Immunomodulatory Effects of Pure Cylindrospermopsin in Rats Orally Exposed for 28 Days
Abstract
1. Introduction
2. Results and Discussion
2.1. Gene Expression Analysis of ILs by RT-qPCR
2.2. Cytokine Serum Levels
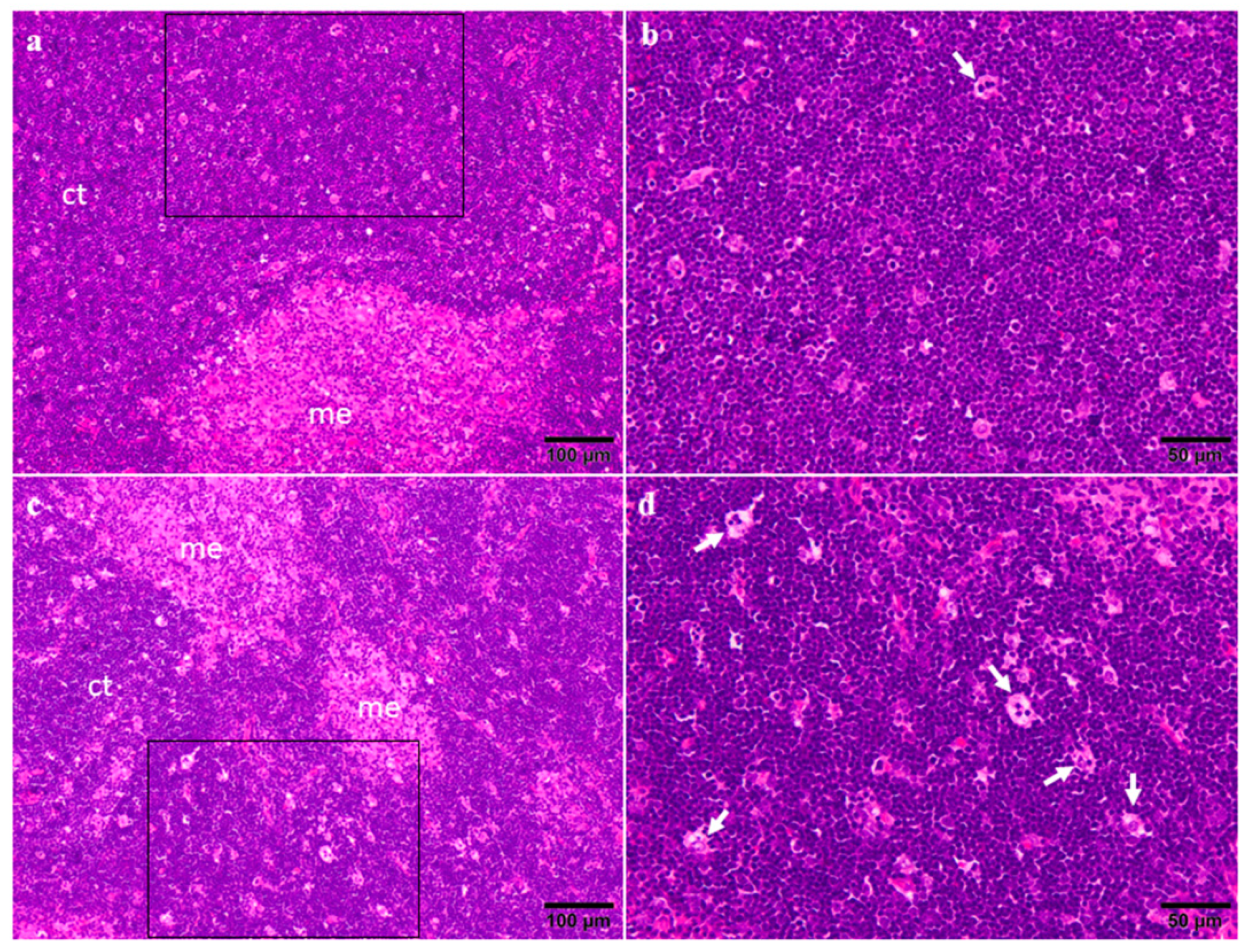
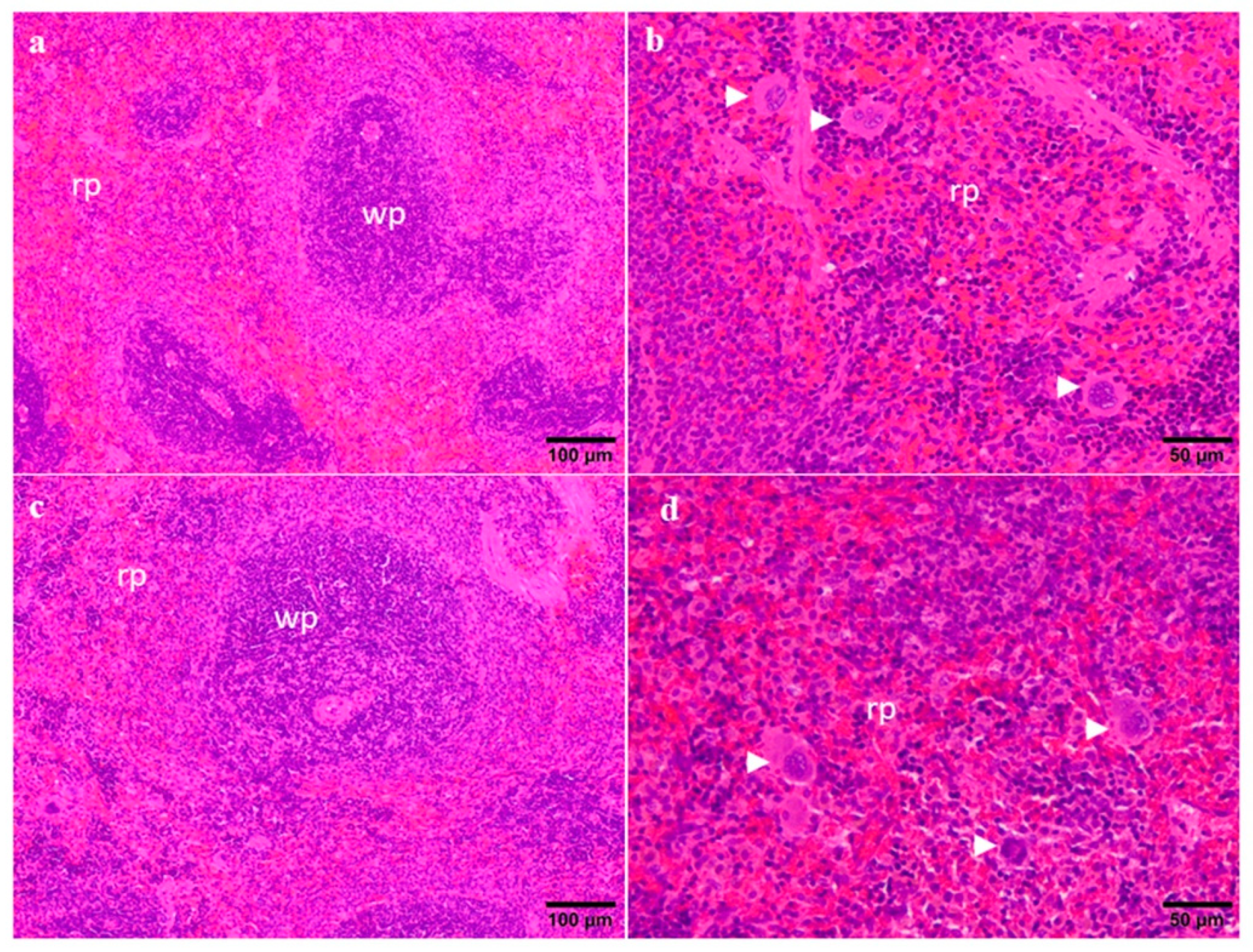
2.3. Organ Weights and Histopathology
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animal Housing and Experimental Set-Up
4.3. Experimental Exposure
4.4. RNA Extraction and Reverse Transcription
4.5. Gene Expression Analysis by Quantitative Real-Time PCR (RT-qPCR)
4.6. Multiplex Assay
4.7. Organ Weights and Histopathology
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sverker, Z.; Lalić, D.; Bojadžija Savić, G.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, G.; Chen, Y.; Jia, N.; Li, R. Four decades of progress in cylindrospermopsin research: The ins and outs of a potent cyanotoxin. J. Hazard. Mater. 2021, 406, 124653. [Google Scholar] [CrossRef] [PubMed]
- Scarlett, K.R.; Kim, S.; Lovin, L.M.; Chatterjee, S.; Scott, J.T.; Brooks, B.W. Global scanning of cylindrospermopsin: Critical review and analysis of aquatic occurrence, bioaccumulation, toxicity and health hazards. Sci. Total Environ. 2020, 738, 139807. [Google Scholar] [CrossRef]
- Kokociński, M.; Cameán, A.M.; Carmeli, S.; Guzmán-Guillén, R.; Jos, A.; Mankiewicz-Boczek, J.; Metcalf, J.S.; Moreno, I.M.; Prieto, A.I.; Sukenik, A. Cylindrospermopsin and congeners. In Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis, 1st ed.; Meriluoto, J., Spoof, L., Codd, G.A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 127–137. [Google Scholar] [CrossRef]
