Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations
Abstract
1. Introduction
2. Results
2.1. First Urinary ZEN Screening on Four JB Breeding Cattle Herds in the Neighborhood
2.2. Follow-up Monthly Monitoring in ZEN Detected JB Breeding Cattle Herd
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
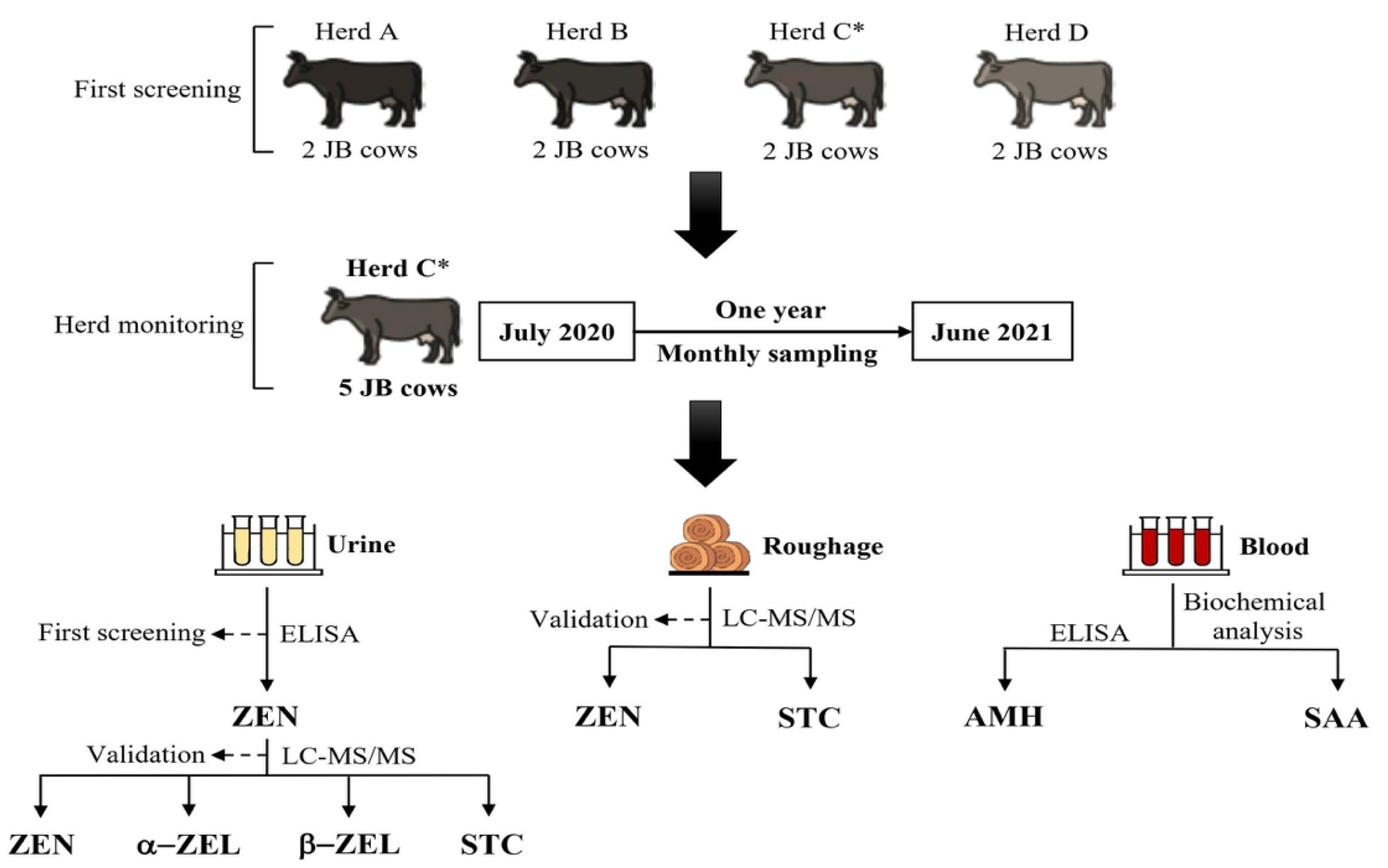
4.2. Screening by Urinary ZEN Monitoring to Detect Cattle Herds Fed with Dietary Roughage with Elevated ZEN Contamination
4.3. Follow-up Monthly Monitoring on the Breeding Cattle Herd with Known Feed Contamination
4.4. Reproductive Records
4.5. Analytical Methods of ZEN in Urine and Feed Samples
4.6. Analytical Methods of AMH and SAA in Serum Samples
4.7. Data Management and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liew, W.P.P.; Mohd-Redzwan, S. Mycotoxin: Its impact on gut health and microbiota. Front. Cell Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef]
- Vandicke, J.; De Visschere, K.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Mycotoxins in Flanders fields: Occurrence and correlations with Fusarium species in whole-plant harvested maize. Microorganism 2019, 7, 571. [Google Scholar] [CrossRef]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of mycotoxins through biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed]
- Raduly, Z.; Szabo, L.; Madar, A.; Pocsi, I.; Csernoch, L. Toxicological and medical aspects of aspergillus-derived mycotoxins entering the feed and food chain. Fronit. Microbiol. 2020, 10, 2908. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in livestock and poultry: Does, toxicokinetics, toxicity and estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Prelusky, D.B.; Warner, R.M.; Trenholm, H.L. Sensitive analysis of the mycotoxin zearalenone and its metabolites in biological fluids by high-performance liquid chromatography. J. Chromatogr. 1989, 494, 267–277. [Google Scholar] [CrossRef]
- Usleber, A.; Renz, V.; Martlbauer, E.; Terplan, G. Studies on the application of enzyme immunoassays for the Fusarium mycotoxins deoxynivalenol, 3-acetyldeoxynivalenol, and zearalenone. J. Vet. Med. B. 1992, 39, 617–627. [Google Scholar] [CrossRef]
- Kleinova, M.; ZELlner, P.; Kahlbacher, H.; Hochsteiner, W.; Lindner, W. Metabolic profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine, liver, and muscle of heifers. J. Agric. Food. Chem. 2002, 50, 4769–4776. [Google Scholar] [CrossRef]
- Takagi, M.; Uno, S.; Kokushi, E.; Shiga, S.; Mukai, S.; Kuriyagawa, T.; Takagaki, K.; Hasunuma, H.; Matsumoto, D.; Okamoto, K.; et al. Measurement of urinary zearalenone concentrations for monitoring natural feed contamination in cattle herds-on farm trials. J. Anim. Sci. 2011, 89, 287–296. [Google Scholar] [CrossRef]
