Carry-Over of Zearalenone and Its Metabolites to Intestinal Tissues and the Expression of CYP1A1 and GSTπ1 in the Colon of Gilts before Puberty
Abstract
:1. Introduction
2. Results
2.1. Experimental Feed
2.2. Clinical Observations
2.3. Concentrations of Zearalenone and Its Metabolites in the Intestinal Tract of Prepubertal Gilts
2.4. Carry-Over Factor (CF)
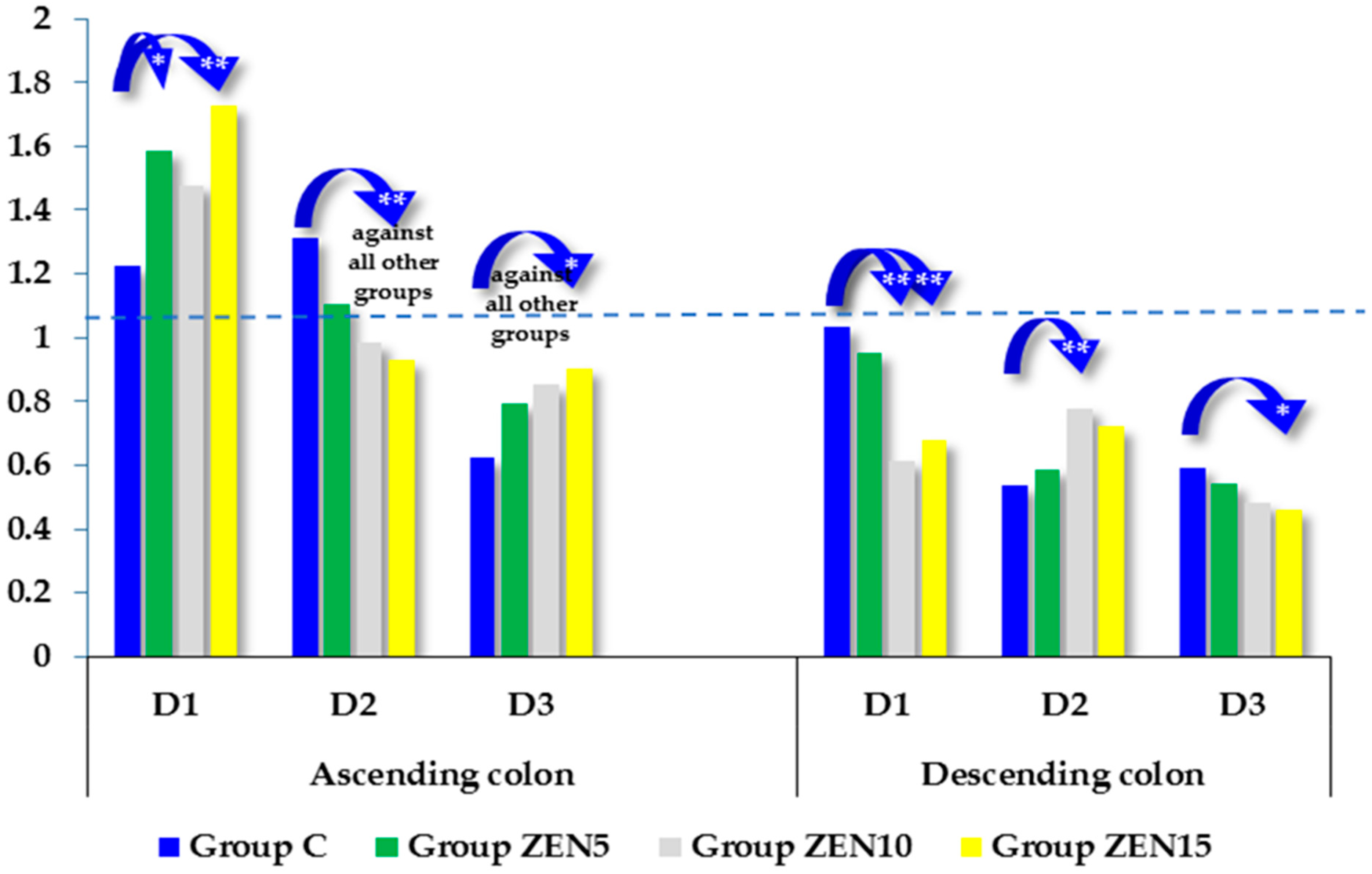
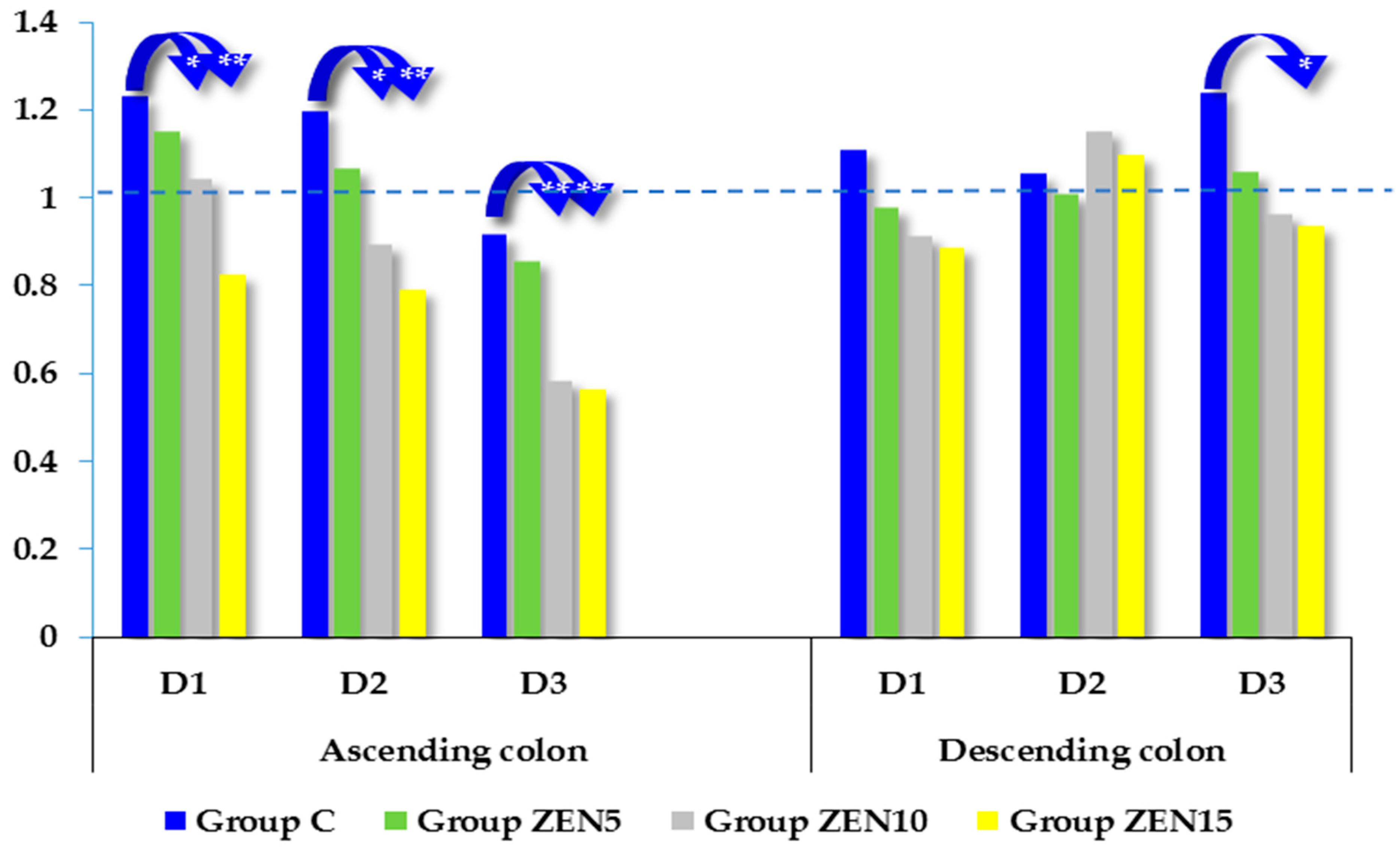
2.5. Expression of CYP1A1 and GSTP1 Genes
3. Discussion
3.1. Zearalenone and its Metabolites
3.1.1. Zearalenone Concentrations
3.1.2. Concentrations of ZEN and Its Metabolites
3.1.3. Carry-Over Factor
3.2. Expression of CYP1A1 and GSTπ1 Genes
4. Summary and Conclusions
5. Materials and Methods
5.1. General Information
5.2. Experimental Feed
Toxicological Analysis of Feed
5.3. Experimental Animals
5.3.1. Toxicological Analysis of Intestinal Tissues
Tissues Samples
Extraction Procedure
Chromatographic Quantification of ZEN and Its Metabolites
Mass Spectrometric Conditions
Carry-Over Factor
Statistical Analysis
5.4. Expression of CYP1A1 and GSTπ1
5.4.1. Sampling and Storage for RNA Extraction
5.4.2. Complete RNA Extraction and cDNA Synthesis
5.4.3. qPCR
5.4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.J.; Vila-Donat, P. Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef] [PubMed]
- Flasch, M.; Bueschl, C.; Del Favero, G.; Adam, G.; Schuhmacher, R.; Marko, D.; Warth, B. Elucidation of xenoestrogen metabolism by non-targeted, stable isotope-assisted mass spectrometry in breast cancer cells. Environ. Int. 2022, 158, 106940. [Google Scholar] [CrossRef] [PubMed]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef]
- Gajęcka, M.; Dąbrowski, M.; Otrocka-Domagała, I.; Brzuzan, P.; Rykaczewska, A.; Cieplińska, K.; Barasińska, M.; Gajęcki, M.T.; Zielonka, Ł. Correlation between the exposure of deoxynivalenol and zearalenone and the immunohistochemical expression of estrogen receptors in the intestinal epithelium and mRNA of selected colonic enzymes in pre-pubertal gilts. Toxicon 2020, 173, 75–93. [Google Scholar] [CrossRef]
- Rykaczewska, A.; Gajęcka, M.; Onyszek, E.; Cieplińska, K.; Dąbrowski, M.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Babuchowski, A.; Gajęcki, M.T.; Zielonka, Ł. Imbalance in the Blood Concentrations of Selected Steroids in Prepubertal Gilts Depending on the Time of Exposure to Low Doses of Zearalenone. Toxins 2019, 11, 561. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Dai, J.; Xu, J.; Yang, J.; Zhang, D. Comparative Cytotoxic Effects and Possible Mechanisms of Deoxynivalenol, Zearalenone and T-2 Toxin Exposure to Porcine Leydig Cells In Vitro. Toxins 2022, 14, 113. [Google Scholar] [CrossRef]
- Rykaczewska, A.; Gajęcka, M.; Dąbrowski, M.; Wiśniewska, A.; Szcześniewska, J.; Gajęcki, M.T.; Zielonka, Ł. Growth performance, selected blood biochemical parameters and body weights of pre-pubertal gilts fed diets supplemented with different doses of zearalenone (ZEN). Toxicon 2018, 152, 84–94. [Google Scholar] [CrossRef]
- Cieplińska, K.; Gajęcka, M.; Dąbrowski, M.; Rykaczewska, A.; Lisieska-Żołnierczyk, S.; Bulińska, M.; Zielonka, Ł.; Gajęcki, M.T. Time-Dependent Changes in the Intestinal Microbiome of Gilts Exposed to Low Zearalenone Doses. Toxins 2019, 11, 296. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, N.; Aoyama, T.; Kimura, S.; Gonzalez, F.J. Targeting nuclear receptors for the treatment of fatty liver disease. Pharmacol. Therapeut. 2017, 179, 142–157. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Obremski, K.; Gajęcka, M.; Gajęcki, M.T.; Zielonka, Ł. Changes in the Subpopulations of Porcine Peripheral Blood Lymphocytes Induced by Exposure to Low Doses of Zearalenone (ZEN) and Deoxynivalenol (DON). Molecules 2016, 21, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mróz, M.; Gajęcka, M.; Przybyłowicz, K.E.; Sawicki, T.; Lisieska-Żołnierczyk, S.; Zielonka, Ł.; Gajęcki, M.T. The Effect of Low Doses of Zearalenone (ZEN) on the Bone Marrow Microenvironment and Haematological Parameters of Blood Plasma in Pre-Pubertal Gilts. Toxins 2022, 14, 105. [Google Scholar] [CrossRef] [PubMed]
