Abstract
Plants are the cradle of the traditional medicine system, assuaging human or animal diseases, and promoting health for thousands of years. However, many plant-derived medicines contain toxic alkaloids of varying degrees of toxicity that pose a direct or indirect threat to human and animal health through accidental ingestion, misuse of plant materials, or through the food chain. Thus, rapid, easy, and sensitive methods are needed to effectively screen these toxic alkaloids to guarantee the safety of plant-derived medicines. Antibodies, due to their inherent specificity and high affinity, have been used as a variety of analytical tools and techniques. This review describes the antigen synthesis and antibody preparation of the common toxic alkaloids in plant-derived medicines and discusses the advances of antibody-based immunoassays in the screening and detection of toxic alkaloids in plants or other related matrices. Finally, the limitations and prospects of immunoassays for toxic alkaloids are discussed.
Key Contribution:
1. Hapten synthesis, bioconjugation, and antibody production for toxic alkaloids are reviewed. 2. Advantages and limitations of different types of immunoassays are summarized.
1. Introduction
In clinical medication practice, the use of plant-derived medicines in the treatment of human or animal diseases, such as inflammation and cancer, has been documented for over 5000 years [1,2,3,4]. Owing to the outstanding advantages, such as the nature of the original compounds, the functionality of a lot of active ingredients, and the low cost of a wide range of sources, plant-derived medicines are becoming more and more popular with consumers worldwide. In the current era of widespread antibiotic resistance, plant-derived medicines such as garlic have remarkably persisted over time and continue to be used today for indications similar to those described historically, which can be used as alternative antibiotic resources [5]. In addition, plant-derived medicines as functional foods have made a significant contribution to human or animal health [6]. It has been estimated by the World Health Organization that up to 80% of the world’s population, mostly in developing countries, rely on plant medicines for primary health care [7,8,9,10]. They are classified as complementary and alternative medicines and are regulated by the Therapeutic Goods Administration in Australia and the Dietary Health and Supplement Education Act under the Food and Drug Administration in the USA [11,12,13,14,15]. As feed additives, plant-derived medicines play an important role in promoting growth, anti-inflammatory, increasing immunity, and other functions [16,17,18,19,20].
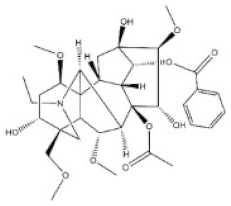
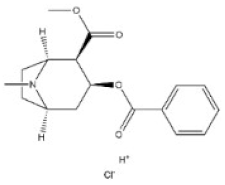
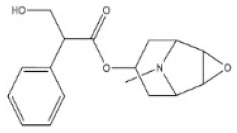
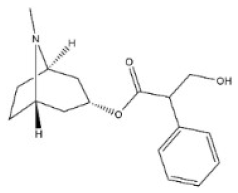
Alkaloids are a very large and chemically inhomogeneous group of nitrogen-containing compounds in plant-derived medicines, which are water-soluble under acidic conditions and lipid-soluble under neutral and basic conditions [21,22]. They have a wide range of pharmacological activities, such as anti-cancer, cardiotonic, analgesic, lower blood pressure, anti-bacterial, and anti-inflammatory [23,24,25,26,27,28]. However, some alkaloids not only have clinical value but also have different degrees of toxicity. Poisoning of people and livestock caused by improper treatment, accidental ingestion, homicide, or accident frequently occurs and seriously limits the safety of clinical use of plant-derived medicines [29]. Morphine, codeine, and cocaine, the first isolated secondary metabolites, have been transformed into important drugs due to the effect of local anesthesia [30,31]. Yet people’s abuse or long-term use of these alkaloids can cause an irregular heart rate and myocardial infarction and disrupt the balance of various physiological tissues in the body. Pyrrolizidine alkaloids are a class of toxic alkaloids with hepatotoxicity, derived from plant families such as Asteraceae, Boraginaceae, or Fabaceae [32,33]. Humans ingested these compounds by ingesting contaminated herbal medicines or teas [33,34]. Incidents of aconitine poisoning caused by improper plant handling or improper ingestion also frequently occur [35,36,37,38]. In addition, toxic alkaloids can indirectly damage human and animal health through the food chain. For instance, atropine is illegally injected into pigs and causes them to drink a lot of water and gain weight in the short term. The substance remains in pork and enters the human body through the food chain, resulting in coma, convulsions, and other toxic phenomena [39]. The related information of the common toxic alkaloids in plant-derived medicines reported in the literature, including their structure, classification, CAS registry number (a unique numeric identifier that is designated only one substance), source, toxicity, and median lethal dose (LD50) of mice and rats, are summarized in Table 1. These toxic alkaloids have various structures, low toxic dose, rapid metabolism in vivo, and no specific clinical manifestations after the poisoning, which directly increase the difficulty of screening and analyzing the related target substances in complex biological systems. At present, there is still a lack of internationally recognized limit specifications and quantitative detection standards for these harmful substances.

Table 1.
The common toxic alkaloids in plant-derived medicines.
A large number of analytical methods based on different analytical techniques have been developed for the determination of toxic alkaloids in plant-derived medicines or other related matrices, e.g., thin-layer chromatography (TLC) [40,41], liquid chromatography-mass spectrometry (LC-MS) [42,43,44,45], gas chromatography-mass spectrometry (GC-MS) [46,47], and ultraviolet spectrophotometry [48]. However, these instrumental methods for monitoring toxic alkaloids have certain disadvantages and limitations, such as being time-consuming, having expensive instruments, and high requirements for the experimental instruments, thus, these methods lack field applicability. More importantly, different toxic alkaloids of the same species are rich in structure and abundant in quantity, and there are isomers, which need to be detected with standard substances as the control. Therefore, it is an urgent task to develop a cheap and reliable method for the effective on-site monitoring of toxic alkaloids in plant-derived medicines or other matrices.
In the late 1960s, the introduction of labeled antibody technology brought many benefits to medical and clinical diagnosis technology [49]. The immunoassay is a rapid detection method based on the specific reaction of antigen and antibody, which amplifies the reaction signal of labeled antigens or antibodies by enzymes, radioactive elements, colloidal gold, or other available labeling materials. They have been routinely used in a wide spectrum of compounds such as proteins, pesticides, veterinary drugs, biomarkers, and heavy metals. The main advantages of immunoassays are that they are inexpensive, suitable for high throughput, and do not require high-tech equipment or professional technicians. Thus, they could be conducted much more economically than the high-tech instrumental methods, and they are continuously expanding, especially in the development of more sensitive, rapid, specific, and robust analytical methods. For toxic alkaloids, literature about antibodies or immunoassays from 1 January 1970 to 31 January 2022 were retrieved from PubMed (http://pubmed.cn/, accessed on 31 January 2022) and Web of Science (http://www.isiknowledge.com/, accessed on 31 January 2022) with the MeSH (Medical Subject Headings) word “aconitine” (for example) and “antibody” or “immunoassay” without language restriction. A total of 453 articles were retrieved. After excluding repetitive articles and literature based on commercial antibodies, 182 articles were used for data extraction and analysis. The application of immunoassay in toxic alkaloids began in the 1970s, and it has mainly gone through four stages: radioimmunoassay (RIA), enzyme-linked immunosorbent assay (ELISA), immunochromatography assay (ICA), and fluoroimmunoassay (FIA), as shown in Figure 1. Yet, it is only widely used in highly toxic or abused toxic alkaloids, such as aconitine, codeine, morphine, etc., and less used in other toxic alkaloids with few reports of poisoning incidents. Furthermore, the structure of toxic alkaloids is complex and the cost of obtaining them from plants is high. Antibody preparation based on the traditional trial-and-error method requires a large number of standard substances, and it is difficult to obtain ideal broad-spectrum antibodies, so the development of immunoassay of toxic alkaloids is slow. This review focuses on the recent advances in the field of immunoassays for the rapid detection of toxic alkaloids, including antigen preparation, antibody production, and various types of methods reported in the literature. Finally, the limitations and prospects for the immunoassay of toxic alkaloids in plant-derived medicines are also discussed.
