Improving the In Vitro Removal of Indoxyl Sulfate and p-Cresyl Sulfate by Coating Diatomaceous Earth (DE) and Poly-vinyl-pyrrolidone-co-styrene (PVP-co-S) with Polydopamine
Abstract
1. Introduction
2. Results and Discussion
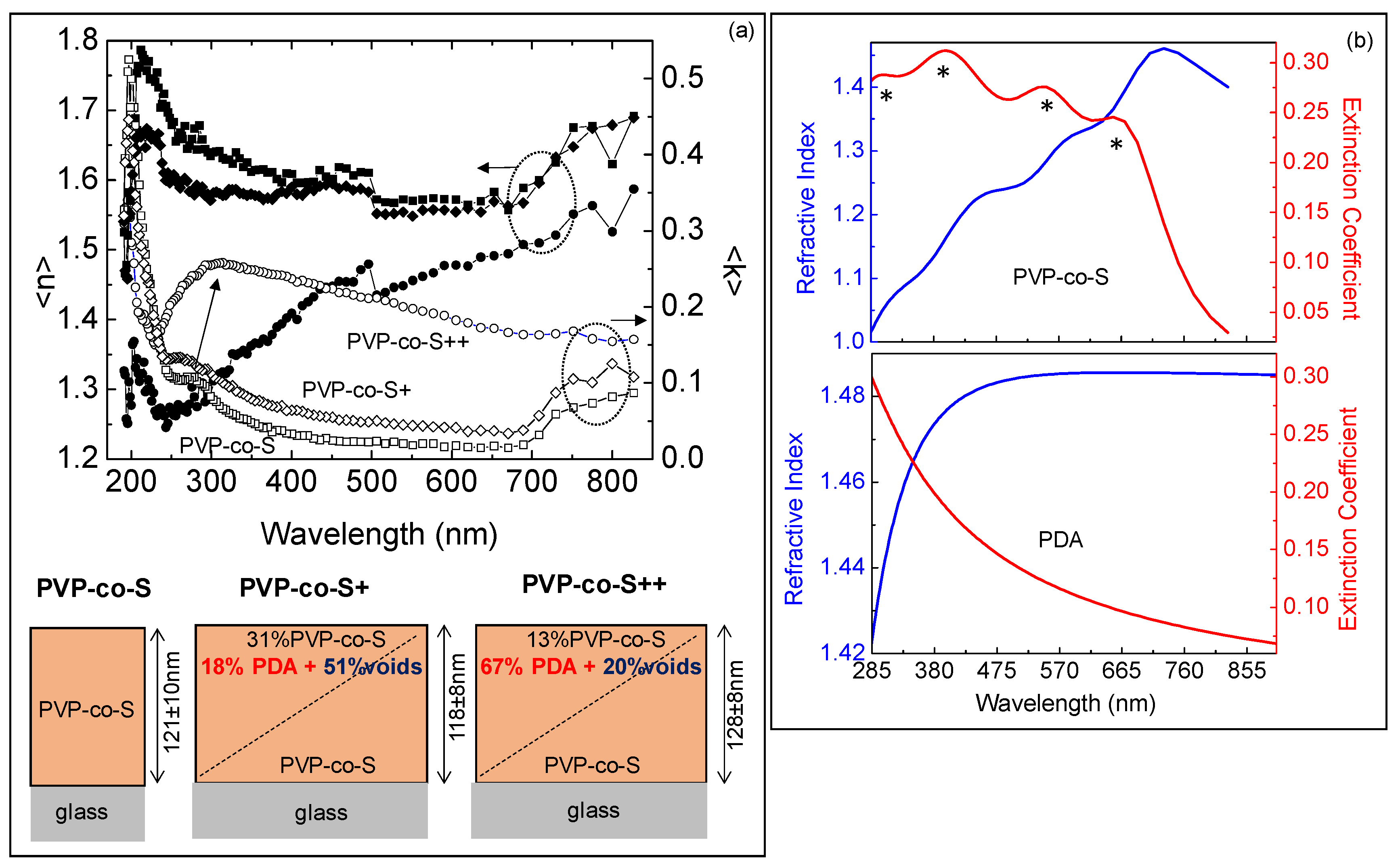
2.1. In-Solution Characterization of PDA-Coated Adsorbent Materials: UV-Vis Spectra
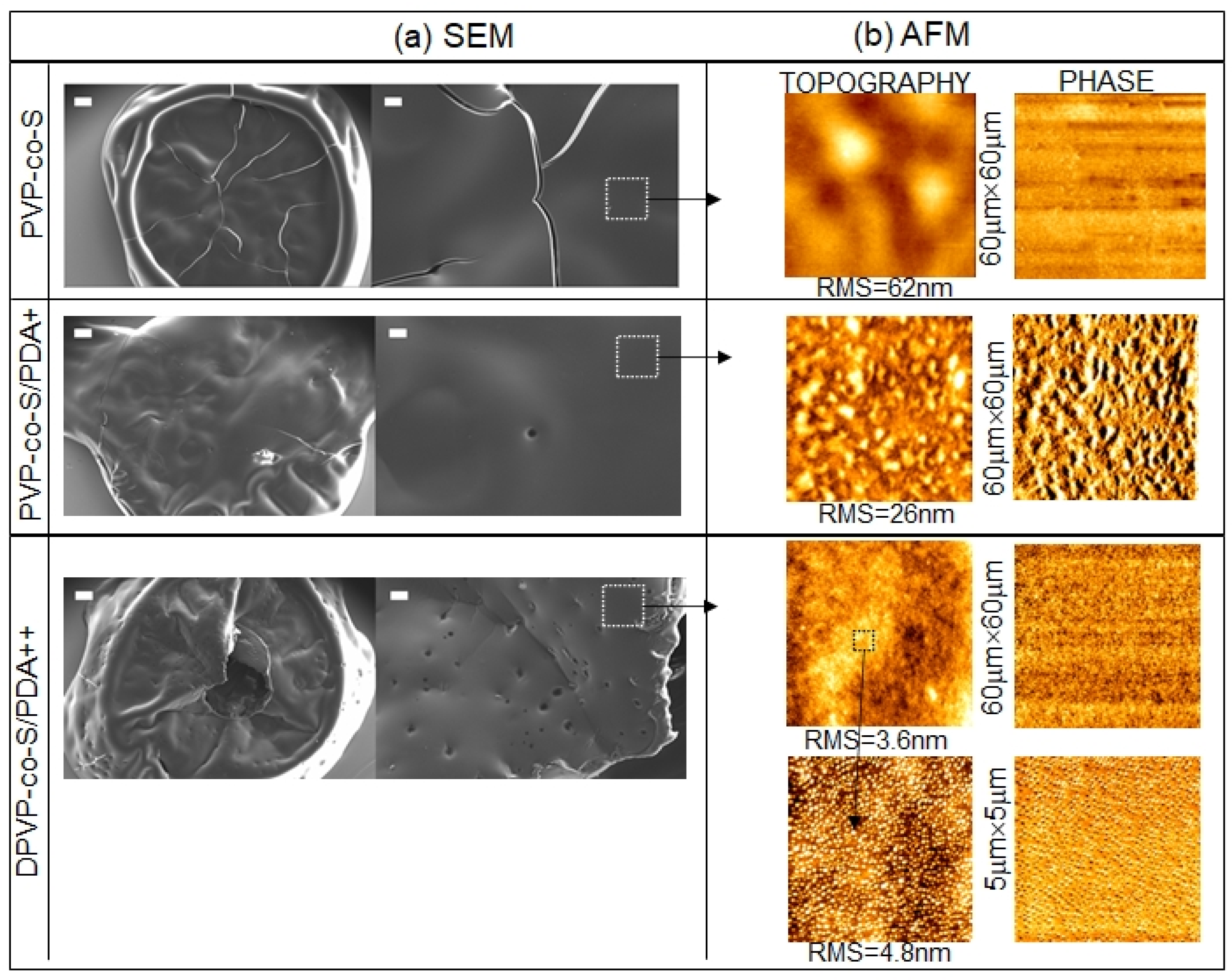
2.2. Solid State Characterization of PDA-Coated Adsorbent Materials: Raman Spectroscopy, AFM and Spectroscopic Ellipsometry
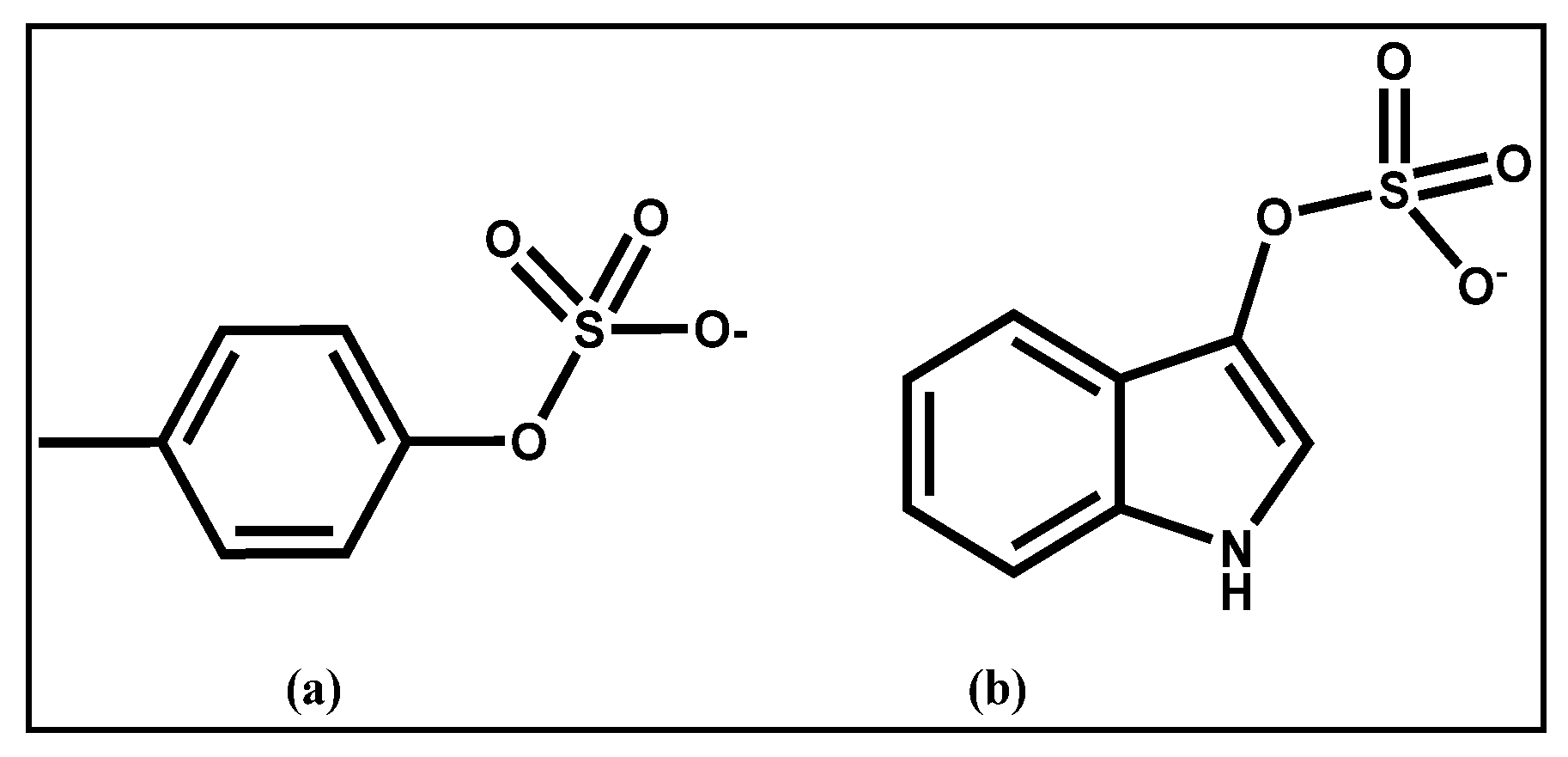
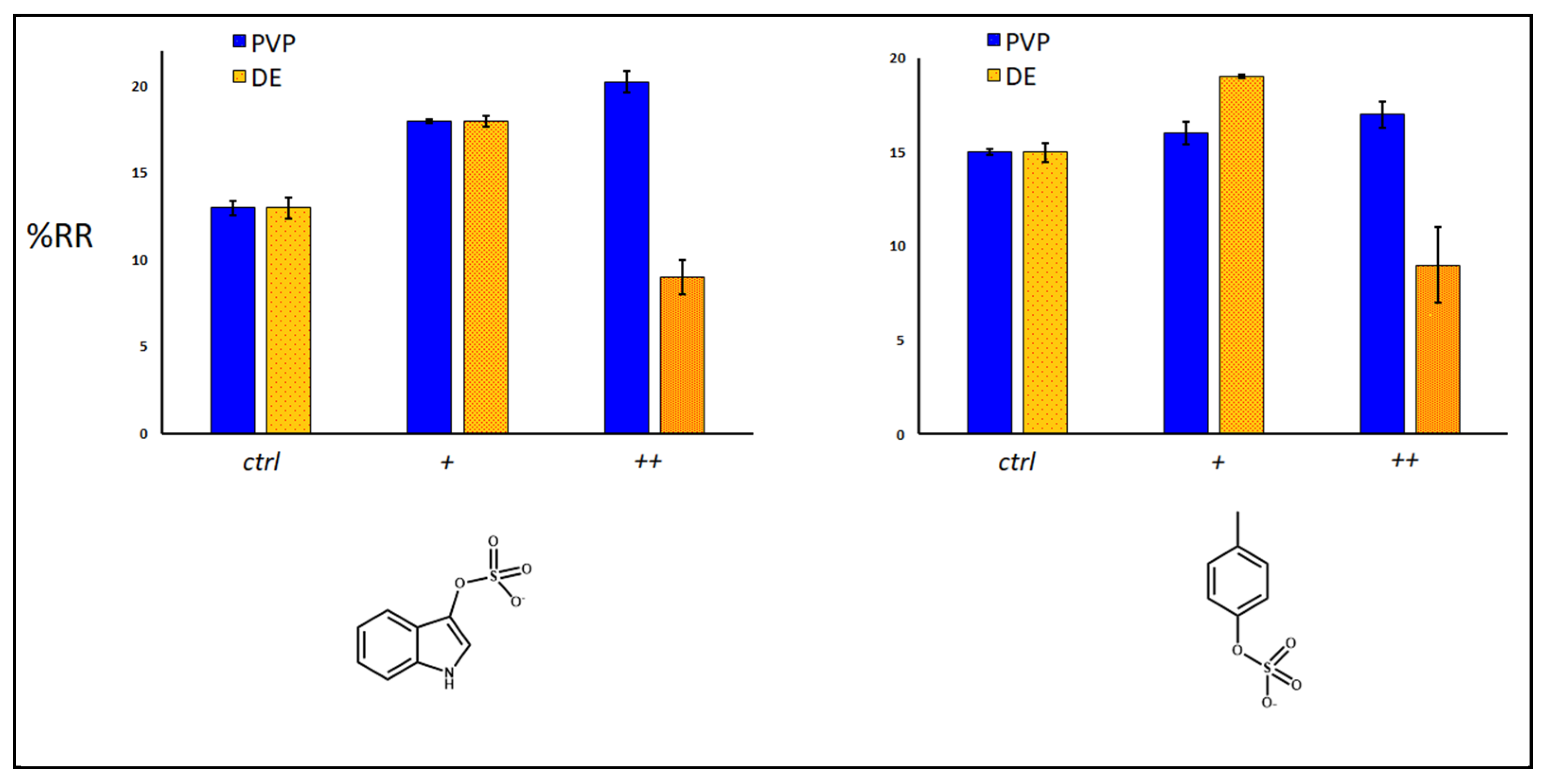
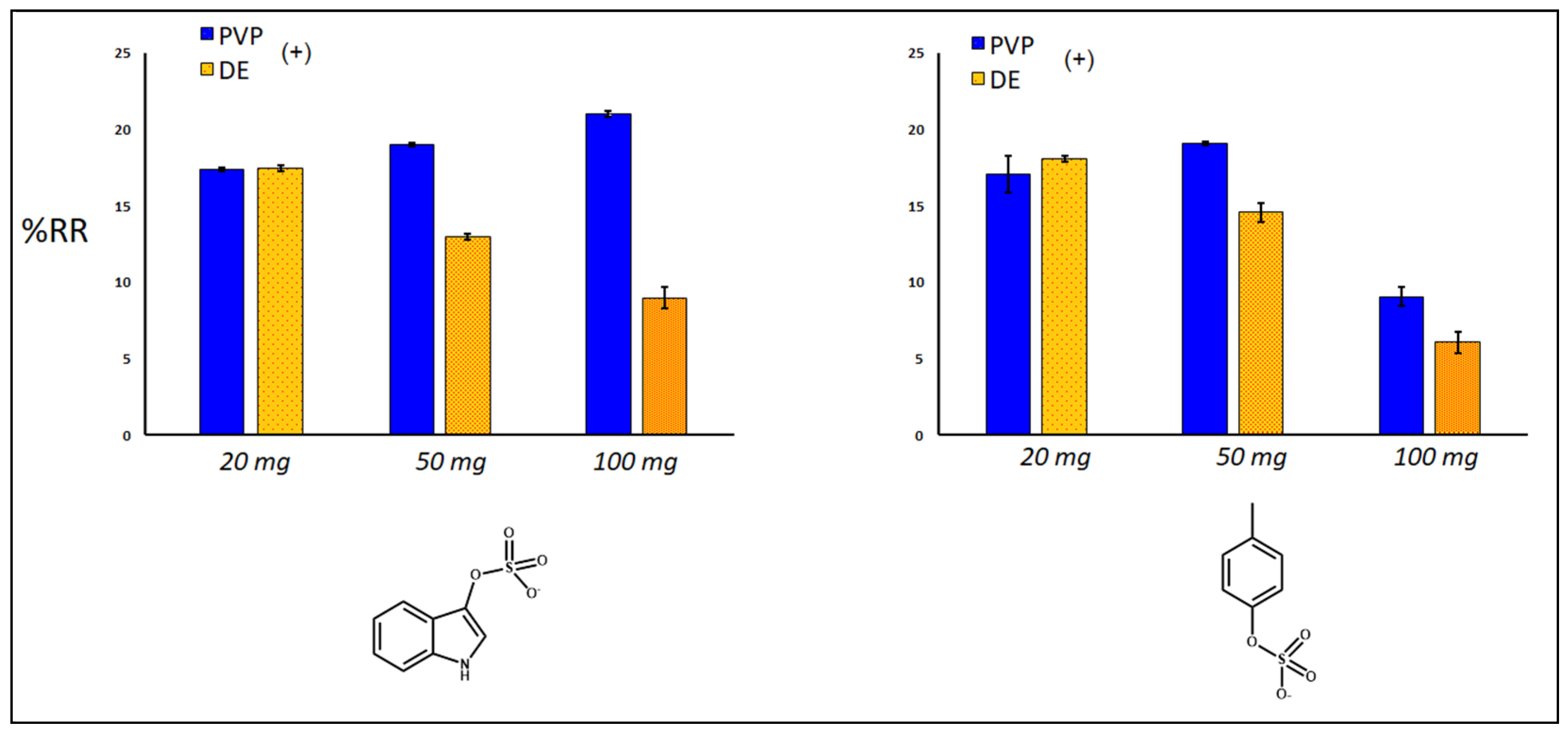
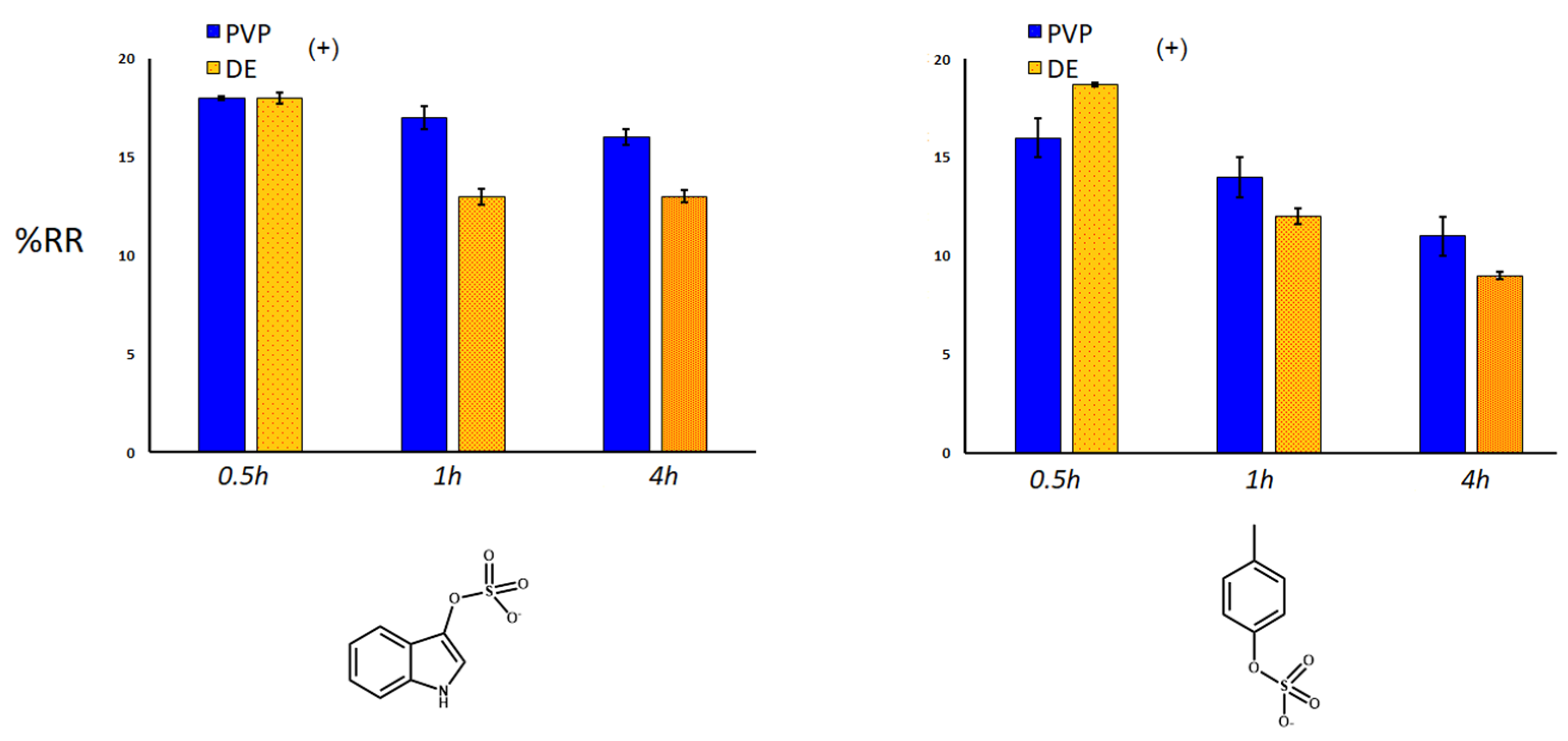
2.3. Removal of Uremic Toxins
3. Conclusions
4. Materials and Methods
4.1. Materials and Equipment
4.2. Material Preparation
4.3. Material Characterization
4.4. In Vitro Adsorption Experiment
4.5. LC-MS/MS for Quantification of PCS and IS
4.6. Statistical Validation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pretorius, C.J.; Mc Whinney, B.C.; Sipinkoski, B.; Johnson, L.A.; Rossi, M.; Campbell, K.L.; Ungerer, J.P.J. Reference ranges and biological variation of free and total serum indoxyl- and p-cresyl sulphate measured with a rapid UPLC fluorescence detection method. Clin. Chim. Acta 2013, 419, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Liabeuf, S.; Drüeke, T.B.; Massy, Z.A. Protein-bound uremic toxins: New insight from clinical studies. Toxins 2011, 3, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Pan, C.-F.; Liu, H.-L.; Chuang, C.-K.; Jayakumar, T.; Wang, T.-J.; Chen, H.-H.; Wu, C.