- Cieplińska, K.; Gajęcka, M.; Nowak, A.; Dąbrowski, M.; Zielonka, Ł.; Gajęcki, M.T. The Genotoxicity of Caecal Water in Gilts Exposed to Low Doses of Zearalenone. Toxins 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozieł, M.J.; Ziaja, M.; Piastowska-Ciesielska, A.W. Intestinal Barrier, Claudins and Mycotoxins. Toxins 2021, 13, 758. [Google Scholar] [CrossRef]
- Agahi, F.; Juan, C.; Font, G.; Juan-García, A. In silico methods for metabolomic and toxicity prediction of zearalenone, α-zearalenone and β-zearalenone. Food Chem. Toxicol. 2020, 146, 111818. [Google Scholar] [CrossRef]
- Pleadin, J.; Lešić, T.; Milićević, D.; Markov, K.; Šarkanj, B.; Vahčić, N.; Kmetič, I.; Zadravec, M. Pathways of Mycotoxin Occurrence in Meat Products: A Review. Processes 2021, 9, 2122. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: Path and Progression to Significance. Int. J. Mol. Sci. 2018, 19, 2871. [Google Scholar] [CrossRef] [Green Version]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. The effect of low monotonic doses of zearalenone on selected reproductive tissues in pre-pubertal female dogs—A review. Molecules 2015, 20, 20669–20687. [Google Scholar] [CrossRef] [Green Version]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. Activity of zearalenone in the porcine intestinal tract. Molecules 2017, 22, 18. [Google Scholar] [CrossRef] [Green Version]
- Stopa, E.; Babińska, I.; Zielonka, Ł.; Gajęcki, M.; Gajęcka, M. Immunohistochemical evaluation of apoptosis and proliferation in the mucous membrane of selected uterine regions in pre-pubertal bitches exposed to low doses of zearalenone. Pol. J. Vet. Sci. 2016, 19, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alassane-Kpembi, I.; Pinton, P.; Oswald, I.P. Effects of Mycotoxins on the Intestine. Toxins 2019, 11, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, H.J.; van den Ham, W.A.; Slob, W.; Pieters, M.N. Conversion Factors Estimating Indicative Chronic No-Observed-Adverse-Effect Levels from Short-Term Toxicity Data. Regul. Toxicol. Pharm. 1996, 23, 249–255. [Google Scholar] [CrossRef]
- Pastoor, T.P.; Bachman, A.N.; Bell, D.R.; Cohen, S.M.; Dellarco, M.; Dewhurst, I.C.; Doe, J.E.; Doerrer, N.G.; Embry, M.R.; Hines, R.N.; et al. A 21st century roadmap for human health risk assessment. Crit. Rev. Toxicol. 2014, 44, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, H.Y.; Peck, C.C.; Yu, K.S.; Lee, H. Determination of the starting dose in the first-in-human clinical trials with monoclonal antibodies: A systematic review of papers published between 1990 and 2013. Drug Des. Dev. Ther. 2016, 10, 4005–4016. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.; Su, Y.; Sun, Y.; Meng, X.; Shi, B.; Shan, A. Response of the nuclear receptors PXR and CAR and their target gene mRNA expression in female piglets exposed to zearalenone. Toxicon 2018, 151, 111–118. [Google Scholar] [CrossRef]
- Nandekar, P.P.; Khomane, K.; Chaudhary, V.; Rathod, V.P.; Borkar, R.M.; Bhandi, M.M.; Srinivas, R.; Sangamwar, A.T.; Guchhait, S.K.; Bansal, A.K. Identification of leads for antiproliferative activity on MDA-MB-435 human breast cancer cells through pharmacophore and CYP1A1-mediated metabolism. Eur. J. Med. Chem. 2016, 115, 82–93. [Google Scholar] [CrossRef]
- Kovacevic, Z.; Sahni, S.; Lok, H.; Davies, M.J.; Wink, D.A.; Richardson, D.R. Regulation and control of nitric oxide (NO) in macrophages: Protecting the “professional killer cell” from its own cytotoxic arsenal via MRP1and GSTP1. BBA Gen. Subj. 2017, 1861, 995–999. [Google Scholar] [CrossRef]