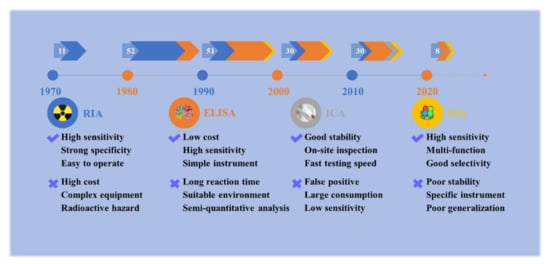
Figure 1.
Development and scientific articles of immunoassays for plant-derived toxic alkaloids. The numbers in white represent the number of articles per decade. RIA: radioimmunoassay; ELISA: enzyme-linked immunosorbent assay; ICA: immune-chromatography assay; FIA: fluoroimmunoassay.
2. Synthesis and Identification of Antigen
2.1. Selection of Hapten
Alkaloids, an important class of small-molecule natural compounds (molecule weight < 1000 Da), usually need to bind to large molecules to induce immune response [50]. In general, if the analyte contains active groups such as amino, carboxyl, hydroxyl, or sulfhydryl, these groups could be directly linked to the carrier protein. Tanaka et al. [51] successfully prepared an antigen that directly coupled the hydroxyl group in the structure of solamargine with bovine serum albumin (BSA). To avoid masking the characteristic structure of small molecules by proteins, a spacer arm is usually introduced, which is generally a non-polar carbon chain, and should not contain other structures with high immune activity, such as benzene rings, heterocycles, halogen atoms, etc. Kiko et al. [52] prepared 3-succinylaconitine by succinylation at the C-3 hydroxyl position of aconitine. The same method has also been used to prepare haptens such as camptothecin [53], morphine [54], retrorsine [55], and tropane [56]. Wang et al. [57] prepared a hapten of methamphetamine by introducing a carboxyl group on the second amino group. Compounds that react with the active groups can also be replaced by bromoacetic acid [58], aminobutyric acid [59], or other substances, with appropriate chain length [57].
However, if there is no active group or the active group greatly influences the specificity and polarity of the compound, haptens for artificial antigen synthesis need to be designed or modified from scratch. Sakurai et al. [60] designed two haptens of cocaine for the preparation of antibodies. The analog incorporated an amido linker functionality in place of the carbomethoxy group at C-2 and benzoyl amino-substituted for the benzyloxy group at C-3 of the cocaine framework. Kikuchi et al. [61] synthesized scopolamine from scopolamine, exposing the amino group at the active site and synthesizing a hapten containing a carboxyl group at the terminal for protein coupling.
Haptens can also be designed by the target’s precursors or metabolites. Sakamoto et al. [62] synthesized a hapten of monocrotaline by using retronecine (a hydrolysate of monocrotaline). Heroin is a highly toxic alkaloid derived from opium. It is first metabolized into monoacetylmorphine (MAM), and MAM is then further converted into morphine [63]. Gandhi et al. [64] synthesized an acidic derivative of MAM and eventually induced antibodies that simultaneously recognize heroin, MAM, and morphine because they have the same common structure. Wang et al. [56] selected the simplest compound with the structure of tropine ring from 12 kinds of atropine alkaloids with similar structures and their structural analogs for hapten design and finally obtained an antibody that can cross-recognize seven kinds of atropine alkaloids.
Designation at different sites in the compound also exposes different fragments of the molecule to immune B cell epitopes, generating antibodies with different recognition properties. Yan et al. [53] designed two haptens based on B- and E-ring modifications to investigate the effects of haptens’ analogs on the sensitivity of immunization. One case is to introduce an active carboxyl group at the C-7 site, and the other case is to introduce a four-carbon spacer arm containing a carboxyl group to connect to the hydroxyl group at the C-20 site. The electron distribution analysis showed that the latter hapten maintained the parent ring structure of camptothecin, and the second haptens were used to obtain monoclonal antibodies with high sensitivity and high titer. Therefore, the stability of haptens is also one of the key factors that cannot be ignored in the process. Trofimov et al. [65] obtained two morphine derivatives, 6-hemisuccinyl and 3-O-carboxymethyl esters, and their antibodies. The results showed that antibodies derived from 3-O-carboxymethyl interacted with morphine more effectively than 6-hemisuccinyl.
Computer-aided molecular modeling is an effective strategy, which can evaluate and select the designed haptens from the aspects of molecular configuration and electronic properties, and ultimately improve the possibility of obtaining the target antibodies. Now, this method has only been preliminarily applied to atropine [56] and pyrrolizidine alkaloids [55], and other related studies have not been reported. The structures of the toxic alkaloids in plant-derived medicines are complex and varied, but the same species has the same parent structure and properties. If the efficient technique can be used to produce monoclonal antibodies (mAbs) of other toxic alkaloids, it is believed that more specific or broad-spectrum target antibodies can be obtained, reducing the waste of laboratory animals and trial-and-error processes.
2.2. Coupling and Identification of Hapten
Currently, the carriers for hapten coupling are mainly proteins, including BSA, ovalbumin (OVA), keyhole limpet hemocyanin (KLH), human serum albumin (HSA), etc. BSA is most commonly used in the toxic alkaloids field due to its stable physicochemical properties, non- dewaterability, low cost, and easy availability, as well as its high lysine content (i.e., high coupling density of hapten and carrier) [52,53,57,58,64,66,67,68,69,70,71,72,73,74]. Similarly, HSA also has a large amount of lysine and more free amino acids, which are widely used [59,75]. KLH is also commonly used as an immunogen protein [53,70,76,77]. It is more expensive than BSA and has poor water solubility, but it has higher molecular weight and better immunogenicity than BSA. Yan et al. screened various carrier proteins to select the most suitable immunogen for the production of mAbs against camptothecin, and KLH showed better immunogenicity than BSA. OVA is characterized by poor solubility, instability, and variability, and contains fewer active groups than BSA, but it is often used as an unrelated carrier protein for antibody screening and immunoassays [53,61,64,73,78,79].
Modified toxic alkaloids and their derivatives are haptens usually carrying carboxyl or amino groups, but a few contain other groups such as aldehyde. The carbodiimide method (Figure 2B) [73,80,81], mixed anhydride method (Figure 2D) [53,82], and active ester method (Figure 2E) [31,52,53,78,83] are the most widespread and universal coupling methods used for hapten containing carboxyl groups. The glutaric dialdehyde methods (Figure 2C) [72,84] are often used for haptens containing amino groups. Cibotti et al. [84] modified the structure of VLB and introduced amino groups at the C-3 position, and successfully conjugated with BSA by the glutaric dialdehyde method to form an immunogen VLBC3-BSA. Antigen VLBC17-BSA was also synthesized by introducing BSA at active aromatic hydrogen C17 in VLB by the Mannich reaction. Comparing the monoclonal antibodies prepared by the two immunogens, the latter antibody is more susceptible to structural changes and has a higher specificity for VLB. Besides, diazo methods (Figure 2A) are commonly used for reactions containing aromatic amine groups, e.g., cocaine [85] and atropine [59]. Stammel et al. [86] reported a method of reductive amination, which is a very effective method for coupling small molecules bearing an amino group to carrier proteins. For haptens containing aldehyde groups, Ishiyama et al. [87] and Putalun et al. [77] used NAIO4 to oxidize the adjacent dihydroxyl groups on the C-3 sugars of solamargine into aldehyde groups. Then it was linked with BSA and KLH to synthesize immune antigen and coated antigen. The connection position and method of the hapten and the carrier protein are important factors that affect the specificity of the resultant antibody. Pontikis et al. (1980) synthesized three haptens conjugated to BSA at different coupling sites on three rings of colchicine. Antibodies exhibited variable cross-reactivity (CR) to the metabolites and structural analogs of colchicine, depending on the site where the colchicine is coupled to the protein carrier. The same work also demonstrated that attaching haptens to different sites, exposing different surfaces opposite the small molecule antigen junction, has a significant effect on the specificity of antibodies [72].