-J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 2012, 225, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Cosola, C.; Rocchetti, M.T.; Cupisti, A.; Gesualdo, L. Microbiota metabolites: Pivotal players of cardiovascular damage in chronic kidney disease. Pharmacol. Res. 2018, 130, 132–142. [Google Scholar] [CrossRef]
- Panichi, V.; Rocchetti, M.T.; Scatena, A.; Rosati, A.; Migliori, M.; Pizzarelli, F.; Gesualdo, L. Long term variation of serum levels of uremic toxins in patients treated by post-dilution high volume on-line hemodiafiltration in comparison to standard low-flux bicarbonate dialysis: Results from the REDERT study. J. Nephrol. 2017, 30, 583–591. [Google Scholar] [CrossRef]
- Krieter, D.H.; Hackl, A.; Rodriguez, A.; Chenine, L.; Moragues, H.L.; Lemke, H.D.; Wanner, C.; Canaud, B. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol. Dial. Transplant. 2010, 25, 212–218. [Google Scholar] [CrossRef]
- Niwa, T. Removal of Protein-Bound Uraemic Toxins by Haemodialysis. Blood Pur. 2013, 35, 20–25. [Google Scholar] [CrossRef]
- Samtleben, W.; Dengler, C.; Reinhardt, B.; Nothdurft, A.; Lemke, H.D. Comparison of the new polyethersulfone high-flux membrane DIAPES® HF800 with conventional high-flux membranes during on-line haemodiafiltration. Nephrol. Dial. Transplant. 2003, 18, 2382–2386. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Cosola, C.; di Bari, I.; Magnani, S.; Galleggiante, V.; Scandiffio, L.; Dalfino, G.; Netti, G.S.; Atti, M.; Corciulo, R.; et al. Efficacy of Divinylbenzenic Resin in Removing Indoxyl Sulfate and P-Cresol Sulfate in Hemodialysis Patients: Results From an In Vitro Study and An In Vivo Pilot Trial (xuanro4-Nature 3.2). Toxins 2020, 12, 170. [Google Scholar] [CrossRef]