- Billat, P.A.; Roger, E.; Faure, S.; Lagarce, F. Models for drug absorption from the small intestine: Where are we and where are we going? Drug Discov. Today 2017. [Google Scholar] [CrossRef] [Green Version]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M.T. A Complementary Analysis of the Effects of Low-Dose Zearalenone Mycotoxicosis on Selected Health Status Indicators in Pre-Pubertal Gilts; Danielewska, A., Maciąg, K., Eds.; Wydawnictwo Naukowe TYGIEL Sp. Zo.o.: Lublin, Poland, 2020; pp. 41–70. ISBN 979-83-66489-21-9. (In Polish) [Google Scholar]
- Gajęcka, M.; Majewski, M.S.; Zielonka, Ł.; Grzegorzewski, W.; Onyszek, E.; Lisieska-Zołnierczyk, S.; Juśkiewicz, J.; Babuchowski, A.; Gajęcki, M.T. Concentration of Zearalenone, Alpha-Zearalenol and Beta-Zearalenol in the Myocardium and the Results of Isometric Analyses of the Coronary Artery in Prepubertal Gilts. Toxins 2021, 13, 396. [Google Scholar] [CrossRef]
- Gajęcka, M.; Mróz, M.; Brzuzan, P.; Onyszek, E.; Zielonka, Ł.; Lipczyńska-Ilczuk, K.; Przybyłowicz, K.E.; Babuchowski, A.; Gajęcki, M.T. Correlations between Low Doses of Zearalenone, Its Carryover Factor and Estrogen Receptor Expression in Different Segments of the Intestines in Pre-Pubertal Gilts—A Study Protocol. Toxins 2021, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Shigeyuki, U.; Dalton, T.P.; Derkenne, S.; Curran, C.P.; Miller, M.L.; Shertzer, H.G.; Nebert, D.W. Oral Exposure to Benzo[a]pyrene in the Mouse: Detoxication by Inducible Cytochrome P450 Is More Important Than Metabolic Activation. Mol. Pharmacol. 2004, 65, 1225–1237. [Google Scholar]
- Basharat, Z.; Yasmin, A. Energy landscape of a GSTP1 polymorph linked with cytological function decay in response to chemical stressors. Gene 2017, 609, 19–27. [Google Scholar] [CrossRef]
- Huszno, J.; Nowara, E.; Suwiński, R. The importance of genetic polymorphisms in cancer chemotherapy. J. Oncol. 2011, 61, 141–149. [Google Scholar]
- Gajęcka, M.; Brzuzan, P.; Otrocka-Domagała, I.; Zielonka, Ł.; Lisieska-Zołnierczyk, S.; Gajęcki, M.T. The Effect of 42-Day Exposure to a Low Deoxynivalenol Dose on the Immunohistochemical Expression of Intestinal ERs and the Activation of CYP1A1 and GSTP1 Genes in the Large Intestine of Pre-pubertal Gilts. Front. Vet. Sci. 2021, 8, 644549. [Google Scholar] [CrossRef]
- Zielonka, Ł.; Gajęcka, M.; Lisieska-Żołnierczyk, S.; Dąbrowski, M.; Gajęcki, M.T. The Effect of Different Doses of Zearalenone in Feed on the Bioavailability of Zearalenone and Alpha-zearalenol, and the Concentrations of Estradiol and Testosterone in the Peripheral Blood of Pre-pubertal Gilts. Toxins 2020, 12, 144. [Google Scholar] [CrossRef] [Green Version]
- Bakhru, S.H.; Furtado, S.; Morello, A.P.; Mathiowitz, E. Oral delivery of proteins by biodegradable nanoparticles. Adv. Drug. Deliv. Rev. 2013, 65, 811–821. [Google Scholar] [CrossRef]
- Lawrenz, B.; Melado, L.; Fatemi, H. Premature progesterone rise in ART-cycles. Reprod. Biol. 2018, 18, 1–4. [Google Scholar] [CrossRef]
- Jakimiuk, E.; Kuciel-Lisieska, G.; Zwierzchowski, W.; Gajęcka, M.; Obremski, K.; Zielonka, Ł.; Skorska-Wyszyńska, E.; Gajęcki, M. Morphometric changes of the reproductive system in gilts during zearalenone mycotoxicosis. Med. Weter. 2006, 62, 99–102. [Google Scholar]
- Rivera, H.M.; Stincic, T.L. Estradiol and the control of feeding behavior. Steroids 2018, 133, 44–52. [Google Scholar] [CrossRef]
- Benagiano, M.; Bianchi, P.; D’Elios, M.M.; Brosens, I.; Benagiano, G. Autoimmune diseases: Role of steroid hormones. Best Pract. Res. Cl. Ob. 2019, 60, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Uvnäs-Moberg, K. The gastrointestinal tract in growth and reproduction. Sci. Am. 1989, 261, 78–83. Available online: https://www.jstor.org/stable/24987325 (accessed on 19 April 2022). [CrossRef] [PubMed]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the Immune Response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Tao, H.; Liu, J.; Zhao, P.; Yang, F.; Lv, Z.; Wang, J. Effects of Bacillus subtilis ZJ-2019-1 on Zearalenone Toxicosis in Female Gilts. Toxins 2021, 13, 788. [Google Scholar] [CrossRef]