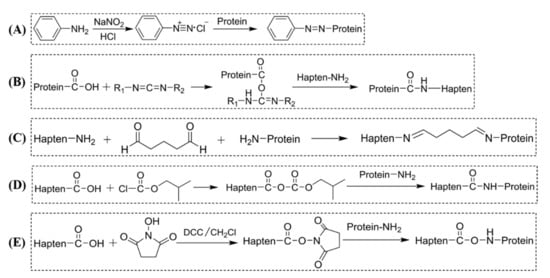
Figure 2.
Common methods for conjugating hapten to the protein of toxic alkaloids. (A) Diazo method; (B) carbodiimide method; (C) glutaric dialdehyde method; (D) mixed anhydride method; (E) active ester method.
The methods commonly used for the identification of toxic alkaloid-carrier protein conjugates are ultraviolet and visible spectrophotometry (UV-Vis), sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS) [52,53,57,81,88,89]. In general, MALDI-TOF-MS is the ideal analytical method for the determination of coupling due to its high sensitivity, small sample quantity, and accurate and reliable results, and the coupling rate can also be measured [55,56,62,90]. Kido et al. used MALDI-TOF-MS to accurately determine the molecular weight range of the conjugate of aconitine derivatives-BSA between 68,700 and 72,800, and the mean fractionation size of the conjugate was 70,500. The BSA fractionation amount was 66,000 and the molecular weight of the aconitine derivative was 4500. The calculated coupling rate ranged from 4 to 9 with an average of 6, which could be used for immunity. However, it is inconvenient for general laboratories because of its complicated operation, high technical requirements, and high cost.
3. Types and Preparation of Antibodies
The research on toxic alkaloids’ antibodies has experienced three stages: polyclonal antibodies (pAbs), monoclonal antibodies (mAbs), and genetic engineering antibodies. The first two antibodies are usually referred to as traditional antibodies, and the third one is called novel antibodies. Through traditional hybridoma technology and display technology (such as phage display, mammalian cell display, and polysome display), antibodies can be successfully obtained.
In the process of an antibody preparation, the effective immunogen and the body’s reaction to the antigen stimulus are the key factors to evaluate whether the antibody can be produced. The adjuvant is a good auxiliary reagent, which may enhance the long-term release of the antigen by functioning as a depot, and may also act as non-specific mediators of immune cell function by stimulating or modulating immune cells. Complete Freund’s adjuvant and incomplete Freund’s adjuvants are often used in the preparation of antibodies against toxic alkaloids [52,55]. A recent study showed that chiral nanoparticles as immune adjuvants could enhance immune response, which provides new ideas for the preparation of toxic alkaloid antibodies [91]. Compared with traditional adjuvants, nanoparticle materials have more kinds and higher safety, and have the potential for a new generation of efficient adjuvants.
Based on the literature analysis, the synthesis of antigens and the preparation of antibodies are summarized in Table 2.

Table 2.
The reported antigen and antibody of toxic alkaloids.
3.1. Polyclonal Antibody
PAbs are antibodies derived from different B cell sources that can recognize multiple epitopes on an antigen. These antibodies are usually produced by immunizing larger mammals, such as rabbits or goats, to collect a greater volume of serum, and the production cost is inexpensive. Polyclonal antibodies generally have a higher affinity than monoclonal antibodies. In 1970, the polyclonal antibody of morphine was first reported, and the technique began to be widely used in the quantitative analysis of other toxic alkaloids [54]. Xu et al. (2015) synthesized aconitine immunogen by the active ester method and prepared polyclonal antibody against aconitine by immunizing New Zealand white rabbits with a serum titer of 1:1,280,000. Yuan et al. (2016) synthesized the immunogen of benzoylmesaconitine using the same method as above. The detection limit of the polyclonal antibody was 139 pg, which could be used to identify monoester and diester alkaloids in aconitum plants. Stamme et al. (1993) immunized rabbits to obtain highly specific and sensitive polyclonal antibodies with a titer of 1:1700, which can be used to detect salsolidine in tissues and blood.
3.2. Monoclonal Antibody
MAbs are identical immunoglobulins, generated from a single B cell clone. These antibodies recognize unique epitopes, or binding sites, on a single antigen. The derivation from a single B cell clone and subsequent targeting of a single epitope is the difference between monoclonal antibodies and polyclonal antibodies. Currently, the preparation and application of monoclonal antibodies in toxic alkaloids are also in a stage of rapid development. Although monoclonal antibodies based on hybridoma technology have obvious disadvantages such as complex preparation, long cycle, and high cost, they are available in unlimited quantities and can be used in commercial immunoassay products. Kikuchi et al. (1991) prepared a highly sensitive monoclonal antibody with a detection limit of 0.2 ng/mL by fusing NS1 myeloma cells with spleen cells of BALB/c mice immunized with scopolamine. The monoclonal antibody to the scopolamine cross-reacts with methscopolamine and Scopolamine Butylbromide, but not with atropine, tropic acid, 6β-hydroxyhyoscyamine, and 7β-hydroxyhyoscyamine. Kim et al. (2004) prepared the monoclonal antibody against berberine which can identify isoquinoline alkaloids in herbal medicines.
3.3. Genetically Engineered Antibody
The genetically engineered antibody is recombinant and cloned into an expression vector, expressed in an appropriate host, and folded into a functional antibody molecule using genetic engineering technology. It has the characteristics of small molecular weight, strong plasticity, low cost, and mass production without animal immunity. It mainly includes chimeric antibodies, humanized antibodies, complete human antibodies, single-chain antibodies fragments, bispecific antibodies, and so on. Currently, only Brennan et al. [100] have reported a single-chain antigen-binding protein (scFv) that can recognize morphine and has been obtained and successfully applied to real samples’ analysis in saliva. In the rapid development of gene-engineered antibodies, they will inevitably become the new favorite in the detection of toxic alkaloids.
4. Detection Assay
Immunoassays for toxic alkaloids have gone through four stages: RIA, ELISA, ICA, and FIA. The schematic diagram of each type of detection is shown in Figure 3. Among these immunoassays, ELISA and ICA are the two most frequently used methods in the routine analysis of toxic alkaloids. A list of immunoassays used for the analysis of toxic alkaloids is shown in Table 3. Each method includes the reference to the original description, the target analyte, the applicable sample matrices, the sample preparation, and the limit of detection (LOD).
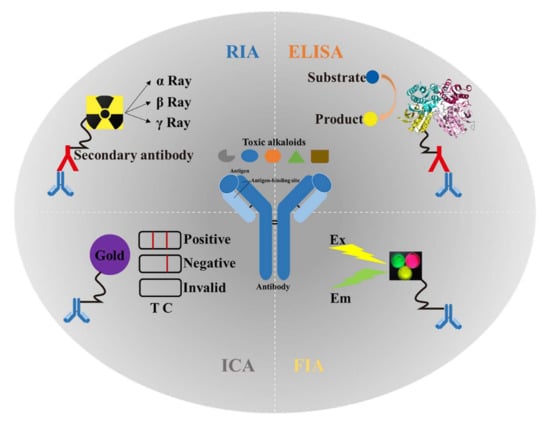
Figure 3.
Application and schematic diagram of four types of immunoassays in the detection of plant-derived toxic alkaloids. RIA: radioimmunoassay; ELISA: enzyme-linked immunosorbent assay; ICA: immuno-chromatography assay; FIA: fluoroimmunoassay; Ex: excitation; Em: emission; T: test line; C: control line.

Table 3.
The reported immunoassays of toxic alkaloids.
4.1. Radioimmunoassay (RIA)
RIA is a new technique for the determination of ultramicro substances in vitro, which combines the high sensitivity, accuracy, and specificity of radioisotope measurement with the specificity of antigen-antibody reaction. In the initial stage of the development of immunoassays, this method was used for the detection of toxic alkaloids, such as morphine [67], vinblastine [89], atropine [97], solasodine [117], and cocaine [118]. Although the method has the advantages of high sensitivity, strong specificity, simple operation, and small sample consumption, the radiation and pollution problems in the analysis process limit the application and development of this method in the detection of toxic alkaloids in plant-derived medicines.
In RIA, the amounts of antibodies are small, and the radioelement labeled antigen competes with the substance to bind to a limited number of antibodies. The sensitivity of the method is high, and the specificity depends on the cross-reactivity of the antibody.