- Sartori, M.; Sharma, A.; Neri, M.; Garzotto, F.; Nalesso, F.; Giavarina, D.; Zancato, M.R.C. New option for the treatment of hyperbilirubinemia: In vitro direct hemoperfusion with the Lixelle S-35. Int. J. Artif. Organs 2014, 37, 816–823. [Google Scholar] [CrossRef]
- Winchester, J.F.; Kellum, J.A.; Ronco, C.; Brady, J.A.; Quartararo, P.J.; Salsberg, J.A.; Levin, N.W. Sorbents in acute renal failure and the systemic inflammatory response syndrome. Blood Purif. 2003, 21, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Libetta, C.; Zucchi, M.; Gori, E.; Sepe, V.; Galli, F.; Meloni, F.; Milanesi, F.; Dal Canton, A. Vitamin E-loaded dialyzer resets PBMC-operated cytokine network in dialysis patients. Kidney Int. 2004, 65, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Ragni, R.; Cicco, S.R.; Vona, D.; Farinola, G.M. Multiple routes to smart nanostructured materials from diatom microalgae: A chemical perspective. Adv. Mater. 2018, 30, 1704289. [Google Scholar] [CrossRef] [PubMed]
- Vona, D.; Cicco, S.R.; Ragni, R.; Leone, G.; Lo Presti, M.; Farinola, G.M. Biosilica/polydopamine/silver nanoparticles composites: New hybrid multifunctional heterostructures obtained by chemical modification of Thalassiosira weissflogii silica shells. MRS Commun. 2018, 8, 911–917. [Google Scholar] [CrossRef]
- Leone, G.; De la Cruz Valbuena, G.; Cicco, S.R.; Vona, D.; Altamura, E.; Ragni, R.; Molotokaite, E.; Cecchin, M.; Cazzaniga, S.; Ballottari, M.; et al. Incorporating a molecular antenna in diatom microalgae cells enhances photosynthesis. Sci. Rep. 2021, 11, 5209. [Google Scholar] [CrossRef]