- Mahmoodzadeh, S.; Dworatzek, E. The Role of 17β-Estradiol and Estrogen Receptors in Regulation of Ca2+ Channels and Mitochondrial Function in Cardiomyocytes. Front. Endocrinol. 2019, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Widodo, O.S.; Etoh, M.; Kokushi, E.; Uno, S.; Yamato, O.; Pambudi, D.; Okawa, H.; Taniguchi, M.; Lamid, M.; Takagi, M. Practical Application of Urinary Zearalenone Monitoring System for Feed Hygiene Management of a Japanese Black Cattle Breeding Herd—The Relationship between Monthly Anti-Müllerian Hormone and Serum Amyloid A Concentrations. Toxins 2022, 14, 143. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, L.; Wang, J.; Tan, Y.; Yu, D.; Chang, X.; Fan, Y.; Zhao, D.; Wang, C.; De Boevre, M.; et al. A QuEChERS-Based Liquid Chromatography Tandem Mass Spectrometry Method for the Simultaneous Determination of Nine Zearalenone-Like Mycotoxins in Pigs. Toxins 2018, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Chodorowski, Z.; Sein Anand, J.; Robakowska, I.; Klimek, J.; Kaletha, K. The role of intestine in detoxification. Przeg. Lek. 2007, 64, 363–364. [Google Scholar]
- Sergent, T.; Ribonnet, L.; Kolosova, A.; Garsou, S.; Schaut, A.; De Saeger, S.; Van Peteghem, C.; Larondelle, Y.; Pussemier, L.; Schneider, Y.J. Molecular and cellular effects of food contaminants and secondary plant components and their plausible interactions at the intestinal level. Food Chem. Toxicol. 2008, 46, 813–841. [Google Scholar] [CrossRef]
- Martın, J.F.; Casqueiro, J.; Liras, P. Secretion systems for secondary metabolites: How producer cells send out messages of intercellular communication. Curr. Opin. Microbiol. 2005, 8, 282–293. [Google Scholar] [CrossRef]
- Balaguer, P.; Delfosse, V.; Bourguet, W. Mechanisms of endocrine disruption through nuclear receptors and related pathways. Curr. Opin. Endocrin. Metab. Res. 2019, 7, 1–8. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endoc. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Jasuja, R. Reproductive and Nonreproductive Actions of Testosterone. Enc. Endocr. Dis. 2019, 2, 721–734. [Google Scholar] [CrossRef]
- Li, P.; Su, R.; Yin, R.; Lai, D.; Wang, M.; Liu, Y.; Zhou, L. Detoxification of Mycotoxins through Biotransformation. Toxins 2020, 12, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, W.; Feng, N.; Wang, Y.; Noll, L.; Xu, S.; Liu, X.; Lu, N.; Zou, H.; Gu, J.; Yuan, Y.; et al. Effects of zearalenone and its derivatives on the synthesis and secretion of mammalian sex steroid hormones: A review. Food Chem. Toxicol. 2019, 126, 262–276. [Google Scholar] [CrossRef]
- Rogowska, A.; Pomastowski, P.; Sagandykova, G.; Buszewski, B. Zearalenone and its metabolites: Effect on human health, metabolism and neutralisation methods. Toxicon 2019, 162, 46–56. [Google Scholar] [CrossRef]
- Tyagi, V.; Scordo, M.; Yoon, R.S.; Liporace, F.A.; Greene, L.W. Revisiting the role of testosterone: Are we missing something? Rev. Urol. 2017, 19, 16–24. [Google Scholar] [CrossRef]
- Alvarez-Ortega, N.; Caballero-Gallardo, K.; Taboada-Alquerque, M.; Franco, J.; Stashenko, E.E.; Juan, C.; Juan-García, A.; Olivero-Verbel, J. Protective Effects of the Hydroethanolic Extract of Fridericia chica on Undifferentiated Human Neuroblastoma Cells Exposed to α-Zearalenol (α-ZEL) and β-Zearalenol (β-ZEL). Toxins 2021, 13, 748. [Google Scholar] [CrossRef]
- Kalemba-Drożdż, M.; Kapiszewska, M. Genetic polymorphism in biosynthesis of estrogens. The risk of hormone-dependent neoplasms. In Środowisko a Gospodarka Hormonalna u Kobiet. Tom I. Zaburzenia w Metabolizmie Estrogenów i ich Konsekwencje; Oficyna Wydawnicza AFM: Kraków, Poland, 2011; pp. 149–161. ISBN 978-83-7571-195-0. Available online: http://hdl.handle.net/11315/744 (accessed on 15 November 2011).