- Hasunuma, H.; Takagi, M.; Kawamura, O.; Taniguchi, C.; Nakamura, M.; Chuma, T.; Uno, S.; Kokushi, E.; Matsumoto, D.; Tshering, C.; et al. Natural contamination of dietary rice straw with zearalenone and urinary zearalenone concentrations in a cattle herd. J. Anim. Sci. 2012, 90, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, Y.; Takagi, M.; Uno, S.; Kokushi, E.; Nakamura, M.; Hasunuma, H.; Shinya, U.; Duguchi, E.; Fink-Gremmels, J. Measurement of sterigmatocystin concentrations in urine for monitoring the contamination of cattle feed. Toxins 2014, 6, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Toda, K.; Uno, S.; Kokushi, E.; Shiiba, A.; Hasunuma, H.; Matsumoto, D.; Ohtani, M.; Yamato, O.; Shinya, U.; Wijayagunawardane, M.; et al. Fructo-oligosaccharide (DFA III) feed supplementation for mitigation of mycotoxin exposure in cattle—clinical evaluation by a urinary zearalenone monitoring system. Toxins 2018, 10, 223. [Google Scholar] [CrossRef]
- Sasazaki, N.; Uno, S.; Kokushi, E.; Toda, K.; Hasunuma, H.; Matsumoto, D.; Miyashita, A.; Yamato, O.; Okawa, H.; Ohtani, M.; et al. Mitigation of sterigmatocystin exposure in cattle by difructose anhydride III feed supplementation and detection of urinary sterigmatocystin and serum amyloid A concentrations. Arch. Anim. Breed. 2021, 64, 257–264. [Google Scholar] [CrossRef]
- Monniaux, D.; di Clemente, N.; Touzé, J.L.; Belville, C.; Rico, C.; Bontoux, M.; Picard, J.Y.; Fabre, S. Intrafollicular steroids and anti-mullerian hormone during normal and cystic ovarian follicular development in the cow. Biol. Reprod. 2008, 79, 387–396. [Google Scholar] [CrossRef]
- Monniaux, D.; Drouilhet, L.; Rico, C.; Estienne, A.; Jarrier, P.; Touze, J.L.; Sapa, J.; Phocas, F.; Dupont, J.; Dalbies-Tran, R.; et al. Regulation of anti-Mullerian hormone production in domestic animals. Reprod. Fertil. Dev. 2013, 25, 1–16. [Google Scholar] [CrossRef]
- Berg, L.C.; Thomsen, P.D.; Andersen, P.H.; Jensen, H.E.; Jacobsen, S. Serum amyloid A is expressed in histologically normal tissues from horses and cattle. Vet. Immunol. Immunopathol. 2011, 144, 155–159. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, F.; Oguejiofor, C.F.; Wang, D.; Dong, S.; Yan, Z. Endometrial expression of the acute phase molecule SAA is more significant than HP in reflecting the severity of endometritis. Res. Vet. Sci. 2018, 121, 130–133. [Google Scholar] [CrossRef]
- Okawa, H.; Monniaux, D.; Mizokami, C.; Fujikura, A.; Takano, T.; Sato, S.; Shinya, U.; Kawashima, C.; Yamato, O.; Fushimi, Y.; et al. Association between anti-Müllerian hormone concentration and inflammation markers in serum during the peripartum period in dairy cows. Animals 2021, 11, 1241. [Google Scholar] [CrossRef]
- Reisinger, N.; Schurer-Waldheim, S.; Mayer, E.; Debevere, S.; Antonissen, G.; Sulyok, M.; Nagl, V. Mycotoxin occurrence in maize silage-A neglected risk for bovine gut health? Toxins 2019, 11, 577. [Google Scholar] [CrossRef]
- Kinkade, C.W.; Rivera-Nunez, Z.; Gorcyzca, L.; Aleksunes, L.M. Impact of Fusarium-derived mycoestrogens on female reproduction: A systematic review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, H.J. Development of an immunoaffinity chromatography and LC-MS/MS method for the determination of 6 zearalenones in animal feed. PLoS ONE 2018, 5, e0193584. [Google Scholar] [CrossRef] [PubMed]