- World Health Organization. Cyanobacterial Toxins: Cylindrospermopsins. Background Document for Development of WHO Guidelines for Drinking-Water Quality and Guidelines for Safe Recreational Water Environments; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/handle/10665/338063https://apps.who.int/iris/handle/10665/338063 (accessed on 13 January 2022).
- Kinnear, S. Cylindrospermopsin: A decade of progress on bioaccumulation research. Mar. Drugs 2010, 8, 542–564. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Jos, Á.; Pichardo, S.; Moreno, I.M.; Cameán, A.M. Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: A review. Food Chem. Toxicol. 2013, 53, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Runnegar, M.T.; Kong, S.-M.; Zhong, Y.-Z.; Lu, S.C. Inhibition of reduced glutathione synthesis by cyanobacterial alkaloid cylindrospermopsin in cultured rat hepatocytes. Biochem. Pharmacol. 1995, 49, 219–225. [Google Scholar] [CrossRef]
- Froscio, S.M.; Humpage, A.R.; Burcham, P.C.; Falconer, I.R. Cylindrospermopsin-induced protein synthesis inhibition and its dissociation from acute toxicity in mouse hepatocytes. Environ. Toxicol. 2003, 18, 243–251. [Google Scholar] [CrossRef]
- Puerto, M.; Jos, Á.; Pichardo, S.; Gutiérrez-Praena, D.; Cameán, A.M. Acute effects of pure cylindrospermopsin on the activity and transcription of antioxidant enzymes in tilapia (Oreochromis niloticus) exposed by gavage. Ecotoxicology 2011, 20, 1852–1860. [Google Scholar] [CrossRef] [PubMed]