- Singh, R.R.; Reindl, K.M. Glutathione S-Transferases in Cancer. Antioxidants 2021, 10, 701. [Google Scholar] [CrossRef]
- Howie, A.F.; Forrester, L.M.; Glancey, M.J.; Schlager, J.J.; Powis, G.; Beckett, G.J.; Hayes, J.D.; Wolf, C.R. Glutathione S-transferase and glutathione peroxidase expression in normal and tumour human tissues. Carcinogenesis 1990, 11, 451–458. [Google Scholar] [CrossRef]
- Bocedi, A.; Noce, A.; Marrone, G.; Noce, G.; Cattani, G.; Gambardella, G.; Di Lauro, M.; Di Daniele, N.; Ricci, G. Glutathione Transferase P1-1 an Enzyme Useful in Biomedicine and as Biomarker in Clinical Practice and in Environmental Pollution. Nutrients 2019, 11, 1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, A.; Gupta, S. The multifaceted role of glutathione S-transferases in cancer. Cancer Lett. 2018, 433, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsen, M.; Thorlacius-Ussing, L.; Vorum, H.; Stender, M.T.; Thorlacius-Ussing, O.; Honoré, B. Proteomic Characterization of Colorectal Cancer Tissue from Patients Identifies Novel Putative Protein Biomarkers. Curr. Issues Mol. Biol. 2021, 43, 1043–1056. [Google Scholar] [CrossRef] [PubMed]
- Hokaiwado, N.; Takeshita, F.; Naiki-Ito, A.; Asamoto, M.; Ochiya, T.; Shirai, T. Glutathione Stransferase pi mediates proliferation of androgen- independent prostate cancer cells. Carcinogenesis 2008, 29, 1134–1138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risks for animal health related to the presence of zearalenone and its modified forms in feed. EFSA J. 2017, 15, 4851. [Google Scholar] [CrossRef] [Green Version]
- Gajęcki, M. Development Project NR12-0080-10 entitled. In The Effect of Experimentally Induced Fusarium Mycotoxicosis on Selected Diagnostic and Morphological Parameters of the Porcine Digestive Tract; The National Centre for Research and Development: Warsaw, Poland, 2013; pp. 1–180. [Google Scholar]
- Heberer, T.; Lahrssen-Wiederholt, M.; Schat, H.; Abraham, K.; Pzyrembel, H.; Henning, K.J.; Schauzu, M.; Braeunig, J.; Goetz, M.; Niemann, L.; et al. Zero tolerances in food and animal feed—Are there any scientificalternatives? A European point of view on aninternational controversy. Toxicol. Lett. 2007, 175, 118–135. [Google Scholar] [CrossRef]
- Smith, D.; Combes, R.; Depelchin, O.; Jacobsen, S.D.; Hack, R.; Luft, J.; Lammens, L.; von Landenberg, F.; Phillips, B.; Pfister, R.; et al. Optimising the design of preliminarytoxicity studies for pharmaceutical safetytesting in the dog. Regul. Toxicol. Pharmacol. 2005, 41, 95–101. [Google Scholar] [CrossRef]
- Barański, W.; Gajęcka, M.; Zielonka, Ł.; Mróz, M.; Onyszek, E.; Przybyłowicz, K.E.; Nowicki, A.; Babuchowski, A.; Gajęcki, M.T. Occurrence of Zearalenone and Its Metabolites in the Blood of High-Yielding Dairy Cows at Selected Collection Sites in Various Disease States. Toxins 2021, 13, 446. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; Tangni, E.K.; Huybrechts, B.; Couck, L.; De Rycke, R.; De Bels, L.; De Saeger, S.; Van den Broeck, W.; Devreese, M.; et al. A Study of Carry-Over and Histopathological Effects after Chronic Dietary Intake of Citrinin in Pigs, Broiler Chickens and Laying Hens. Toxins 2020, 12, 719. [Google Scholar] [CrossRef]
- Tohno, M.; Shimasato, T.; Moue, M.; Aso, H.; Watanabe, K.; Kawai, Y.; Yamaguchi, T.; Saito, T.; Kitazawa, H. Toll-like receptor 2 and 9 are expressed and functional in gut associated lymphoid tissues of presuckling newborn swine. Vet. Res. 2006, 37, 791–812. [Google Scholar] [CrossRef] [Green Version]