- Panasiuk, L.; Jedziniak, P.; Pietruazka, K.; Piatkowska, M.; Bocian, L. Frequency and levels of regulated and emerging mycotoxins in silage in Poland. Mycotoxin Res. 2019, 35, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Nualkaw, K.; Poapolathep, S.; Zhang, Z.; Zhang, Q.; Giorgi, M.; Li, P.; Logrieco, F.; Poapolathep, A. Simultaneous determination of multiple mycotoxins in swine, poultry and dairy feeds using ultra high-performance liquid chromatography-tandem mass spectrometry. Toxins 2020, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Guerre, P. Mycotoxin and gut microbiota interactions. Toxins 2020, 12, 769. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Minervini, F.; Giannoccaro, A.; Fornelli, F.; Dell’ Aquila, M.E.; Minoia, P.; Visconti, A. Influence of mycotoxin zearalenone and its derivatives (alpha and beta zearalenol) on apoptosis and proliferation of cultured granulosa cells from equine ovaries. Reprod. Biol. Endocrinol. 2006, 4, 62. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, H.; Guo, C.; Lu, Y.; Deng, S.; Yang, Y.; Wei, Q.; Wen, L.; He, Z. Zearalenone induces apoptosis and necrosis in porcine granulosa cells via a caspase-3- and caspase-9-dependent mitochondrial signaling pathway. J. Cell Physiol. 2012, 227, 1814–1820. [Google Scholar] [CrossRef]
- Zhang, G.L.; Feng, Y.L.; Song, J.L.; Zhou, X.S. Zearalenone: A mycotoxin with different toxic effect in domestic and laboratory animals’ granulosa cells. Front. Genet. 2018, 9, 667. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Li, C.; Yang, F.; Wang, G. Understanding the toxin effects of β-zearalenol and HT-2 on bovine granulosa cells using iTRAQ-based proteomics. Animals 2020, 10, 130. [Google Scholar] [CrossRef]
- Pistol, G.C.; Gras, M.A.; Marin, D.E.; Israel-Roming, F.; Stancu, M.; Taranu, I. Natural feed contaminant zearalenone decreases the expressions of important pro- and anti-inflammatory mediators and mitogen-activated protein kinase/NF-kB signaling molecules in pigs. Br. J. Nutr. 2014, 111, 452–464. [Google Scholar] [CrossRef]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the immune response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Lee, P.-Y.; Liu, C.-C.; Wang, S.-C.; Chen, K.-Y.; Lin, T.-C.; Liu, P.-L.; Chiu, C.-C.; Chen, I.-C.; Lai, Y.-H.; Cheng, W.-C. Mycotoxin zearalenone attenuates innate immune responses and suppresses NLRP3 inflammasome activation in LPS-activated macrophages. Toxins 2021, 13, 593. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Kubosaki, A.; Takahashi, Y.; Yanai, M.; Konuma, R.; Uehara, S.; Chiba, T.; Watanabe, M.; Terajima, J.; Sugita-Konishi, Y. Distribution of sterigmatocystin-producing aspergilli in Japan. Food Safety 2018, 6, 67–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, M.; Aoyama, K.; Ishibashi, T. Sterigmatocystin and aflatoxin B1 contamination of corn, soybean meal, and formula feed in Japan. Mycotoxin Res. 2018, 34, 21–27. [Google Scholar] [CrossRef]