- Guzmán-Guillén, R.; Prieto, A.I.; Vasconcelos, V.M.; Cameán, A.M. Cyanobacterium producing cylindrospermopsin cause oxidative stress at environmentally relevant concentrations in sub-chronically exposed tilapia (Oreochromis niloticus). Chemosphere 2013, 90, 1184–1194. [Google Scholar] [CrossRef]
- Diez-Quijada, L.; Llana-Ruiz-Cabello, M.; Cătunescu, M.G.; Puerto, M.; Moyano, R.; Jos, A.; Cameán, A.M. In vivo genotoxicity evaluation of cylindrospermopsin in rats using a combined micronucleus and comet assay. Food Chem. Toxicol. 2019, 132, 110664. [Google Scholar] [CrossRef]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar] [CrossRef]
- Terao, K.; Ohmori, S.; Igarashi, K.; Ohtani, I.; Watanabe, M.F.; Harada, K.I.; Ito, E.; Watanabe, M. Electron microscopic studies on experimental poisoning in mice induced by cylindrospermopsin isolated from blue-green alga Umezakia natans. Toxicon 1994, 32, 833–843. [Google Scholar] [CrossRef]
- Seawright, A.A.; Nolan, C.C.; Shaw, G.R.; Chiswell, R.K.; Norris, R.L.; Moore, M.R.; Smith, M.J. The oral toxicity for mice of the tropical cyanobacterium Cylindrospermopsis raciborskii (Wolonszynska). Environ. Toxicol. 1999, 14, 135–142. [Google Scholar] [CrossRef]
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “common suspects” for the Dalmatian pelican (Pelecanus crispus) deaths in a Mediterranean reconstructed reservoir. Environ. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef]
- World Health Organization & International Programme on Chemical Safety. Guidance for Immunotoxicity Risk Assessment for Chemicals; World Health Organization: Geneva, Switzerland, 2012; Available online: https://apps.who.int/iris/handle/10665/330098 (accessed on 13 January 2022).