- Arukwe, A. Toxicological housekeeping genes: Do they really keep the house? Environ. Sci. Technol. 2006, 40, 7944–7949. [Google Scholar] [CrossRef] [PubMed]
- Spachmo, B.; Arukwe, A. Endocrine and developmental effects in Atlantic salmon (Salmo salar) exposed to perfluorooctane sulfonic or perfluorooctane carboxylic acids. Aquat. Toxicol. 2012, 108, 112–124. [Google Scholar] [CrossRef] [PubMed]

| Exposure Dates | Feed Intake (kg/day) | Total ZEN Doses in Groups (µg/kg BW) | Tissues | Group ZEN5 (ng/g) | Carry-Over Factor | Group ZEN10 (ng/g) | Carry-Over Factor | Group ZEN15 (ng/g) | Carry-Over Factor |
|---|---|---|---|---|---|---|---|---|---|
| D1 | 0.8 | 80.5/161.9/242.7 | Duodenum | 12.81 ± 8.49 | 1 × 10−4 | 64.36 ± 71.16 | 3 × 10−4 | 48.35 ± 43.92 | 1 × 10−4 |
| Jejunum | 15.59 ± 20.40 | 1 × 10−4 | 15.24 ± 13.65 | 9 × 10−5 | 13.31 ± 4.92 | 5 × 10−5 | |||
| Ileum | 3.13 ± 0.69 | 3 × 10−5 | 4.67 ± 4.50 | 2 × 10−5 | 18.36 ± 13.81 c | 7 × 10−5 | |||
| Cecum | 15.07 ± 8.14 | 1 × 10−4 | 6.84 ± 4.18 | 4 × 10−5 | 47.99 ± 34.30 d | 1 × 10−4 | |||
| CFG | 14.74 ± 7.59 | 1 × 10−4 | 8.63 ± 5.09 | 5 × 10−5 | 33.13 ± 48.53 | 1 × 10−4 | |||
| CPG | 18.24 ± 12.39 | 2 × 10−4 | 9.67 ± 4.95 | 5 × 10−5 | 28.48 ± 17.20 | 1 × 10−4 | |||
| Transverse colon | 4.59 ± 2.74 | 5 × 10−5 | 4.30 ± 2.99 | 2 × 10−5 | 31.13 ± 20.40 c,d | 1 × 10−4 | |||
| Descending colon | 6.11 ± 3.67 | 7 × 10−5 | 4.50 ± 2.68 | 2 × 10−5 | 31.69 ± 38.52 | 1 × 10−4 | |||
| D2 | 1.1 | 101.01/196.9/298.2 | Duodenum | 7.67 ± 4.56 | 7 × 10−5 | 46.41 ± 33.57 c | 2 × 10−4 | 8.23 ± 10.24 d | 2 × 10−5 |
| Jejunum | 6.81 ± 5.80 | 6 × 10−5 | 4.95 ± 1.94 | 2 × 10−5 | 5.56 ± 5.97 | 1 × 10−5 | |||
| Ileum | 3.26 ± 2.32 | 3 × 10−5 | 3.30 ± 2.07 | 1 × 10−5 | 11.66 ± 5.46 cc, dd | 3 × 10−5 | |||
| Cecum | 8.52 ± 6.13 | 8 × 10−5 | 12.56 ± 10.45 | 6 × 10−5 | 38.11 ± 38.07 | 1 × 10−4 | |||
| CFG | 3.68 ± 1.06 aa | 3 × 10−5 | 12.13 ± 9.63 | 6 × 10−5 | 35.15 ± 37.92 | 1 × 10−4 | |||
| CPG | 4.73 ± 1.76 a | 4 × 10−5 | 8.22 ± 8.53 | 4 × 10−5 | 24.82 ± 19.84 | 8 × 10−5 | |||
| Transverse colon | 2.19 ± 1.31 | 2 × 10−5 | 7.64 ± 3.91 | 3 × 10−5 | 9.39 ± 8.98 | 3 × 10−5 | |||
| Descending colon | 2.23 ± 1.18 | 2 × 10−5 | 7.31 ± 4.09 | 3 × 10−5 | 18.35 ± 13.56 c | 6 × 10−5 | |||
| D3 | 1.6 | 128.3/481.4/716.7 | Duodenum | 8.84 ± 4.20 | 6 × 10−5 | 39.84 ± 2.22 cc | 8 × 10−5 | 57.34 ± 8.98 cc,dd | 8 × 10−5 |
| Jejunum | 2.83 ± 4.26 | 2 × 10−5 | 3.02 ± 3.76 | 6 × 10−6 | 8.03 ± 0.98 | 1 × 10−5 | |||
| Ileum | 4.10 ± 2.87 | 3 × 10−5 | 8.34 ± 0.94 c | 1 × 10−5 | 15.11 ± 0.66 cc,dd | 2 × 10−5 | |||
| Cecum | 4.46 ± 4.73 | 3 × 10−5 | 17.17 ± 20.89 | 3 × 10−5 | 39.86 ± 2.30 cc | 5 × 10−5 | |||
| CFG | 1.76 ± 2.07 aa | 1 × 10−5 | 16.73 ± 22.78 | 3 × 10−5 | 18.10 ± 2.50 cc, d | 6 × 10−5 | |||
| CPG | 2.21 ± 1.56 a | 1 × 10−5 | 8.91 ± 2.80 cc | 1 × 10−5 | 18.17 ± 2.78 cc,dd | 2 × 10−5 | |||
| Transverse colon | 1.10 ± 1.15 | 8 × 10−6 | 22.70 ± 12.87 a, b,c | 4 × 10−5 | 20.19 ± 1.30 c | 2 × 10−5 | |||
| Descending colon | 2.30 ± 1.35 | 1 × 10−5 | 13.26 ± 16.42 | 2 × 10−5 | 18.94 ± 2.11 | 2 × 10−5 |
| Exposure Date | Tissues | Group ZEN5 (ng/g) | Carry-Over Factor | Group ZEN10 (ng/g) | Carry-Over Factor | Group ZEN15 (ng/g) | Carry-Over Factor |
|---|---|---|---|---|---|---|---|
| D1 | Duodenum | 3.72 ± 1.68 | 4 × 10−5 | 6.25 ± 2.57 | 3 × 10−5 | 6.66 ± 6.32 | 2 × 10−5 |