4.2. Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA is an immunoassay method using biological enzymes as markers, combining the specificity of the antigen-antibody reaction with the highly efficient catalytic amplification of enzyme. It is a common analytical biochemistry immunoassay, and also the most widely used method in the field of an immunoassay for toxic alkaloids in plant-derived medicines. Unlike macromolecules containing multiple epitopes, toxic alkaloids are generally detected by competitive ELISA because there is only one characteristic functional group of toxic alkaloids that can be recognized by antibodies.
In ELISA, the selectivity and specificity of the method depend on the antibodies. As the reaction in the buffer, antibodies and antigens can compete and combine under the best conditions, and the recognition regions of antibodies in the buffer will not be affected by the coupling methods. Therefore, the sensitivity of this method is high. In addition, the sensitivity of this method is also affected by some reaction conditions such as enzyme quality, substrate selection, etc. These conditions need to be optimized to improve the detection performance of the method.
4.3. Immunochromatography Assay (ICA)
ICA is an immunoassay method based on the reversible reaction of antigens and antibodies labeled with gold nanoparticles as tracers. Yusalul et al. (2019) established an ICA method based on the high sensitivity and specificity antibody of monocrotaline, which is time-saving and 16 times more sensitive than ELISA. This method also has many other advantages, such as convenience, low cost, no need for special instruments and pieces of equipment, accurate results, high sensitivity, point-of-care detection, etc., so it develops rapidly. However, there are also shortcomings such as a narrow linear range and insufficient system stability. Therefore, it is necessary to repeat experiments to investigate the influencing factors. This method may also be the most promising tool for the rapid preliminary screening analysis for toxic alkaloids in the future.
In ICA, the interaction between the tracers and antibodies is electrostatic adsorption, and few antibodies are effectively coupled. Thus, the sensitivity is low. Generally, the sensitivity is improved by increasing the number of antibodies within a certain range. Furthermore, it is easy to produce non-specific adsorption, and the cross-reaction rate of some compounds may be increased, resulting in interference of selectivity.
4.4. Fluoroimmunoassay (FIA)
FIA is a method that combines the specificity of the immunological response and the sensitivity of the fluorescence technique. Fluorescent nanomaterials have been widely used in clinical diagnosis and imaging due to their broad-spectrum, low toxicity, and strong versatility. In recent years, there have been few reports on the application of fluorescence immunoassays in the detection of toxic alkaloids. The sensitivity of detection can be improved when it is used to label antigens or antibodies. Zheng et al. (2021) prepared fluorescent gold nanoclusters, conjugated with mAb as a label probe, and used in establishing a qualitative and quantitative lateral flow immunoassay for the determination of four pyrrolizidine alkaloids (retrorsine, platyphylline, senecionine, integerrimine) in honey. The LOD was 0.083 μg/kg, and the sensitivity was increased over ten times that of ELISA. Zhang et al. (2020) developed a sensitive, environmental-friendly carbon quantum dots-based FIA for the detection of morphine. It can be applied as a sensitive and convenient tool for the rapid detection of morphine. There are also preliminary studies on colchicine [111]. Given the abundance and diversity of fluorescent nanomaterials, FIA will be of interest to researchers in the future.
FIA has higher sensitivity than the above methods, which depend on the unique properties of the fluorescent materials. However, FIA is less stable than IAC and ELISA, so it is necessary to optimize the reaction conditions such as probe dosage, antibody dosage, buffer solution, pH, and so on to achieve stable and efficient detection performance. Its specificity and selectivity also depend on antibody performance. However, some fluorescent molecules can also recognize non-target objects, leading to non-specific adsorption in the detection process, resulting in false results.
5. Prospect
Plant-derived medicines have a long history in the prevention and treatment of human diseases. Now, in the prevention and control of COVID-19, plant-derived medicines have the efficacy of clearing away heat and detoxification, invigorating qi and promoting blood circulation, purifying the lung, and regulating immune function [119]. Toxic alkaloids are one of the important ingredients of plant-derived medicines, and even some of them are the main sources of their medicinal ingredients. Thus, there is a great demand for developing inexpensive and reliable assay methods for effective monitoring of toxic alkaloids to ensure the safe and rational use of plant-derived medicines.
Compared with the traditional chromatographic analysis methods for toxic alkaloids, such as TLC, LC-MS, or GC-MS, immunoassays have inevitable disadvantages. Antibodies are the core reagent of immunoassays. They need a long time to prepare for specificity and need proper storage conditions, which limit the rapid development of immunoassays. Even so, immunoassays have the significant advantages of being fast, relatively inexpensive, and robust enough for in-field measurements, without expensive special personnel for analysis and maintenance. Due to the complex structure and large quantity of plant-derived alkaloids, it is difficult to obtain sufficient standard toxic alkaloids as reference calibrants. Immunoassays could achieve the high-throughput analysis of toxic alkaloids and their structural analogs based on the broad-spectrum antibodies. Besides, the antibodies used in immunoassays can be combined with a variety of nanomaterials or biological probes to achieve a significant increase in sensitivity. Thus, immunoassays can provide strong support for rapid and effective monitoring of toxic alkaloids.
To overcome the existing deficiencies, more in-depth and multifaceted studies in antigen-antibody-based immunoassays are needed. The affinity of the antigen and antibody is the premise of the immunoassay. Much effort has been invested in the development of antibodies against various toxic alkaloids. The antibodies currently developed only specifically identify or cross-identify a small number of highly similar compounds but fail to effectively achieve a high-throughput analysis. Properties of small molecules will influence the antibody response, such as the degree of molecular hydrophobicity, spatial conformation, electron distribution, and physical parameters, which have been researched in antibacterial synergists [120] and veterinary drugs [121]. Modeling the antibody structure and genetic modification of the binding site with site-directed mutagenesis is also a good strategy. Besides, the study of the interaction between the antigen and antibody by molecular modeling and a quantitative structure-activity relationship analysis can reverse guide the design and synthesis of haptens. The research has been conducted on phenylurea herbicides [122] and sulfonamides [123]. Most traditional antibodies used in immunoassays are polyclonal or monoclonal antibodies obtained from immunized mice or rabbits. In recent years, antibodies such as scFv, antigen-binding fragment, or nanobodies obtained from non-immunized animals have attracted more and more attention and have the potential to replace traditional antibodies used in immunoassays. The sensitivity and specificity of antibodies are the key factors that determine the linear range of detection. In addition to antibodies, another problem that restricts the application of immunoassays for toxic alkaloids’ detection is the probe. In recent years, researchers have focused on the development of different types of nanoparticle labels to be used instead of traditionally labeled probes such as enzymes or colloidal gold. These labels include fluorescent probe modified AuNCs, colloidal carbon, and new fluorescence proteins that improve the sensitivity of immunoassays [124,125]. The non-covalent binding force of the antigen and antibody can be detected by amplification of other signals such as electrical signals or fluorescence signals. Most of these new nano-sized labels have not yet been used to develop immunoassays for the detection of toxic alkaloids. Metal-organic frameworks (MOF) have the characteristics of porosity and a large specific surface area, which can be modified with organic ligands to realize the adsorption of a class of compounds with similar properties. This may be an effective method for sample preparation in the future, especially for the extraction of a class of toxic alkaloids with the same properties. In summary, it is necessary to carry out more studies in the field of materials and immune analysis models to optimize new strategies, achieve high-throughput detection of multiple residues of toxic alkaloids, and accelerate the transformation from laboratory technology to real-time, portable, and intelligent field technology, to meet the growing demand for safety monitoring of plant-derived medicines.