- Della Rosa, G.; Vona, D.; Aloisi, A.; Ragni, R.; Di Corato, R.; Lo Presti, M.; Cicco, S.R.; Altamura, E.; Taurino, A.; Catalano, M.; et al. Luminescent silica based nanostructures from in vivo Iridium-doped diatoms microalgae. ACS Sustain. Chem. Eng. 2018, 7, 2207–2215. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; De Giglio, E.; Cometa, S.; Mattioli-Belmonte, M.; Palumbo, F.; Ragni, R.; Farinola, G.M. Chemically Modified Diatoms Biosilica for Bone Cell Growth with Combined Drug-Delivery and Antioxidant Properties. ChemPlusChem 2015, 80, 1104–1112. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Leone, G.; De Giglio, E.; Bonifacio, M.R.; Cometa, S.; Fiore, S.; Palumbo, F.; Ragni, R.; Farinola, G.M. In vivo functionalization of diatom biosilica with sodium alendronate as osteoactive material. Mater. Sci. Eng. C 2019, 104, 109897. [Google Scholar] [CrossRef]
- Leone, G.; Vona, D.; Lo Presti, M.; Urbano, L.; Cicco, S.; Gristina, R.; Palumbo, F.; Ragni, R.; Farinola, G.M. Ca2+-in vivo doped biosilica from living Thalassiosira weissflogii diatoms: Investigation on Saos-2 biocompatibility. Mater. Res. Soc. 2017, 2, 1047–1058. [Google Scholar] [CrossRef]
- Cicco, S.R.; Vona, D.; Leone, G.; Lo Presti, M.; Palumbo, F.; Altamura, E.; Ragni, R.; Farinola, G.M. From polydisperse diatomaceous earth to biosilica with specific morphologies by glucose gradient/dialysis: A natural material for cell growth. MRS Commun. 2017, 7, 214–220. [Google Scholar] [CrossRef]
- Vona, D.; Leone, G.; Ragni, R.; Palumbo, F.; Evidente, A.; Vurro, M.; Farinola, G.M.; Cicco, S.R. Diatoms Biosilica as Efficient Drug-Delivery System. MRS Adv. 2016, 1, 3825–3830. [Google Scholar] [CrossRef]