- Kobayashi, N.; Sakurai, K.; Nakarai, R.; Shigaki, K.; Horikawa, K.; Honda, M.; Sugiura, Y.; Watanabe, M.; Takino, M.; Sugita-Konishi, Y. Microflora of mycotoxigenic fungi in rice grains in Kyushu region of Japan and their changes during storage under non-controlled conditions. Biocont. Sci. 2019, 24, 161–166. [Google Scholar] [CrossRef]
- Yoshinari, T.; Takeuchi, H.; Kosugi, M.; Taniguchi, M.; Waki, M.; Hashiguchi, S.; Fujiyoshi, T.; Shichinohe, Y.; Nakajima, M.; Ohnishi, T.; et al. Determination of sterigmatocystin in foods in Japan: Method validation and occurrence data. Food Addit. Contam. Part A 2019, 36, 1404–1410. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 37, 326–341. [Google Scholar] [CrossRef]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2584. [Google Scholar] [CrossRef]
- Weaver, H.A.; Kurtz, H.T.; Behrens, J.C.; Robinson, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, J.C. Effect of zearalenone of dairy cows. Am. J. Vet. Res. 1986, 47, 659–662. [Google Scholar]
- Weaver, H.A.; Kurtz, H.T.; Behrens, J.C.; Robinson, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, J.C. Effects of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 1986, 47, 1395–1397. [Google Scholar] [PubMed]
- Fink-Gremmels, J. The role of mycotoxins in the health and performance of dairy cows. Vet. J. 2008, 176, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Mukai, S.; Kuriyagawa, T.; Takagaki, K.; Uno, S.; Kokushi, E.; Otoi, T.; Budiyanto, A.; Shirasuna, K.; Miyamoto, A.; et al. Detection of zearalenone and its metabolites in naturally contaminated follicular fluids by using LC/MS/MS and in vitro effects of zearalenone on oocyte maturation in cattle. Reprod. Toxicol. 2008, 26, 164–169. [Google Scholar] [CrossRef]
- Takagi, M.; Hirai, T.; Shiga, S.; Uno, S.; Kokushi, E.; Otoi, T.; Deguchi, E.; Tshering, C.; Fink-Gremmels, J. Relationship between urinary zearalenone concentration and embryo production in superovulated cattle. Arch. Anim. Breed. 2013, 36, 360–366. [Google Scholar] [CrossRef]
- FAMIC (Food and Agricultural Materials Inspection Center, Japan). Shiryobunsekikijun. FAMIC, Saitama. Available online: http://www.famic.go.jp/ffis/feed/bunseki/bunsekikijun.html (accessed on 20 April 2012). (In Japanese).
- Fushimi, Y.; Monniaux, D.; Takagi, M. Efficacy of a single measurement of plasma anti-Muüllerian hormone concentration for ovum pick-up donor selection of Japanese Black heifers in herd breeding programs. J. Reprod. Dev. 2019, 65, 369–374. [Google Scholar] [CrossRef]
 : calving, (f) total; mean urinary ZEN concentration and AMH from five cows, (g) monthly changes of urinary ZEN, its metabolites, and STC concentrations measured by LC-MS/MS, (h) monthly changes of ZEN and STC concentrations in the dietary roughage measured by LC-MS/MS.
