectants against abrin induced lethality. Interdiscip. Toxicol. 2018, 11, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, K.; Jadhav, S.E.; Bhutia, Y.D.; Kumar, O.; Kaul, R.K.; Shrivastava, N. Purification and dose-dependent toxicity study of abrin in swiss albino male mice. Cell. Mol. Biol. 2015, 61, 36–44. [Google Scholar] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, H.; Ma, B.; Luo, L.; Guo, L.; Zhang, P.; Zhao, Y.; Wang, L.; Xie, J. Neutralizing Monoclonal Antibody, mAb 10D8, Is an Effective Detoxicant against Abrin-a Both In Vitro and In Vivo. Toxins 2022, 14, 164. https://doi.org/10.3390/toxins14030164
Li Z, Xu H, Ma B, Luo L, Guo L, Zhang P, Zhao Y, Wang L, Xie J. Neutralizing Monoclonal Antibody, mAb 10D8, Is an Effective Detoxicant against Abrin-a Both In Vitro and In Vivo. Toxins. 2022; 14(3):164. https://doi.org/10.3390/toxins14030164
Chicago/Turabian StyleLi, Zhi, Hua Xu, Bo Ma, Li Luo, Lei Guo, Pingping Zhang, Yong Zhao, Lili Wang, and Jianwei Xie. 2022. "Neutralizing Monoclonal Antibody, mAb 10D8, Is an Effective Detoxicant against Abrin-a Both In Vitro and In Vivo" Toxins 14, no. 3: 164. https://doi.org/10.3390/toxins14030164
APA StyleLi, Z., Xu, H., Ma, B., Luo, L., Guo, L., Zhang, P., Zhao, Y., Wang, L., & Xie, J. (2022). Neutralizing Monoclonal Antibody, mAb 10D8, Is an Effective Detoxicant against Abrin-a Both In Vitro and In Vivo. Toxins, 14(3), 164. https://doi.org/10.3390/toxins14030164