- Lo Presti, M.; Ragni, R.; Vona, D.; Leone, G.; Cicco, S.; Farinola, G.M. In vivo doped biosilica from living Thalassiosira weissflogii diatoms with a triethoxysilyl functionalized red emitting fluorophore. MRS Adv. 2018, 3, 1509–1517. [Google Scholar] [CrossRef]
- Vona, D.; Ragni, R.; Altamura, E.; Albanese, P.; Giangregorio, M.M.; Cicco, S.R.; Farinola, G.M. Light emitting biosilica by in vivo functionalization of phaeodactylum tricornutum diatom microalgae with organometallic complexes. Appl. Sci. 2021, 11, 3327. [Google Scholar] [CrossRef]
- Ardizzone, A.; Blasi, D.; Vona, D.; Rosspeintner, A.; Punzi, A.; Altamura, E.; Grimaldi, N.; Sala, S.; Vauthey, E.; Farinola, G.M.; et al. Highly Stable and Red-Emitting Nanovesicles Incorporating Lipophilic Diketopyrrolopyrroles for Cell Imaging. Chem. Eur. J. 2018, 24, 11386–11392. [Google Scholar] [CrossRef]
- Vicente-Garcia, C.; Losacco, V.; Vona, D.; Cicco, S.R.; Altamura, E.; Farinola, G.M.; Ragni, R. Straightforward and effective removal of phenolic compounds from water by Polydopamine coated Diatomaceous Earth. In International Workshop on Metrology for the Sea; Learning to Measure Sea Health Parameters (MetroSea); IEEE: New York City, NY, USA, 2021; pp. 27–31. [Google Scholar]
- Buscemi, G.; Vona, D.; Ragni, R.; Comparelli, R.; Trotta, M.; Milano, F.; Farinola, G.M. Polydopamine/Ethylenediamine Nanoparticles Embedding a Photosynthetic Bacterial Reaction Center for Efficient Photocurrent Generation. Adv. Sustain. Syst. 2021, 5, 2000303. [Google Scholar] [CrossRef]
- Vona, D.; Cicco, S.R.; Ragni, R.; Vincente-Garcia, C.; Leone, G.; Giangregorio, M.M.; Palumbo, F.; Altamura, E.; Farinola, G.M. Polydopamine coating of living diatom microalgae. Photochem. Photobiol. Sci. 2022, 21, 945–958. [Google Scholar] [CrossRef]