: calving, (f) total; mean urinary ZEN concentration and AMH from five cows, (g) monthly changes of urinary ZEN, its metabolites, and STC concentrations measured by LC-MS/MS, (h) monthly changes of ZEN and STC concentrations in the dietary roughage measured by LC-MS/MS.
 : calving, (f) total; mean urinary ZEN concentration and AMH from five cows, (g) monthly changes of urinary ZEN, its metabolites, and STC concentrations measured by LC-MS/MS, (h) monthly changes of ZEN and STC concentrations in the dietary roughage measured by LC-MS/MS.
: calving, (f) total; mean urinary ZEN concentration and AMH from five cows, (g) monthly changes of urinary ZEN, its metabolites, and STC concentrations measured by LC-MS/MS, (h) monthly changes of ZEN and STC concentrations in the dietary roughage measured by LC-MS/MS.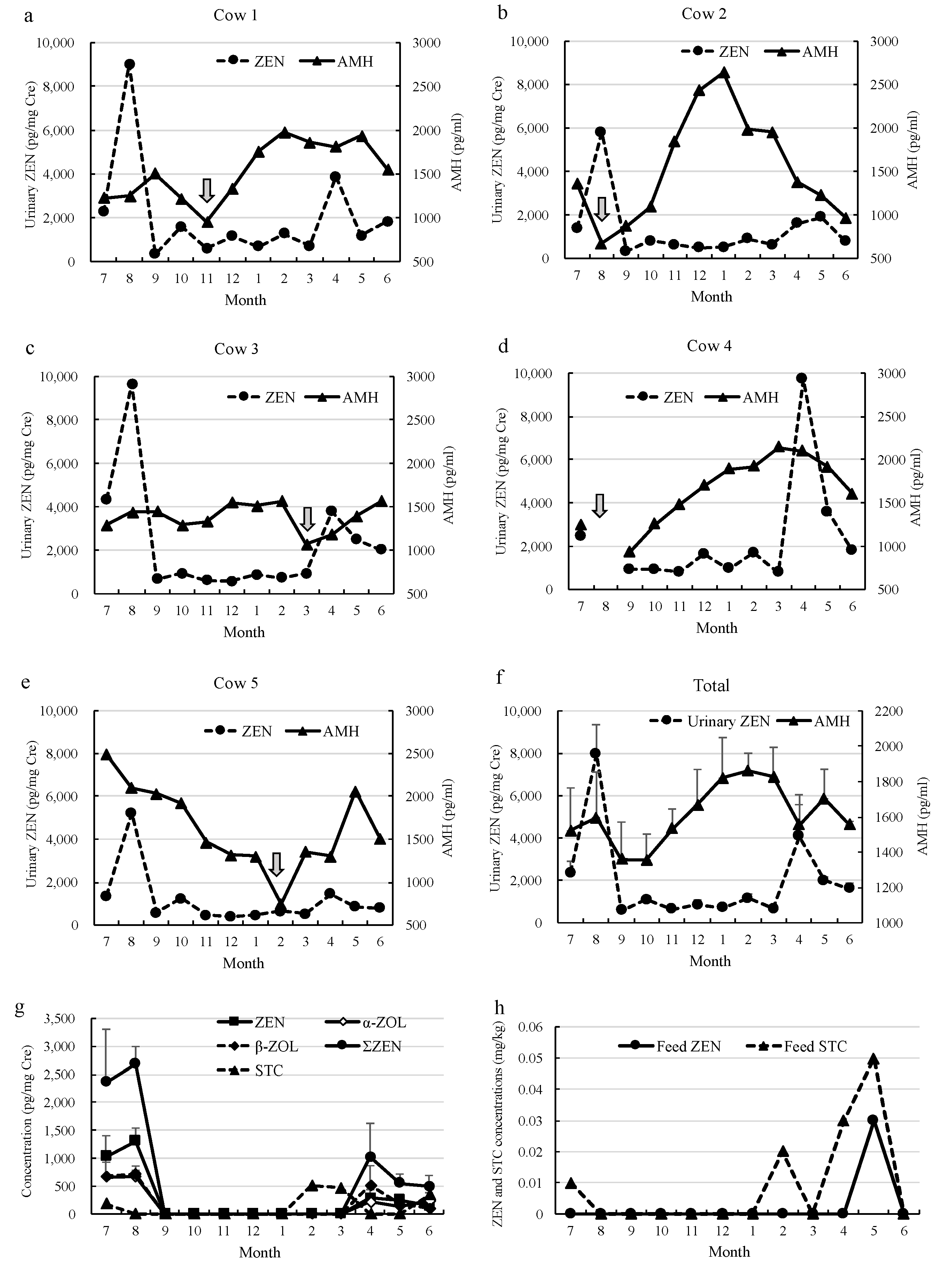
| ELISA | LC-MS/MS | LC-MS/MS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cow | Urinary ZEN Concentrations (pg/mL) | ZEN/Cre | ZEN/Cre | α-ZEL/Cre | β-ZEL/Cre | ΣZEN/Cre | STC/Cre | ZEN in Roughage (mg/kg) | STC in Roughage (mg/kg) |
| A1 | 2132.6 | 3280.9 | ND | ND | ND | ND | ND | ND | ND |
| A2 | 1637.6 | 930.5 | ND | ND | ND | ND | ND | ||
| B1 | 1937.9 | 983.7 | ND | ND | ND | ND | ND | ND | <0.04 ** |
| B2 | 1528.5 | 979.8 | ND | ND | ND | ND | ND | ||
| C1 | >20,250 | >23,011.4 * | 14,363.6 | 10,772.7 | 16,454.5 | 41,590.9 | 659.1 | 1.34 | 0.08 |
| C2 | >20,250 | >21,315.8 * | 11,915.8 | 8526.3 | 4736.8 | 25,178.9 | 442.1 | ||
| D1 | 1931.2 | 3862.4 | ND | ND | ND | ND | ND | ND | ND |
| D2 | 985.2 | 1669.8 | ND | ND | ND | ND | ND | ||
| ZEN | AMH | SAA | ||||
|---|---|---|---|---|---|---|
| Date | Geometric Mean | 95% CI | Arithmetic Mean | 95% CI | Geometric Mean | 95% CI |
| 2020/7 | 2142.2 | 1373.8–3340.4 | 1521.6 | 1165.7–1877.5 | 2.8 | 1.5–4.9 |
| 2020/8 | 8056.5 | 4853.3–13373.8 | 1594.5 | 1135.0–2053.9 | 2.5 | 1.2–5.2 |
| 2020/9 | 521.1 | 334.2–812.6 | 1358.0 | 1002.1–1713.9 | 2.9 | 1.6–5.1 |
| 2020/10 | 1065.7 | 683.4–1661.7 | 1356.6 | 1000.7–1712.5 | 4.0 | 2.2–7.1 |