Author Contributions
Conceptualization, Z.R. and H.J.; writing-original draft preparation, Z.R.; literature researching, Z.R., H.Z. and Z.W.; validation, Z.R., X.C. and L.Y.; supervision, H.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of the People’s Republic of China (No. 2019YFC1604902).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Newman, D.J.; Cragg, G.M.; Snader, K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000, 17, 215–234. [Google Scholar] [PubMed]
- Berlin, C. Herbal medicine. Clin. Pediatr. 2001, 40, 271–272. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, N. Traditional Chinese medicine: A brief outline. J. Chem. Inf. Comput. Sci. 2002, 42, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Licciardi, P.V.; Underwood, J.R. Plant-derived medicines: A novel class of immunological adjuvants. Int. Immunopharmacol. 2011, 11, 390–398. [Google Scholar] [CrossRef]
- Magryś, A.; Olender, A.; Tchórzewska, D. Antibacterial properties of Allium sativum L. against the most emerging multidrug-resistant bacteria and its synergy with antibiotics. Arch. Microbiol. 2021, 203, 2257–2268. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Ncube, B.; Okem, A.; Mulaudzi, R.B.; Van Staden, J. Toxicology of some important medicinal plants in southern Africa. Food Chem. Toxicol. 2013, 62, 609–621. [Google Scholar] [CrossRef]
- Borchers, A.T.; Hackman, R.M.; Keen, C.L.; Stern, J.S.; Gershwin, M.E. Complementary medicine: A review of immunomodulatory effects of Chinese herbal medicines. Am. J. Clin. Nutr. 1997, 66, 1303–1312. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J.; Snader, K.M. Natural products in drug discovery and development. J. Nat. Prod. 1997, 60, 52–60. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity of South African herbal teas: Rooibos (Aspalathus linearis) and honeybush (Cyclopia intermedia). Phytother. Res. 2007, 21, 1–16. [Google Scholar] [CrossRef]
- Ozorio, P. World Health Organization encourages traditional medicine in the third world. Dev. Dir. 1979, 2, 16. [Google Scholar]
- Fennell, C.W.; Lindsey, K.L.; McGaw, L.J.; Sparg, S.G.; Stafford, G.I.; Elgorashi, E.E.; Grace, O.M.; van Staden, J. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J. Ethnopharmacol. 2004, 94, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Matthews, H.B.; Lucier, G.W.; Fisher, K.D. Medicinal herbs in the United States: Research needs. Environ. Health Perspect. 1999, 107, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Talalay, P.; Talalay, P. The importance of using scientific principles in the development of medicinal agents from plants. Acad. Med. 2001, 76, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, H.; Oliver, C.; Nathan, P.J. A review of the status of Western herbal medicine in Australia. J. Herb. Pharmacother. 2002, 2, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.J. Research on the Problems and Strategies of the Internationalization of Chinese Medicine; Shandong University of Traditional Chinese Medicine: Jinan, China, 2018. [Google Scholar]
- Kallon, S.; Li, X.; Ji, J.; Chen, C.; Xi, Q.; Chang, S.; Xue, C.; Ma, J.; Xie, Q.; Zhang, Y. Astragalus polysaccharide enhances immunity and inhibits H9N2 avian influenza virus in vitro and in vivo. J. Anim. Sci. Biotechnol. 2013, 4, 22. [Google Scholar] [CrossRef]
- Cheng, J.; Li, Q.; Shi, W.; Zhong, X. Effects of Huangqi Maxingshigan decoction on infectious laryngotracheitis in chickens. Ital. J. Anim. Sci. 2011, 10, 124–130. [Google Scholar] [CrossRef]
- Xu, C.L.; Zhao, Y.F.; Shang, X.Y.; Niu, W.N. The effects of supplementing diets with Atractylodes macrocephala Koidz rhizomes on growth performance and immune function in piglets. J. Anim. Feed Sci. 2012, 21, 302–312. [Google Scholar] [CrossRef]
- Brambilla, G.; Filippis, S.D. Trends in animal feed composition and the possible consequences on residue tests. Anal. Chim. Acta. 2005, 529, 7–13. [Google Scholar] [CrossRef]
- Hao, C.Q.; Ma, Y.; Feng, F.; Yang, J.; Gao, Q.X.; Bu, J.J.; Xin, G.S. Primary biological function and application of Chinese herbal feed additives. Pratacultural Sci. 2020, 37, 1638–1645. [Google Scholar]
- Pelletier, S.W. Alkaloids: Chemical and Biological Perspectives. Pergamon 1999, 13, 163–236. [Google Scholar]
- Verpoorte, R. Encyclopedia of Analytical Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Bello-Ramírez, A.M.; Buendía-Orozco, J.; Nava-Ocampo, A.A. A QSAR analysis to explain the analgesic properties of Aconitum alkaloids. Fundam. Clin. Pharmacol. 2003, 17, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Ding, R.; Chu, Z.Y.; Zhang, M.B.; Liu, X.Y.; Xie, S.H.; Zhai, Y.J.; Wang, Y.D. Role of berberine in anti-bacterial as a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC Complement. Altern. Med. 2014, 14, 89. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Ono, M.; Baba, M. Potent inhibition of HIV type 1 replication by an antiinflammatory alkaloid, cepharanthine, in chronically infected monocytic cells. AIDS Res. Hum. Retrov. 1998, 14, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Ramesha, B.T.; Suma, H.K.; Senthilkumar, U.; Priti, V.; Ravikanth, G.; Vasudeva, R.; Kumar, T.R.; Ganeshaiah, K.N.; Shaanker, R.U. New plant sources of the anti-cancer alkaloid, camptothecine from the Icacinaceae taxa, India. Phytomedicine 2013, 20, 521–527. [Google Scholar] [CrossRef]
- Li, Q.L.; Zhao, S.; Sun, L.T.; Liu, X.L.; Wang, C.Y.; Li, Y.M.; Li, C.; Zhang, D. Effective components of ginseng aconite in cardiotonic effect. J. Chang. Univ. Chin. Med. 2015, 31, 463–465. [Google Scholar]
- Li, G.D. Veratrine Antihypertensive Effect and Mechanism of the Experimental Study; Yanbian University: Yanji, China, 2007. [Google Scholar]
- Green, B.T.; Lee, S.T.; Panter, K.E.; Brown, D.R. Piperidine alkaloids: Human and food animal teratogens. Food Chem. Toxicol. 2012, 50, 2049–2055. [Google Scholar] [CrossRef]
- Carnaval, T.G.; Sampaio, R.M.; Lanfredi, C.B.; Borsatti, M.A.; Adde, C.A. Effects of opioids on local anesthesia in the rat: A codeine and tramadol study. Braz. Oral Res. 2013, 27, 455–462. [Google Scholar] [CrossRef]
- Wang, L.M.; Zhang, Z.; Yao, R.Z.; Wang, G.L. The Role of Intrathecal Morphine for Postoperative Analgesia in Primary Total Joint Arthroplasty under Spinal Anesthesia: A Systematic Review and Meta-Analysis. Pain Med. 2021, 22, 1473–1484. [Google Scholar] [CrossRef]
- Ning, J.; Chen, L.; Rietjens, I. Role of toxicokinetics and alternative testing strategies in pyrrolizidine alkaloid toxicity and risk assessment; state-of-the-art and future perspectives. Food Chem. Toxicol. 2019, 131, 110572. [Google Scholar] [CrossRef]
- Schrenk, D.; Gao, L.; Lin, G.; Mahony, C.; Mulder, P.P.J.; Peijnenburg, A.; Pfuhler, S.; Rietjens, I.; Rutz, L.; Steinhoff, B.; et al. Pyrrolizidine alkaloids in food and phytomedicine: Occurrence, exposure, toxicity, mechanisms, and risk assessment—A review. Food Chem. Toxicol. 2020, 136, 111107. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, B.L. Safety considerations of traditional Chinese medicine containing pyrrolizidine alkaloids. Chin. J. Clin. Pharmacol. 2020, 36, 84–86. [Google Scholar]