- Lo Presti, M.; Giangregorio, M.M.; Ragni, R.; Giotta, L.; Guascito, M.R.; Comparelli, R.; Fanizza, E.; Tangorra, R.R.; Agostiano, A.; Losurdo, M.; et al. Photoelectrodes with Polydopamine Thin Films Incorporating a Bacterial Photoenzyme. Adv. Electron. Mater. 2020, 6, 2000140. [Google Scholar] [CrossRef]
- Fantacci, S.; De Angelis, F.; Nazeeruddin, M.K.; Gratzel, M. Electronic and Optical Properties of the Spiro-MeOTAD Hole Conductor in Its Neutral and Oxidized Forms: A DFT/TDDFT Investigation. J. Phys. Chem. C 2011, 115, 23126–23133. [Google Scholar] [CrossRef]
- Shin, W.S.; Joo, M.K.; Kim, S.C.; Park, S.M.; Jin, S.H.; Shim, J.M.; Lee, J.K.; Lee, J.W.; Gal, Y.S.; Jenekhe, S.A.; et al. Synthesis and electro-optical properties of spiro-bifluorenylvinylene-based polymers for light-emitting diodes applications. J. Mater. Chem. 2006, 16, 4123–4132. [Google Scholar] [CrossRef]
- Sanchez, C.O.; Sobarzo, P.; Gatica, N. Electronic and structural properties of polymers based on phenylene vinylene and thiophene units. Control of the gap by gradual increases of thiophene moieties. N. J. Chem. 2015, 39, 7979–7987. [Google Scholar] [CrossRef]
- Hedl, E.; Fabijani, I.; Raki´, I.S.; Vadla, I.; Sancho-Parramon, J. Fabrication by spin-coating and optical characterization of poly (styrene-co-acrylonitrile) thin films. Coatings 2021, 11, 1015. [Google Scholar] [CrossRef]