| 2020/11 | 627.1 | 392.9–1000.9 | 1532.9 | 1134.9–1930.8 | 3.4 | 1.8–6.4 |
| 2020/12 | 720.8 | 462.2–1123.9 | 1665.8 | 1309.9–2021.7 | 3.1 | 1.7–5.4 |
| 2021/1 | 676.9 | 434.1–1055.4 | 1820.8 | 1464.9–2176.7 | 4.9 | 2.8–8.7 |
| 2021/2 | 995.8 | 623.9–1589.5 | 1860.0 | 1462.1–2257.9 | 3.1 | 1.6–5.8 |
| 2021/3 | 669.9 | 419.7–1069.2 | 1828.1 | 1430.1–2226.0 | 5.2 | 2.8–9.8 |
| 2021/4 | 3194.8 | 2048.8–4981.8 | 1553.8 | 1197.9–1909.7 | 3.3 | 1.9–5.9 |
| 2021/5 | 1763.7 | 1131.0–2750.2 | 1704.4 | 1348.5–2060.3 | 3.0 | 1.7–5.3 |
| 2021/6 | 1414.2 | 885.9–2257.7 | 1554.7 | 1156.8–1952.6 | 2.2 | 1.2–4.2 |
| AMH | |||||
|---|---|---|---|---|---|
| β | 95% CI | p-Value | |||
| Simple correlation | |||||
| ZEN | −0.085 | −0.787 | – | 0.617 | 0.793 |
| Time-lagged correlation | |||||
| ZEN (lag 1 month) | −0.449 | −1.112 | – | 0.214 | 0.160 |
| AMH Change over one Month | |||||
|---|---|---|---|---|---|
| β | 95% CI | p-Value | |||
| Time-lagged correlation | |||||
| ZEN (lag 0) | −0.024 | −0.744 | – | 0.695 | 0.941 |
| ZEN (lag 1 month) | −0.377 | −1.039 | – | 0.285 | 0.230 |
| SAA | |||||
|---|---|---|---|---|---|
| β | 95% CI | p-Value | |||
| Simple regression | |||||
| ZEN | −0.400 | −1.046 | – | 0.246 | 0.198 |
| Time-lagged regression | |||||
| ZEN (lag 1 month) | −0.333 | −1.029 | – | 0.364 | 0.308 |
| SAA Change over one Month | |||||
|---|---|---|---|---|---|
| β | 95% CI | p-Value | |||
| Time-lagged regress | |||||
| ZEN (lag 0) | −0.245 | −0.941 | – | 0.450 | 0.446 |
| ZEN (lag 1 month) | 0.065 | −0.654 | – | 0.784 | 0.843 |
| Birthday | 2017 (Pre) | 2018 (Pre) | 2019 (Pre) * | 2020 (Post) ** | 2021 (Post) | |
|---|---|---|---|---|---|---|
| Cow 1 | 9 January 2016 | - | 351 | 335 | 349 | 333 |
| Cow 2 | 8 November 2014 | 690 | - | 380 | 321 | 349 |
| Cow 3 | 7 April 2014 | 346 | 392 | 437 | 334 | 346 |
| Cow 4 | 15 July 2015 | - | 600 | - | 377 | 355 |
| Cow 5 | 27 December 2016 | - | - | 420 | - | 385 |
| Mean of 5 cows | 518.0 ± 172.0 | 447.7 ± 77.1 | 393.0 ± 22.7 | 345.3 ± 12.0 | 353.6 ± 8.6 | |