| Jejunum | 2.85 ± 2.78 | 3 × 10−5 | 5.24 ± 3.95 | 3 × 10−5 | 9.62 ± 1.66 | 3 × 10−5 | |
| Ileum | 1.07 ± 0.21 | 1 × 10−5 | 6.46 ± 7.25 | 3 × 10−5 | 2.88 ± 3.21 | 1 × 10−5 | |
| Cecum | 3.45 ± 1.05 | 4 × 10−5 | 12.01 ± 4.36 cc | 7 × 10−5 | 11.85 ± 2.04 cc | 4 × 10−5 | |
| CFG | 3.15 ± 0.49 | 3 × 10−5 | 5.58 ± 1.83 | 3 × 10−5 | 4.59 ± 2.78 | 1 × 10−5 | |
| CPG | 3.98 ± 2.43 | 4 × 10−5 | 6.88 ± 2.65 | 4 × 10−5 | 6.41 ± 4.58 | 2 × 10−5 | |
| Transverse colon | 1.07 ± 0.95 | 1 × 10−5 | 2.26 ± 1.74 | 1 × 10−5 | 2.05 ± 0.99 | 8 × 10−6 | |
| Descending colon | 1.48 ± 0.60 | 1 × 10−5 | 1.47 ± 0.80 | 9 × 10−6 | 2.68 ± 3.04 | 1 × 10−5 | |
| D2 | Duodenum | 6.43 ± 2.36 | 6 × 10−5 | 9.12 ± 8.11 | 4 × 10−5 | 6.97 ± 0.31 | 2 × 10−5 |
| Jejunum | 5.36 ± 3.87 | 5 × 10−5 | 22.37 ± 20.81 | 1 × 10−4 | 14.28 ± 1.92 a | 4 × 10−5 | |
| Ileum | 6.58 ± 7.01 | 6 × 10−5 | 4.46 ± 2.99 | 2 × 10−5 | 8.49 ± 2.52 | 2 × 10−5 | |
| Cecum | 11.23 ± 4.47 aa | 1 × 10−4 | 22.74 ± 17.44 | 1 × 10−4 | 35.59 ± 14.60 a | 1 × 10−4 | |
| CFG | 6.01 ± 2.01 a | 5 × 10−5 | 10.80 ± 10.41 | 5 × 10−5 | 10.88 ± 1.74 aa | 3 × 10−5 | |
| CPG | 7.01 ± 2.76 | 6 × 10−5 | 11.86 ± 4.88 | 6 × 10−5 | 27.08 ± 15.25 c | 9 × 10−5 | |
| Transverse colon | 2.42 ± 1.59 | 2 × 10−5 | 5.86 ± 2.61 | 2 × 10−5 | 6.65 ± 1.55 c | 2 × 10−5 | |
| Descending colon | 1.51 ± 0.88 | 1 × 10−5 | 2.75 ± 1.74 | 1 × 10−5 | 1.07 ± 0.40 | 3 × 10−6 | |
| D3 | Duodenum | 3.72 ± 1.68 | 2 × 10−5 | 30.00 ± 22.55 c | 6 × 10−5 | 20.12 ± 4.65 aa, bb | 2 × 10−5 |
| Jejunum | 2.85 ± 2.78 | 2 × 10−5 | 16.42 ± 5.54 cc | 3 × 10−5 | 19.64 ± 1.82 aa, b, cc | 2 × 10−5 | |
| Ileum | 6.69 ± 1.68 | 5 × 10−5 | 4.60 ± 4.00 | 9 × 10−6 | 7.32 ± 4.10 | 1 × 10−5 | |
| Cecum | 3.45 ± 1.05 bb | 2 × 10−5 | 49.47 ± 9.75 aa, bb, cc | 1 × 10−4 | 37.04 ± 5.08 a, cc | 5 × 10−5 | |
| CFG | 3.15 ± 0.49 b | 2 × 10−5 | 12.67 ± 8.59 c | 2 × 10−5 | 15.56 ± 1.34 aa, b, c | 2 × 10−5 | |
| CPG | 3.98 ± 2.43 | 3 × 10−5 | 25.84 ± 10.70 a, c | 5 × 10−5 | 16.13 ± 11.21 | 2 × 10−5 | |
| Transverse colon | 0.80 ± 0.94 | 6 × 10−6 | 8.08 ± 7.40 | 1 × 10−5 | 5.91 ± 6.88 | 8 × 10−6 | |
| Descending colon | 0.78 ± 0.74 | 6 × 10−6 | 7.00 ± 5.59 | 1 × 10−5 | 5.53 ± 3.86 | 4 × 10−6 |
| Exposure Date | Tissue | Group ZEN5 (ng/g) | Carry-Over Factor | Group ZEN10 (ng/g) | Carry-over Factor | Group ZEN15 (ng/g) | Carry-Over Factor |
|---|---|---|---|---|---|---|---|
| D1 | Duodenum | 0.16 ± 0.09 | 1 × 10−6 | 3.49 ± 6.14 | 2 × 10−5 | 0.29 ± 0.07 | 1 × 10−6 |
| Jejunum | 0.07 ± 0.08 | 8 × 10−7 | 2.17 ± 3.61 | 1 × 10−5 | 0.12 ± 0.02 | 4 × 10−7 | |
| Ileum | 0 | 0 | 0.64 ± 0.77 | 3 × 10−6 | 0.02 ± 0.03 | 8 × 10−8 | |
| Cecum | 0.14 ± 0.04 | 1 × 10−6 | 1.19 ± 0.20 cc | 7 × 10−6 | 0.54 ± 0.10 cc, dd | 2 × 10−6 | |
| CFG | 0.13 ± 0.07 | 1 × 10−6 | 0.44 ± 0.28 | 2 × 10−6 | 0.30 ± 0.02 | 1 × 10−6 | |
| CPG | 0.18 ± 0.10 | 2 × 10−6 | 2.37 ± 3.66 | 1 × 10−5 | 0.27 ± 0.09 | 1 × 10−6 | |
| Transverse colon | 0.12 ± 0.18 | 1 × 10−6 | 0.77 ± 1.10 | 4 × 10−6 | 0.09 ± 0.06 | 3 × 10−7 | |
| Descending colon | 0.02 ± 0.01 | 2 × 10−7 | 0.22 ± 0.17 | 1 × 10−6 | 0.05 ± 0.01 | 2 × 10−7 | |
| D2 | Duodenum | 0.08 ± 0.10 | 7 × 10−7 | 0.26 ± 0.17 | 1 × 10−6 | 0.08 ± 0.09 aa | 2 × 10−7 |
| Jejunum | 0.07 ± 0.10 | 6 × 10−7 | 1.11 ± 0.11 cc | 5 × 10−6 | 0.69 ± 0.11 aa, cc, dd | 2 × 10−6 | |
| Ileum | 0.14 ± 0.11 | 1 × 10−6 | 0.27 ± 0.18 | 1 × 10−6 | 0.05 ± 0.06 | 1 × 10−7 | |
| Cecum | 0.30 ± 0.19 | 2 × 10−6 | 1.07 ± 1.00 | 5 × 10−6 | 1.10 ± 0.26 a | 3 × 10−6 | |