- Ibrahim, M.A.A. Cell biology of the immune system. In Goodman’s Medical Cell Biology, 4th ed.; Goodman, S.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 337–360. ISBN 9780128179277. [Google Scholar] [CrossRef]
- Holsapple, M.P.; Kaminski, N.E. Immune system. In Encyclopedia of Toxicology, 2nd ed.; Philip Wexler, P., Ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 573–596. [Google Scholar] [CrossRef]
- Harff, C.; Panoskaltsis-Mortari, A. Tissue engineering of the lymphoid organs. J. Immunol. Regen. Med. 2021, 13, 100049. [Google Scholar] [CrossRef]
- Thapa, P.; Farber, L.D. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef]
- Lewis, S.M.; Williams, A.; Eisenbarth, S.C. Structure and function of the immune system in the spleen. Sci. Immunol. 2019, 4, eaau6085. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Mo, X.; Liu, J.; Ye, S.; Zeng, X.; Chen, D. Thymic function in the regulation of T cells, and molecular mechanisms underlying the modulation of cytokines and stress signaling (Review). Mol. Med. Rep. 2017, 16, 7175–7184. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Castro, F.; Cardoso, A.P.; Madeira Gonçalves, R.; Serre, A.; Oliveira, M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, J.; Pascual, V.; O’Garra, A. From IL-2 to IL-37: The expanding spectrum of antiinflammatory cytokines. Nat. Immunol. 2012, 13, 925–931. [Google Scholar] [CrossRef]
- Sierosławska, A. Immunotoxic, genotoxic and carcinogenic effects of cyanotoxins. Cent. Eur. J. Immunol. 2010, 35, 105–110. [Google Scholar]
- Pichardo, S.; Cameán, A.M.; Jos, A. In vitro toxicological assessment of cylindrospermopsin: A review. Toxins 2017, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Diez-Quijada, L.; Benítez-González, M.d.M.; Puerto, M.; Jos, A.; Cameán, A.M. Immunotoxic effects induced by microcystins and cylindrospermopsin: A review. Toxins 2021, 13, 711. [Google Scholar] [CrossRef]
- Shaw, G.R.; Seawright, A.A.; Moore, M.R.; Lam, P.K.S. Cylindrospermopsin, a cyanobacterial alkaloid: Evaluation of its toxicological activity. Ther. Drug Monit. 2000, 22, 89–92. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. First report of cylindrospermopsin effect on human peripheral blood lymphocytes proliferation in vitro. Cent. Eur. J. Immunol. 2012, 37, 314–317. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Wiktorowicz, K. Toxicity of cylindrospermopsin in human lymphocytes: Proliferation, viability and cell cycle studies. Toxicol. Vitr. 2014, 28, 968–974. [Google Scholar] [CrossRef]
- Poniedziałek, B.; Rzymski, P.; Karczewski, J. The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. Vitr. 2015, 29, 926–932. [Google Scholar] [CrossRef]
- Sieroslawska, A.; Rymuszka, A.; Adaszek, L. Effects of cylindrospermopsin on the phagocytic cells of the common carp (Cyprinus carpio L.). J. Appl. Toxicol. 2015, 35, 1406–1414. [Google Scholar] [CrossRef]
- Moosova, Z.; Pekarova, M.; Sindlerova, L.S.; Vasicek, O.; Kubala, L.; Blaha, L.; Adamovsky, O. Immunomodulatory effects of cyanobacterial toxin cylindrospermopsin on innate immune cells. Chemosphere 2019, 226, 439–446. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4; OECD Publishing: Paris, France, 2008. [Google Scholar] [CrossRef]
- Slaats, J.; ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regener. 2019, 39, 12. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.; Gao, W.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef]