- Tajibieke, A.; Gulinaer. Poisoning and Treatment of Aconitum Carmichaeli. Grass Feed. Livest. 2012, 02, 46–47. [Google Scholar]
- Yan, D.J.; Zhou, Q.W.; Lu, H.; Wu, C.C.; Zhao, B.Y.; Cao, D.D.; Ma, F.; Liu, X.X. The disaster, ecological distribution and control of poisonous weeds in natural grasslands of Xinjiang Uygur Autonomous region. Sci. Agric. Sin. 2015, 48, 565–582. [Google Scholar]
- Chen, F.R.; Zou, D.J.; Shan, R.L.; Xu, Z.L.; Ma, L.H.; Mei, Z.N.; Huang, X.J. A literature analysis on the causes and detoxification methods of aconitine-containing plants poisoning in recent 10 years. Lishizhen Med. Mater. Med. Res. 2012, 23, 3116–3118. [Google Scholar]
- Huang, W.Q.; Zeng, X.F.; Zhang, R.L.; Li, L.H.; Hong, S.J. Forensic analysis of deaths ascribed to acute aconitine poisoning in yunnan province: 4 cases report. J. Kunming Med. Univ. 2015, 36, 142–144. [Google Scholar]
- Sun, P.; Gao, X.K.; Zheng, X. Investigation report of food poisoning caused by eating pork containing atropine. Henan J. Prev. Med. 1998, 9, 315–316. [Google Scholar]
- Yan, Y.M.; Luo, C.H.; Fang, L.H.; Lei, X.L.; Liu, W.; Mo, Z.X. HPLC and UV determinations of hirsutine and total alkaloids in four species plants of Uncaria Genus in Guangdong province. Lishizhen Med. Mater. Med. Res. 2012, 23, 2974–2977. [Google Scholar]
- Mroczek, T.; Ndjoko-Ioset, K.; Głowniak, K.; Miętkiewicz-Capała, A.; Hostettmann, K. Investigation of Symphytum cordatum alkaloids by liquid–liquid partitioning, thin-layer chromatography and liquid chromatography–ion-trap mass spectrometry. Anal. Chim. Acta. 2006, 566, 157–166. [Google Scholar] [CrossRef]
- Beales, K.A.; Betteridge, K.; Colegate, S.M.; Edgar, J.A. Solid-phase extraction and LC-MS analysis of pyrrolizidine alkaloids in honeys. J. Agric. Food. Chem. 2004, 52, 6664–6672. [Google Scholar] [CrossRef]
- Beike, J.; Frommherz, L.; Wood, M.; Brinkmann, B.; Köhler, H. Determination of aconitine in body fluids by LC-MS-MS. Int. J. Legal. Med. 2004, 118, 289–293. [Google Scholar] [CrossRef]
- Bystrowska, B.; Adamczyk, P.; Moniczewski, A.; Zaniewska, M.; Fuxe, K.; Filip, M. LC/MS/MS evaluation of cocaine and its metabolites in different brain areas, peripheral organs and plasma in cocaine self-administering rats. Pharm. Rep. 2012, 64, 1337–1349. [Google Scholar] [CrossRef]
- Hamscher, G.; Priess, B.; Nau, H.; Panariti, E. Determination of colchicine residues in sheep serum and milk using high-performance liquid chromatography combined with electrospray ionization ion trap tandem mass spectrometry. Anal. Chem. 2005, 77, 2421–2425. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.M.; Xiang, B.; Xiao, X.Q.; Ge, J.W.; Chen, Z.; Yang, L.P.; Wei, F. Study on chemical constituents of lindera aggregate by GC-MS and UPLC-ESI-MS/MS. J. Chin. Med. Sci. 2016, 39, 2229–2236. [Google Scholar]
- Zhong, S.H.; Ren, X.X.; Huang, J.; Luan, Y.J.; Yu, Z.S. Detection of four foodborne toxic alkaloids in putrefied blood by QuEChERS-UPLC-MS/MS. Chem. Res. Appl. 2020, 32, 24–31. [Google Scholar]
- Newman, C.I.; Giordano, B.C.; Copper, C.L.; Collins, G.E. Microchip micellar electrokinetic chromatography separation of alkaloids with UV-absorbance spectral detection. Electrophoresis 2008, 29, 803–810. [Google Scholar] [CrossRef]
- Emon, J.M.V. Immunoassay and Other Bioanalytical Techniques; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Fodey, T.L.; Greer, N.M.; Crooks, S.R. Antibody production: Low dose immunogen vs. low incorporation hapten using salmeterol as a model. Anal. Chim. Acta 2009, 637, 328–332. [Google Scholar] [CrossRef]
- Tanaka, H.; Putalun, W.; Tsuzaki, C.; Shoyama, Y. A simple determination of steroidal alkaloid glycosides by thin-layer chromatography immunostaining using monoclonal antibody against solamargine. FEBS Lett. 1997, 404, 279–282. [Google Scholar] [CrossRef]
- Kido, K.; Edakuni, K.; Morinaga, O.; Tanaka, H.; Shoyama, Y. An enzyme-linked immunosorbent assay for aconitine-type alkaloids using an anti-aconitine monoclonal antibody. Anal. Chim. Acta. 2008, 616, 109–114. [Google Scholar] [CrossRef]
- Yan, L.; Nan, X.; Zhang, C.; Wang, H.; Huang, X.; Hu, J.; Liu, Y. Development of an enzyme-linked immunosorbent assay for camptothecin. Mol. Med. Rep. 2019, 20, 959–966. [Google Scholar] [CrossRef]
- Spector, S.; Parker, C.W. Morphine: Radioimmunoassay. Science 1970, 168, 1347–1348. [Google Scholar] [CrossRef]
- Zheng, P.M.; Peng, T.; Wang, J.Y.; Zhang, J.; Wang, Z.L.; Zhang, Y.F.; Ren, Z.H.; Wang, S.H.; Jiang, H.Y. Fluorescent lateral flow immunoassay based on gold nanocluster for detection of pyrrolizidine alkaloids. Microchim. Acta. 2021, 188, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, P.; Wang, J.; He, S.; Ren, Z.; Zhang, Y.; Xiong, J.; Jiang, H. Indirect competitive enzyme-linked immunosorbent assay based on a broad-spectrum monoclonal antibody for tropane alkaloids detection in pig urine, pork and cereal flours. Food Chem. 2021, 337, 127617. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhai, S.; Liu, C.; Wang, X.; Yang, Y.; Tu, Y. A convenient electrochemiluminescent immunosensor for detecting methamphetamine antibody. Anal. Sci. 2019, 35, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Gao, L.; Shu, M.L.; Liu, J.H.; Yu, B.Y. Development of a highly sensitive and specific ELISA method for the determination of l-corydalmine in SD rats with monoclonal antibody. J. Chromatogr. B 2018, 1073, 163–169. [Google Scholar] [CrossRef]
- Virtanen, R.; Kanto, J.; Iisalo, E. Radioimmunoassay for atropine and l-hyoscyamine. Acta Pharmacol. Toxicol. 1980, 47, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, M.; Wirsching, P.; Janda, K.D. Design and synthesis of a cocaine-diamide hapten for vaccine development. Tetrahedron Lett. 1996, 37, 5479–5482. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Irie, M.; Yoshimatsu, K.; Ishimaru, K.; Sawada, J. A monoclonal antibody to scopolamine and its use for competitive enzyme-linked immunosorbent assay. Phytochemistry 1991, 30, 3273–3276. [Google Scholar] [CrossRef]
- Sakamoto, S.; Nagamitsu, R.; Yusakul, G.; Miyamoto, T.; Tanaka, H.; Morimoto, S. Ultrasensitive immunoassay for monocrotaline using monoclonal antibody produced by N, N′-carbonyldiimidazole mediated hapten-carrier protein conjugates. Talanta 2017, 168, 67–72. [Google Scholar] [CrossRef]
- Dasgupta, A. Alcohol, Drugs, Genes and the Clinical Laboratory; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Gandhi, S.; Sharma, P.; Capalash, N.; Verma, R.S.; Suri, C.R. Group-selective antibodies based fluorescence immunoassay for monitoring opiate drugs. Anal. Bioanal. Chem. 2008, 392, 215–222. [Google Scholar] [CrossRef]