- Lee, H.A.; Park, E.; Lee, H. Polydopamine and Its Derivative Surface Chemistry in Material Science: A Focused Review for Studies at KAIST. Adv. Mater. 2020, 32, 1907505. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Niu, H.; Ma, Y.; Zeng, T.; Cai, Y.; Meng, Z. Preparation of polydopamine coated Fe3O4 nanoparticles and their application for enrichment of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2013, 1283, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Aresta, A.; Cicco, R.S.; Vona, D.; Farinola, G.M.; Zambonin, C. Mussel Inspired Polydopamine as Silica Fibers Coating for Solid Phase Microextraction. Separations 2022, 9, 194. [Google Scholar] [CrossRef]
- Kumar, A.; Malik, A.K.; Tewary, D.K.; Singh, B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal. Chim. Acta 2008, 610, 1–14. [Google Scholar] [CrossRef]
- Amiri, A. Solid-phase microextraction-based sol–gel technique. Trends Anal. Chem. 2016, 75, 57–74. [Google Scholar] [CrossRef]
- Kim, T.Y.; Alhooshani, K.; Kabir, A.; Fries, D.P.; Malik, A. High pH-resistant, surface-bonded sol–gel titania hybrid organic–inorganic coating for effective on-line hyphenation of capillary microextraction (in-tube solid-phase microextraction) with high-performance liquid chromatography. J. Chromatogr. A 2004, 1047, 165–174. [Google Scholar] [CrossRef]
- Krieter, D.H.; Devine, E.; Körner, T.; Rüth, M.; Wanner, C.; Raine, M.; Jankowski, J.; Lemke, H.-D. Haemodiafiltration at increased plasma ionic strength for improved protein-bound toxin removal. Acta Physiol. (Oxf) 2017, 219, 510–520. [Google Scholar] [CrossRef]
- Madero, M.; Cano, K.B.; Campos, I.; Tao, X.; Maheshwari, V.; Brown, J.; Cornejo, B.; Handelman, G.; Thijssen, S.; Kotanko, P. Removal of protein-bound uremic toxins during hemodialysis using a binding competitor. Clin. J. Am. Soc. Nephrol. 2019, 14, 394–402. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cicco, S.R.; Giangregorio, M.M.; Rocchetti, M.T.; di Bari, I.; Mastropaolo, C.; Labarile, R.; Ragni, R.; Gesualdo, L.; Farinola, G.M.; Vona, D. Improving the In Vitro Removal of Indoxyl Sulfate and p-Cresyl Sulfate by Coating Diatomaceous Earth (DE) and Poly-vinyl-pyrrolidone-co-styrene (PVP-co-S) with Polydopamine. Toxins 2022, 14, 864. https://doi.org/10.3390/toxins14120864
Cicco SR, Giangregorio MM, Rocchetti MT, di Bari I, Mastropaolo C, Labarile R, Ragni R, Gesualdo L, Farinola GM, Vona D. Improving the In Vitro Removal of Indoxyl Sulfate and p-Cresyl Sulfate by Coating Diatomaceous Earth (DE) and Poly-vinyl-pyrrolidone-co-styrene (PVP-co-S) with Polydopamine. Toxins. 2022; 14(12):864. https://doi.org/10.3390/toxins14120864
Chicago/Turabian StyleCicco, Stefania Roberta, Maria Michela Giangregorio, Maria Teresa Rocchetti, Ighli di Bari, Claudio Mastropaolo, Rossella Labarile, Roberta Ragni, Loreto Gesualdo, Gianluca Maria Farinola, and Danilo Vona. 2022. "Improving the In Vitro Removal of Indoxyl Sulfate and p-Cresyl Sulfate by Coating Diatomaceous Earth (DE) and Poly-vinyl-pyrrolidone-co-styrene (PVP-co-S) with Polydopamine" Toxins 14, no. 12: 864. https://doi.org/10.3390/toxins14120864
APA StyleCicco, S. R., Giangregorio, M. M., Rocchetti, M. T., di Bari, I., Mastropaolo, C., Labarile, R., Ragni, R., Gesualdo, L., Farinola, G. M., & Vona, D. (2022). Improving the In Vitro Removal of Indoxyl Sulfate and p-Cresyl Sulfate by Coating Diatomaceous Earth (DE) and Poly-vinyl-pyrrolidone-co-styrene (PVP-co-S) with Polydopamine. Toxins, 14(12), 864. https://doi.org/10.3390/toxins14120864