- Dembic, Z. Cytokines of the immune system: Interleukins. In The Cytokines of the Immune System; Dembic, Z., Ed.; Academic Press: Cambridge, MA, US, 2015; pp. 143–239. ISBN 9780124199989. [Google Scholar] [CrossRef]
- Liao, W.; Lin, J.-X.; Leonard, W.J. IL-2 family cytokines: New insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr. Opin. Immunol. 2011, 23, 598–604. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef]
- Choy, E.; Rose-John, S. Interleukin-6 as a multifunctional regulator: Inflammation, immune response, and fibrosis. J. Scleroderma Relat. Disord. 2017, 2, S1–S5. [Google Scholar] [CrossRef]
- Tumor necrosis factor alfa. In Meyler’s Side Effects of Drugs, 16th ed.; Aronson, J.K., Ed.; Elsevier: Amsterdam, The Netherlands; pp. 230–232. ISBN 9780444537164. [CrossRef]
- Diez-Quijada, L.; Moyano, R.; Molina-Hernández, V.; Cameán, A.M.; Jos, Á. Evaluation of toxic effects induced by repeated exposure to Cylindrospermopsin in rats using a 28-day feeding study. Food Chem. Toxicol. 2021, 151, 112108. [Google Scholar] [CrossRef]
- Mahdavi Sharif, P.; Jabbari, P.; Razi, S.; Keshavarz-Fathi, M.; Rezai, N. Importance of TNF-alpha and its alterations in the development of cancers. Cytokine 2020, 130, 155066. [Google Scholar] [CrossRef] [PubMed]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor necrosis factor α and regulatory T cells in oncoimmunology. Front. Immunol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Reem, G.H.; Yeh, N.-H. Interleukin 2 regulates expression of its receptor and synthesis of gamma interferon by human T lymphocytes. Science 1984, 225, 429–430. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J.L.; Escalante, N.K.; Jude, K.M.; Sotolongo Bellon, J.; Su, L.; Horton, T.M.; Tsutsumi, N.; Berardinelli, S.J.; Haltiwanger, R.S.; Piehler, J.; et al. Structure of the IFNγ receptor complex guides design of biased agonists. Nature 2019, 567, 56–60. [Google Scholar] [CrossRef]
- Wang, T.; Hu, Y.; Wangkahart, E.; Liu, F.; Wang, A.; Zahran, E.; Maisey, K.R.; Liu, M.; Xu, Q.; Imarai, M.; et al. Interleukin (IL)-2 is a key regulator of T helper 1 and T helper 2 cytokine expression in fish: Functional characterization of two divergent IL2 paralogs in salmonids. Front. Immunol. 2018, 9, 1683. [Google Scholar] [CrossRef] [PubMed]
- Kuper, C.F.; Harleman, J.H.; Richter-Reichelm, H.B.; Vos, J.G. Histopathologic approaches to detect changes indicative of immunotoxicity. Toxicol. Pathol. 2000, 28, 454–466. [Google Scholar] [CrossRef]
- Xu, H.; McClain, S.; Medina, S.; Lauer, F.T.; Douillet, C.; Liu, K.J.; Hudson, L.G.; Stýblo, M.; Burchiel, S.W. Differential sensitivities of bone marrow, spleen and thymus to genotoxicity induced by environmentally relevant concentrations of arsenite. Toxicol. Lett. 2016, 262, 55–61. [Google Scholar] [CrossRef]
- Cao, L.; Huang, F.; Massey, I.Y.; Wen, C.; Zheng, S.; Xu, S.; Yang, F. Effects of Microcystin-LR on the microstructure and inflammation-related factors of jejunum in mice. Toxins 2019, 11, 482. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Xie, P.; Zhang, X.; Tang, R.; Gao, Y.; Li, D.; Li, L. In vivo studies on the immunotoxic effects of microcystins on rabbit. Environ. Toxicol. 2010, 27, 83–89. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Xiao, W.; Ye, X.; Zhong, Q.; Gu, K. Comparison of response indices to toxic microcystin-LR in blood of mice. Chemosphere 2013, 92, 563–569. [Google Scholar] [CrossRef]