- Trofimov, A.V.; Sokolov, A.V.; Rak, A.Y.; Ischenko, A.M.; Kudling, T.V.; Vakhrushev, A.V.; Gorbunov, A.A. Epitope specificity of two anti-morphine monoclonal antibodies: In vitro and in silico studies. J. Mol. Recognit. 2020, 33, e2846. [Google Scholar] [CrossRef]
- Fliniaux, M.A.; Jacquin-Dubreuil, A. A competitive solid-phase enzyme immunoassay for the evaluation of tropane alkaloids in plant material, using anti-DL-tropic acid antibodies. Planta Med. 1987, 53, 87–90. [Google Scholar] [CrossRef]
- Gintzler, A.R.; Mohacsi, E.; Spector, S. Radioimmunoassay for the simultaneous determination of morphine and codeine. Eur. J. Pharmacol. 1976, 38, 149–156. [Google Scholar] [CrossRef]
- Haurowitz, F. The Chemistry and Function of Proteins; Academic Press: New York, NY, USA, 1963. [Google Scholar]
- Kashanian, S.; Shams, A.; Ghahremani, H.; Paknejad, M. Preparation and Characterization of a Monoclonal Antibody Against Morphine. Monoclon. Antibodies Immunodiagn. Immunother. 2015, 34, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Edmond Rouan, S.K.; Otterness, I.G.; Cunningham, A.C.; Rhodes, C.T. Specific, high affinity colchicine binding monoclonal antibodies: Development and characterization of the antibodies. Hybridoma 1989, 8, 435–448. [Google Scholar] [CrossRef]
- Sabouraud, A.; Cano, N.; Scherrmann, J.M. Radioimmunoassay of colchicine with antisera exhibiting variable cross-reactivity. Ther. Drug Monit. 1994, 16, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, K.; Ji, L.Y.; Takatori, T.; Aoki, K.; Hirose, Y.; Kuroiwa, Y. Development of monoclonal antibodies reactive with methamphetamine raised against a new antigen. J. Immunoass. 1991, 12, 277–292. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.B.; Zhong, P.; Nie, J.L.; Li, J.S.; Qu, L.N.; Li, Y.H.; Kan, G.H. Preparation and identification of specific and high-affinity monoclonal antibodies against morphine. Hybrid. Hybridomics 2002, 21, 197–201. [Google Scholar] [CrossRef]
- Zhao, H. The preparation of aconitine N-hydroxysuccinimide esters and their reactivities with bovine serum albumin. J. Shenyang Pharm. Univ. 2000, 17, 26–31. [Google Scholar]
- Yusakul, G.; Sakamoto, S.; Chanpokapaiboon, K.; Tanaka, H.; Morimoto, S. Preincubation format for a sensitive immunochromatographic assay for monocrotaline, a toxic pyrrolizidine alkaloid. Phytochem. Anal. 2019, 30, 653–660. [Google Scholar] [CrossRef]
- Roseman, D.M.; Wu, X.; Kurth, M.J. Enzyme-linked immunosorbent assay detection of pyrrolizidine alkaloids: Immunogens based on quaternary pyrrolizidinium salts. Bioconjug. Chem. 1996, 7, 187–195. [Google Scholar] [CrossRef]
- Putalun, W.; Tanaka, H.; Yahara, S.; Lhieochaiphan, S.; Shoyama, Y. Survey of solasodine-type glycoalkaloids by western blotting and ELISA using anti-solamargine monoclonal antibody. Biol. Pharm. Bull. 2000, 23, 72–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dillon, P.P.; Daly, S.J.; Manning, B.M.; O’Kennedy, R. Immunoassay for the determination of morphine-3-glucuronide using a surface plasmon resonance-based biosensor. Biosens. Bioelectron. 2003, 18, 217–227. [Google Scholar] [CrossRef]
- Yang, T.B.; Yuan, Y.H.; Zhong, P.; Qu, L.N.; Yang, B.; Li, Y.H.; Ju, G. Group-selective immunoassay for the detection of morphine in urine. Hybrid. Hybridomics 2004, 23, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, K.; Piek, K.; Stöckigt, J.; Weiler, E.W. Monoclonal antibody-based enzyme immunoassay for the quantitative determination of the tropane alkaloid, scopolamine. Planta Med. 1992, 58, 68–72. [Google Scholar] [CrossRef]
- Pontikis, R.; Scherrmann, J.M.; Nguyen, H.N.; Boudet, L.; Pichat, L. Radioimmunoassay for colchicine: Synthesis and properties of three haptens. J. Immunoass. 1980, 1, 449–461. [Google Scholar] [CrossRef]
- Wainer, B.H.; Fitch, F.W.; Rothberg, R.M.; Fried, J. Morphine-3-succinyl—Bovine serum albumin: An immunogenic hapten-protein conjugate. Science 1972, 176, 1143–1145. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, L.; Yuan, S.; Xu, Y.; Hua, M. Preparation of Immunogen and Polyclonal Antibodies against Aconitine. Chin. J. Pharm. 2015, 46, 578–580. [Google Scholar]
- Cibotti, M.C.; Freier, C.; Andrieux, J.; Plat, M.; Cosson, L.; Bohuon, C. Monoclonal antibodies to bis-indole alkaloids of Catharanthus roseus and their use in enzyme-linked immuno-sorbent-assays. Phytochemistry 1990, 29, 2109–2114. [Google Scholar] [CrossRef]
- Chen, P.; Watt, D.S.; Tai, H.H. Improved sensitivity of enzyme immunoassay for cocaine and benzoylecgonine using heterologous hapten-enzyme conjugates. Res. Commun. Alcohol Subst. Abuse. 1994, 15, 71–82. [Google Scholar]
- Stammel, W.; Müller, R.; Thomas, H. Demonstration of highly specific and sensitive antibodies to a naturally occurring tetrahydroisoquinoline alkaloid, salsolidine. Int. J. Biochem. 1993, 25, 917–927. [Google Scholar] [CrossRef]
- Ishiyama, M.; Shoyama, Y.; Murakami, H.; Shinohara, H. Production of monoclonal antibodies and development of an ELISA for solamargine. Cytotechnology 1996, 18, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Lapinjoki, S.P.; Veräjänkorva, H.M.; Huhtikangas, A.E.; Lehtola, T.J.; Lounasmaa, M. An enzyme-linked immunosorbent assay for the antineoplastic agent vincristine. J. Immunoass. 1986, 7, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Teale, J.D.; Clough, J.M.; Marks, V. Radioimmunoassay of vinblastine and vincristine. Br. J. Clin. Pharmacol. 1977, 4, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Tanaka, H.; Shoyama, Y. Immunoquantitative analysis for berberine and its related compounds using monoclonal antibodies in herbal medicines. Analyst 2004, 129, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.G.; Wang, X.X.; Wang, W.W.; Sun, M.Z.; Choi, W.J.; Kim, J.; Hao, C.L.; Li, S.; Qu, A.H.; Lu, M.R.; et al. Enantiomer-dependent immunological response to chiral nanoparticles. Nature 2022, 601, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xu, Y.; Yuan, S.; Xu, Y.; Hua, M. An enzyme-linked immunosorbent assay for monoester-type aconitic alkaloids and its application in the pharmacokinetic study of benzoylhypaconine in rats. J. Asian Nat. Prod. Res. 2018, 20, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Jing, L.; Zuo, W.; Wang, J.; Wang, Y. Preparation and Identification of the Complete Antigen of Double Ester Aconitine. J. Henan Agric. Sci. 2014, 43, 156–159. [Google Scholar]
- Abdelshafi, N.A.; Panne, U.; Schneider, R.J. Screening for cocaine on Euro banknotes by a highly sensitive enzyme immunoassay. Talanta 2017, 165, 619–624. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Inoue, H.; Chikuma, T.; Itoh, J.; Makino, Y.; Hojo, H. A rapid enzyme immunoassay for cocaine and benzoylecgonine using glucose oxidase. J. Health Sci. 2001, 47, 419–423. [Google Scholar] [CrossRef][Green Version]
- Nuntawong, P.; Tanaka, H.; Sakamoto, S.; Morimoto, S. ELISA for the Detection of the Prohibited Doping Agent Higenamine. Planta Med. 2020, 86, 760–766. [Google Scholar] [CrossRef]