| Mean of the pre- and post- monitorin | 439.0 ± 41.2 a (n = 9) | 349.9 ± 6.9 b (n = 9) | ||||
| Herd | Date of Sample Collection | Forage Feeds/Day | Formula Feeds/Day |
|---|---|---|---|
| A (n = 2) (Both 12 y) * | 10 July 2019 | Home-grown rice straw 2 kg, Home-grown WCS (rice) 6 kg, Home-grown Italian ryegrass 4 kg Total: 12 kg | Commercially available concentrates 4 kg |
| B (n = 2) (3 y and 5 y) | 24 June 2019 | Home-grown rice straw 10 kg, Mixed of Italian ryegrass and Orchard grass 10 kg Total: 20 kg | Commercially available concentrates 1 kg, Wheat bran 1 kg, Maize 1 kg |
| C (n = 2) (8 y and 10 y) | 19 August 2019 | Home-grown rice straw 12~14 kg, Orchard grass 10 kg (once a week) Total: 12~14 kg | Commercially available concentrates 3 kg Wheat 0.5–1 kg |
| D (n = 2) (9 m and 10 m) | 11 July 2019 | Imported Oats-hey 2.25 kg, Bermuda-grass 2.25 kg Total: 4.5 kg | Commercially available concentrates 4.5 kg |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widodo, O.S.; Etoh, M.; Kokushi, E.; Uno, S.; Yamato, O.; Pambudi, D.; Okawa, H.; Taniguchi, M.; Lamid, M.; Takagi, M. Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations. Toxins 2022, 14, 143. https://doi.org/10.3390/toxins14020143
Widodo OS, Etoh M, Kokushi E, Uno S, Yamato O, Pambudi D, Okawa H, Taniguchi M, Lamid M, Takagi M. Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations. Toxins. 2022; 14(2):143. https://doi.org/10.3390/toxins14020143
Chicago/Turabian StyleWidodo, Oky Setyo, Makoto Etoh, Emiko Kokushi, Seiichi Uno, Osamu Yamato, Dhidhi Pambudi, Hiroaki Okawa, Masayasu Taniguchi, Mirni Lamid, and Mitsuhiro Takagi. 2022. "Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations" Toxins 14, no. 2: 143. https://doi.org/10.3390/toxins14020143
APA StyleWidodo, O. S., Etoh, M., Kokushi, E., Uno, S., Yamato, O., Pambudi, D., Okawa, H., Taniguchi, M., Lamid, M., & Takagi, M. (2022). Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations. Toxins, 14(2), 143. https://doi.org/10.3390/toxins14020143