- Dar, H.Y.; Lone, Y.; Koiri, R.K.; Mishra, P.K.; Srivastava, R.K. Microcystin-leucine arginine (MC-LR) induces bone loss and impairs bone micro-architecture by modulating host immunity in mice: Implications for bone health. Environ. Pollut. 2018, 238, 792–802. [Google Scholar] [CrossRef]
- Humpage, A.R.; Falconer, I.R. Oral toxicity of the cyanobacterial toxin cylindrospermopsin in male swiss albino mice: Determination of no observed adverse effect level for deriving a drinking water guideline value. Environ. Toxicol. 2003, 18, 94–103. [Google Scholar] [CrossRef]
- Falconer, I.R.; Hardy, S.J.; Humpage, A.R.; Froscio, S.M.; Tozer, G.J.; Hawkins, P.R. Hepatic and renal toxicity of the blue-green alga (cyanobacterium) Cylindrospermopsis raciborskii in male swiss albino mice. Environ. Toxicol. 1999, 14, 143–150. [Google Scholar] [CrossRef]
- Guzmán-Guillén, R.; Prieto, A.I.; Vázquez, C.M.; Vasconcelos, V.; Cameán, A.M. The protective role of l-carnitine against cylindrospermopsin-induced oxidative stress in tilapia (Oreochromis niloticus). Aquat. Toxicol. 2013, 132–133, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, P.R.; Chandrasena, N.R.; Jones, G.J.; Humpage, A.R.; Falconer, I.R. Isolation and toxicity of Cylindrospermosis raciborskii from an ornamental lake. Toxicon 1997, 35, 341–346. [Google Scholar] [CrossRef]
- Norris, R.L.; Eaglesham, G.K.; Pierens, G.; Shaw, G.R.; Smith, M.J.; Chiswell, R.K.; Seawright, A.A.; Moore, M.R. Deoxycylindrospermopsin, an analog of cylindrospermopsin from Cylindrospermopsis raciborskii. Environ. Toxicol. 1999, 14, 163–165. [Google Scholar] [CrossRef]
- Seifert, M.; McGregor, G.; Eaglesham, G.; Wickramasinghe, W.; Shaw, G. First evidence for the production of cylindrospermopsin and deoxycylindrospermopsin by the freshwater benthic cyanobacterium, Lyngbya wollei (Farlow ex Gomont) Speziale and Dyck. Harmful Algae 2007, 6, 73–80. [Google Scholar] [CrossRef]
- Chernoff, N.; Hill, D.J.; Chorus, I.; Diggs, D.L.; Huang, H.; King, D.; Lang, J.R.; Le, T.-T.; Schmid, J.E.; Travlos, G.S.; et al. Cylindrospermopsin toxicity in mice following a 90-d oral exposure. J. Toxicol. Environ. Health Part A 2018, 81, 549–566. [Google Scholar] [CrossRef] [PubMed]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 13 January 2022).
- Decision 2020/569/EU. Commission Implementing Decision (EU) 2020/569 of 16 April 2020 Establishing a Common Format and Information Content for the Submission of the Information to be Reported by Member States Pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the Protection of Animals Used for Scientific Purposes and Repealing Commission Implementing Decision 2012/707/EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32020D0569 &from=EN (accessed on 13 January 2022).
- Real Decreto 1386/2018. Real Decreto 1386/2018, de 19 de Noviembre, por el que se Modifica el Real Decreto 53/2013, de 1 de Febrero, por el que se Establecen las Normas Básicas Aplicables Para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia, Vol. 280. BOE Núm. 2018. Available online: https://www.boe.es/boe/dias/2018/11/20/pdfs/BOE-A-2018-15797.pdf (accessed on 13 January 2022).