- Wurzburger, R.J.; Miller, R.L.; Boxenbaum, H.G.; Spector, S. Radioimmunoassay of atropine in plasma. J. Pharmacol. Exp. Ther. 1977, 203, 435–441. [Google Scholar] [PubMed]
- Poulev, A.; Deusneumann, B.; Bombardelli, E.; Zenk, M.H. Immunoassays for the Quantitative-Determination of Colchicine. Planta Med. 1994, 60, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Torres, O.B.; Jalah, R.; Rice, K.C.; Li, F.; Antoline, J.F.; Iyer, M.R.; Jacobson, A.E.; Boutaghou, M.N.; Alving, C.R.; Matyas, G.R. Characterization and optimization of heroin hapten-BSA conjugates: Method development for the synthesis of reproducible hapten-based vaccines. Anal. Bioanal. Chem. 2014, 406, 5927–5937. [Google Scholar] [CrossRef] [PubMed]
- Brennan, J.; Dillon, P.; O’Kennedy, R. Production, purification and characterisation of genetically derived scFv and bifunctional antibody fragments capable of detecting illicit drug residues. J. Chromatogr. B 2003, 786, 327–342. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Mehraby, M.; Zarbakhsh, M.; Farzaneh, H. Design and synthesis of a morphine-6-succinyl-bovine serum albumin hapten for vaccine development. Biotechnol. Appl. Biochem. 1999, 30, 139–146. [Google Scholar]
- Usagawa, T.; Itoh, Y.; Hifumi, E.; Takeyasu, A.; Nakahara, Y.; Uda, T. Characterization of morphine-specific monoclonal antibodies showing minimal cross-reactivity with codeine. J. Immunol. Methods 1993, 157, 143–148. [Google Scholar] [CrossRef]
- Langer, T.; Mostl, E.; Chizzola, R.; Gutleb, R. A competitive enzyme immunoassay for the pyrrolizidine alkaloids of the senecionine type. Planta Med. 1996, 62, 267–271. [Google Scholar] [CrossRef]
- Sethi, V.S.; Burton, S.S.; Jackson, D.V. A sensitive radioimmunoassay for vincristine and vinblastine. Cancer Chemother. Pharmacol. 1980, 4, 183–187. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Kuang, H.; Liu, L. Development of an ic-ELISA and an immunochromatographic strip assay for the detection of aconitine. Food Agric. Immunol. 2020, 31, 243–254. [Google Scholar] [CrossRef]
- Nakayama, H.; Kenjjou, N.; Shigetoh, N.; Ito, Y. Fluorescence Immunoassay for Cocaine Detection. Monoclon. Antibodies Immunodiagn. Immunother. 2016, 35, 83–85. [Google Scholar] [CrossRef]
- Susan, V.D.H.; Calavia, P.G.; Hardwick, S.; Hudson, S.; Wolff, K.; Russell, D.A. A competitive enzyme immunoassay for the quantitative detection of cocaine from banknotes and latent fingermarks. Forensic Sci. Int. 2015, 250, 1–7. [Google Scholar]
- Wenger, B.; Kugelbrey, K.; Gao, H.; Sigrist, H.; Voirin, G. Au-labeled antibodies to enhance the sensitivity of a refractometric immunoassay: Detection of cocaine. Biosens. Bioelectron. 2012, 34, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.G.; Eremenko, A.V.; Kühn, A.; Kürzinger, K.; Makower, A.; Scheller, F.W. Automated amplified flow immunoassay for cocaine. Anal. Chem. 1998, 70, 4624–4630. [Google Scholar] [CrossRef] [PubMed]
- Elmar, W.W.; Stöckigt, J.; Zenk, M.H. Radioimmunoassay for the quantitative determination of scopolamine. Phytochemistry 1981, 20, 2009–2016. [Google Scholar]
- Zvereva, E.A.; Zherdev, A.V.; Formanovsky, A.A.; Abuknesha, R.A.; Eremin, S.A.; Dzantiev, B.B. Fluorescence polarization immunoassay of colchicine. J. Pharm. Biomed. Anal. 2018, 159, 326–330. [Google Scholar] [CrossRef]
- Liu, M.; Lu, J.R.; He, Y.H.; Du, J.X. Molecular imprinting–chemiluminescence sensor for the determination of brucine. Anal. Chim. Acta. 2005, 541, 97–102. [Google Scholar] [CrossRef]
- Guteneva, N.V.; Znoyko, S.L.; Orlov, A.V.; Nikitin, M.P.; Nikitin, P.I. Rapid lateral flow assays based on the quantification of magnetic nanoparticle labels for multiplexed immunodetection of small molecules: Application to the determination of drugs of abuse. Mikrochim. Acta 2019, 186, 621. [Google Scholar] [CrossRef]
- Gandhi, S.; Caplash, N.; Sharma, P.; Raman Suri, C. Strip-based immunochromatographic assay using specific egg yolk antibodies for rapid detection of morphine in urine samples. Biosens. Bioelectron. 2009, 25, 502–505. [Google Scholar] [CrossRef]
- Chapman, D.J.; Joel, S.P.; Aherne, G.W. Evaluation of a differential radioimmunoassay technique for the determination of morphine and morphine-6-glucuronide in human plasma. J. Pharm. Biomed. Anal. 1994, 12, 353–360. [Google Scholar] [CrossRef]
- Duan, Y.; Luo, J.; Liu, C.; Shan, L.; Dou, X.; Yang, S.; Yang, M. Rapid identification of triptolide in Tripterygium wilfordii products by gold immunochromatographic assay. J. Pharm. Biomed. Anal. 2019, 168, 102–112. [Google Scholar] [CrossRef]
- Weiler, E.W.; Kruger, H.; Zenk, M.H. Use of immunoassay in plant sciences.13. Radioimmunoassay for the determination of the steroidal alkaloid solasodine and related-compounds in living plants and Herbarium specimens. Planta Med. 1980, 39, 112–124. [Google Scholar] [CrossRef]
- Mule, S.J.; Jukofsky, D.; Kogan, M.; Depace, A.; Verebey, K. Evaluation of radioimmunoassay for benzoylecgonine (a cocaine metabolite) in human urine. Clin. Chem. 1977, 23, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Li, R.F.; Hou, Y.L.; Huang, J.C.; Pan, W.Q.; Ma, Q.H.; Shi, Y.X.; Li, C.F.; Zhao, J.; Jia, Z.H.; Jiang, H.M.; et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2). Pharmacol. Res. 2020, 156, 104761. [Google Scholar]
- Li, H.; Ma, S.; Zhang, X.; Li, C.; Dong, B.; Mujtaba, M.G.; Wei, Y.; Liang, X.; Yu, X.; Wen, K.; et al. Generic hapten synthesis, broad-specificity monoclonal antibodies preparation, and ultrasensitive ELISA for five antibacterial synergists in chicken and milk. J. Agric. Food Chem. 2018, 66, 11170–11179. [Google Scholar] [CrossRef]
- Wen, K.; Bai, Y.; Wei, Y.; Li, C.; Shen, J.; Wang, Z. Influence of Small Molecular Property on Antibody Response. J. Agric. Food Chem. 2020, 68, 10944–10950. [Google Scholar] [CrossRef]
- Yuan, M.; Liu, B.; Liu, E.; Sheng, W.; Zhang, Y.; Crossan, A.; Kennedy, I.; Wang, S. Immunoassay for phenylurea herbicides: Application of molecular modeling and quantitative structure-activity relationship analysis on an antigen-antibody interaction study. Anal. Chem. 2011, 83, 4767–4774. [Google Scholar] [CrossRef]
- Wang, Z.H.; Ding, S.Y.; Zhang, S.X.; Shen, J.Z. Structure-activity relationship of 17 sulfonamides binding to antibody by molecular modeling technique. Acta Chim. Sin. 2008, 66, 2613–2619. [Google Scholar]
- Goryacheva, I.Y.; Lenain, P.; Saeger, S.D. Nanosized labels for rapid immunotests. TrAC—Trend. Anal. Chem. 2013, 46, 30–43. [Google Scholar] [CrossRef]
- Peng, T.; Wang, J.; Zhao, S.; Xie, S.; Yao, K.; Zheng, P.; Wang, S.; Ke, Y.; Jiang, H. A fluorometric clenbuterol immunoassay based on the use of organic/inorganic hybrid nanoflowers modified with gold nanoclusters and artificial antigen. Microchim. Acta. 2018, 185, 366. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).