| CFG | 0.17 ± 0.13 | 7 × 10−7 | 0.45 ± 0.29 | 2 × 10−6 | 0.24 ± 0.16 | 8 × 10−7 | |
| CPG | 0.13 ± 0.11 | 1 × 10−6 | 0.41 ± 0.12 | 2 × 10−6 | 0.46 ± 0.53 | 1 × 10−6 | |
| Transverse colon | 0.03 ± 0.03 | 1 × 10−7 | 0.27 ± 0.09 cc | 1 × 10−6 | 0.25 ± 0.05 cc | 8 × 10−7 | |
| Descending colon | 0.03 ± 0.02 | 1 × 10−7 | 0.23 ± 0.27 | 1 × 10−6 | 0.08 ± 0.04 | 2 × 10−7 | |
| D3 | Duodenum | 1.11 ± 0.67 aa, bb | 8 × 10−6 | 2.21 ± 0.73 | 5 × 10−6 | 3.06 ± 0.17 aa, cc | 4 × 10−6 |
| Jejunum | 0.91 ± 0.45 aa, bb | 7 × 10−6 | 2.46 ± 1.51 | 5 × 10−6 | 3.08 ± 0.16 aa, bb | 4 × 10−6 | |
| Ileum | 0.21 ± 0.28 | 1 × 10−6 | 0.89 ± 1.32 | 1 × 10−6 | 0.61 ± 0.52 | 8 × 10−7 | |
| Cecum | 0.63 ± 0.24 aa | 4 × 10−6 | 1.18 ± 0.38 | 2 × 10−6 | 1.15 ± 0.19 a | 1 × 10−6 | |
| CFG | 0.48 ± 0.34 | 3 × 10−6 | 1.37 ± 0.18 a, b, cc | 2 × 10−6 | 1.66 ± 0.21 a, b, cc | 2 × 10−6 | |
| CPG | 0.47 ± 0.41 | 3 × 10−6 | 0.27 ± 0.20 | 5 × 10−7 | 0.80 ± 0.13 | 1 × 10−6 | |
| Transverse colon | 0.20 ± 0.14 | 1 × 10−6 | 0.76 ± 0.59 | 1 × 10−6 | 1.06 ± 0.20 aa, bb | 1 × 10−6 | |
| Descending colon | 0.16 ± 0.16 | 1 × 10−6 | 0.53 ± 0.19 | 1 × 10−6 | 0.79 ± 0.11 aa, bb, c | 1 × 10−6 |
| Parameters | Composition Declared by the Manufacturer (%) |
|---|---|
| Soybean meal | 16 |
| Wheat | 55 |
| Barley | 22 |
| Wheat bran | 4.0 |
| Chalk | 0.3 |
| Zitrosan | 0.2 |
| Vitamin–mineral premix 1 | 2.5 |
| Analyte | Precursor | Quantification Ion | Confirmation Ion | LOD (ng mL−1) | LOQ (ng mL−1) | Linearity (%R2) |
|---|---|---|---|---|---|---|
| ZEN | 317.1 | 273.3 | 187.1 | 0.03 | 0.1 | 0.999 |
| α-ZEL | 319.2 | 275.2 | 160.1 | 0.3 | 0.9 | 0.997 |
| β-ZEL | 319.2 | 275.2 | 160.1 | 0.3 | 1 | 0.993 |
| Primer | Sequence (5′→3′) | Amplicon Length (bp) | References | |
|---|---|---|---|---|
| CYP1A1 | Forward | cagagccgcagcagccaccttg | 226 | [68] |
| Reverse | ggctcttgcccaaggtcagcac | |||
| GSTP1 | Forward | acctgcttcggattcaccag | 178 | [68] |
| Reverse | ctccagccacaaagccctta | |||
| β-Actin | Forward | catcaccatcggcaaaga | 237 | [73] |
| Reverse | gcgtagaggtccttcctgatgt | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mróz, M.; Gajęcka, M.; Brzuzan, P.; Lisieska-Żołnierczyk, S.; Leski, D.; Zielonka, Ł.; Gajęcki, M.T. Carry-Over of Zearalenone and Its Metabolites to Intestinal Tissues and the Expression of CYP1A1 and GSTπ1 in the Colon of Gilts before Puberty. Toxins 2022, 14, 354. https://doi.org/10.3390/toxins14050354
Mróz M, Gajęcka M, Brzuzan P, Lisieska-Żołnierczyk S, Leski D, Zielonka Ł, Gajęcki MT. Carry-Over of Zearalenone and Its Metabolites to Intestinal Tissues and the Expression of CYP1A1 and GSTπ1 in the Colon of Gilts before Puberty. Toxins. 2022; 14(5):354. https://doi.org/10.3390/toxins14050354
Chicago/Turabian StyleMróz, Magdalena, Magdalena Gajęcka, Paweł Brzuzan, Sylwia Lisieska-Żołnierczyk, Dawid Leski, Łukasz Zielonka, and Maciej T. Gajęcki. 2022. "Carry-Over of Zearalenone and Its Metabolites to Intestinal Tissues and the Expression of CYP1A1 and GSTπ1 in the Colon of Gilts before Puberty" Toxins 14, no. 5: 354. https://doi.org/10.3390/toxins14050354
APA StyleMróz, M., Gajęcka, M., Brzuzan, P., Lisieska-Żołnierczyk, S., Leski, D., Zielonka, Ł., & Gajęcki, M. T. (2022). Carry-Over of Zearalenone and Its Metabolites to Intestinal Tissues and the Expression of CYP1A1 and GSTπ1 in the Colon of Gilts before Puberty. Toxins, 14(5), 354. https://doi.org/10.3390/toxins14050354