- Dhawan, S.S.; Xia, S.; Tait, D.S.; Bundgaard, C.; Bowman, E.; Brown, V.J. Oral dosing of rodents using a palatable tablet. Psychopharmacology 2018, 235, 1527–1532. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Length, R.V. Emmeans: Estimated Marginal Means, aka Least-Squares Means; R Package Version 1.7.0; 2021. The Comprehensive R Archive Network (CRAN) Repository; Institute for Statistics and Mathematics of WU (Wirtschaftsuniversität Wien): Vienna, Austria, 2021. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]


| MALE | FEMALE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Group 1 (0 µg/Kg/day) | Group 2 (18.75 µg/Kg/day) | Group 3 (37.5 µg/Kg/day) | Group 4 (75 µg/Kg/day) | Group 1 (0 µg/Kg/day) | Group 2 (18.75 µg/Kg/day) | Group 3 (37.5 µg/Kg/day) | Group 4 (75 µg/Kg/day) | ||
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | ||
| IL-1β | MEAN | 192.01 | 205.92 | 236.28 | 169.93 | 218.09 | 190.12 | 262.53 | 300.43 |
| SD | 45.21 | 56.19 | 56.98 | 33.89 | 41.22 | 61.96 | 159.78 | 146.09 | |
| K.W χ2 = 7.825 p = 0.35; N.S. | |||||||||
| IL-2 | MEAN | 7587.473 | 9125.263 | 10128.74 && | 9417.15 | 8988.63 | 4805.75 | 5649.99 | 8134.93 |
| SD | 984.78 | 1312.51 | 735.55 | 1131.08 | 1147.15 | 1670.85 | 1533.65 | 1596.87 | |
| K.W χ2 = 31.053 && p < 0.01 | |||||||||
| IL-6 | MEAN | 1027.99 | 1123.73 | 1369.89 && | 1202.74 | 1172.01 | 637.66 | 782.97 | 1092.62 |
| SD | 187.34 | 93.79 | 137.42 | 126.98 | 179.73 | 191.24 | 135.18 | 222.13 | |
| K.W χ2 = 31.492 && p < 0.01 | |||||||||
| TNF-α | MEAN | 653.40 | 786.23 | 908.90 & | 837.63 | 845.67 | 358.23 ** | 487.09 | 784.34 |
| SD | 66.40 | 141.69 | 112.18 | 151.93 | 91.41 | 124.78 | 174.91 | 107.19 | |
| K.W χ2 = 31.287 ** p < 0.01; & p < 0.05 | |||||||||
| IFN-γ | MEAN | 509.18 | 520.04 | 617.72&& | 546.59 | 551.73 | 330.11 | 364.92 | 480.6 |
| SD | 106.23 | 40.34 | 64.04 | 45.84 | 79.45 | 92.84 | 68.47 | 96.20 | |
| K.W χ2 = 29.699 && p < 0.01 | |||||||||
| ORGAN WEIGHT DATA SUMMARY | |||||||||||
| MALE | FEMALE | ||||||||||
| Group 1 | Group 2 | Group 3 | Group 4 | Group 1 | Group 2 | Group 3 | Group 4 | ||||
| (0 µg/Kg/day) | (18.75 µg/Kg/day) | (37.5 µg/Kg/day) | (75 µg/Kg/day) | (0 µg/Kg/day) | (18.75 µg/Kg/day) | (37.5 µg/Kg/day) | (75 µg/Kg/day) | ||||
| N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | N = 6 | ||||
| THYMUS (g) | MEAN | 1.1 | 1.0 | 1.0 | 1.1 | THYMUS (g) | MEAN | 0.7 | 0.8 | 0.9 | 0.9 |
| SD | 0.1 | 0.1 | 0.2 | 0.1 | SD | 0.0 | 0.2 | 0.1 | 0.1 | ||
| F (20.3) = 1.276 p = 0.31; N.S. | F (20.3) = 2.745 p = 0.07; N.S. | ||||||||||
| SPLEEN (g) | MEAN | 1.1 | 1.1 | 1.1 | 1.2 | SPLEEN (g) | MEAN | 0.6 | 0.7 | 0.7 | 0.7 |
| SD | 0.1 | 0.1 | 0.2 | 0.1 | SD | 0.0 | 0.1 | 0.1 | 0.0 | ||
| F (20.3) = 2.118 p = 0.13; N.S. | F (20.3) = 1.808 p = 0.18; N.S. | ||||||||||
| ORGAN WEIGHT/BODY WEIGHT RATIO DATA SUMMARY | |||||||||||
| MALE | FEMALE | ||||||||||
| THYMUS (g) | MEAN | 0.27 | 0.25 | 0.25 | 0.28 | THYMUS (g) | MEAN | 0.30 | 0.29 | 0.34 | 0.36 |
| SD | 0.02 | 0.02 | 0.04 | 0.02 | SD | 0.01 | 0.06 | 0.04 | 0.04 | ||
| F (20.3) = 1.913 p = 0.16; N.S. | F (20.3) = 3.681 p = 0.06; N.S. | ||||||||||
| SPLEEN (g) | MEAN | 0.25 | 0.26 | 0.26 | 0.31 * | SPLEEN (g) | MEAN | 0.26 | 0.25 | 0.27 | 0.29 |
| SD | 0.02 | 0.02 | 0.05 | 0.02 | SD | 0.02 | 0.04 | 0.02 | 0.02 | ||
| F (20.3) = 3.795 * p < 0.05 | K.W χ2 = 5.410 p = 0.14; N.S. | ||||||||||
| ORGAN WEIGHT/BRAIN WEIGHT RATIO DATA SUMMARY | |||||||||||
| MALE | FEMALE | ||||||||||
| THYMUS (g) | MEAN | 54.7 | 51.8 | 48.0 | 54.4 | THYMUS (g) | MEAN | 37.7 | 40.3 | 46.1 | 45.6 |
| SD | 3.1 | 4.8 | 9.2 | 4.3 | SD | 3.5 | 7.9 | 7.5 | 6.3 | ||
| F (20.3) = 1.702 p = 0.20; N.S. | F (20.3) = 2.366 p = 0.10; N.S. | ||||||||||
| SPLEEN (g) | MEAN | 50.9 | 55.1 | 51.3 | 60.6 | SPLEEN (g) | MEAN | 33.6 | 35.1 | 36.4 | 36.7 |
| SD | 3.9 | 3.4 | 9.6 | 6.8 | SD | 3.7 | 4.6 | 1.7 | 1.5 | ||
| F (20.3) = 2.916 p = 0.06; N.S. | F (20.3) = 1.216 p = 0.33; N.S. | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez-Quijada, L.; Casas-Rodriguez, A.; Guzmán-Guillén, R.; Molina-Hernández, V.; Albaladejo, R.G.; Cameán, A.M.; Jos, A. Immunomodulatory Effects of Pure Cylindrospermopsin in Rats Orally Exposed for 28 Days. Toxins 2022, 14, 144. https://doi.org/10.3390/toxins14020144
Diez-Quijada L, Casas-Rodriguez A, Guzmán-Guillén R, Molina-Hernández V, Albaladejo RG, Cameán AM, Jos A. Immunomodulatory Effects of Pure Cylindrospermopsin in Rats Orally Exposed for 28 Days. Toxins. 2022; 14(2):144. https://doi.org/10.3390/toxins14020144
Chicago/Turabian StyleDiez-Quijada, Leticia, Antonio Casas-Rodriguez, Remedios Guzmán-Guillén, Verónica Molina-Hernández, Rafael G. Albaladejo, Ana María Cameán, and Angeles Jos. 2022. "Immunomodulatory Effects of Pure Cylindrospermopsin in Rats Orally Exposed for 28 Days" Toxins 14, no. 2: 144. https://doi.org/10.3390/toxins14020144
APA StyleDiez-Quijada, L., Casas-Rodriguez, A., Guzmán-Guillén, R., Molina-Hernández, V., Albaladejo, R. G., Cameán, A. M., & Jos, A. (2022). Immunomodulatory Effects of Pure Cylindrospermopsin in Rats Orally Exposed for 28 Days. Toxins, 14(2), 144. https://doi.org/10.3390/toxins14020144









