Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology
Abstract
1. Introduction
2. Results
2.1. Potassium Content in Raw Samples
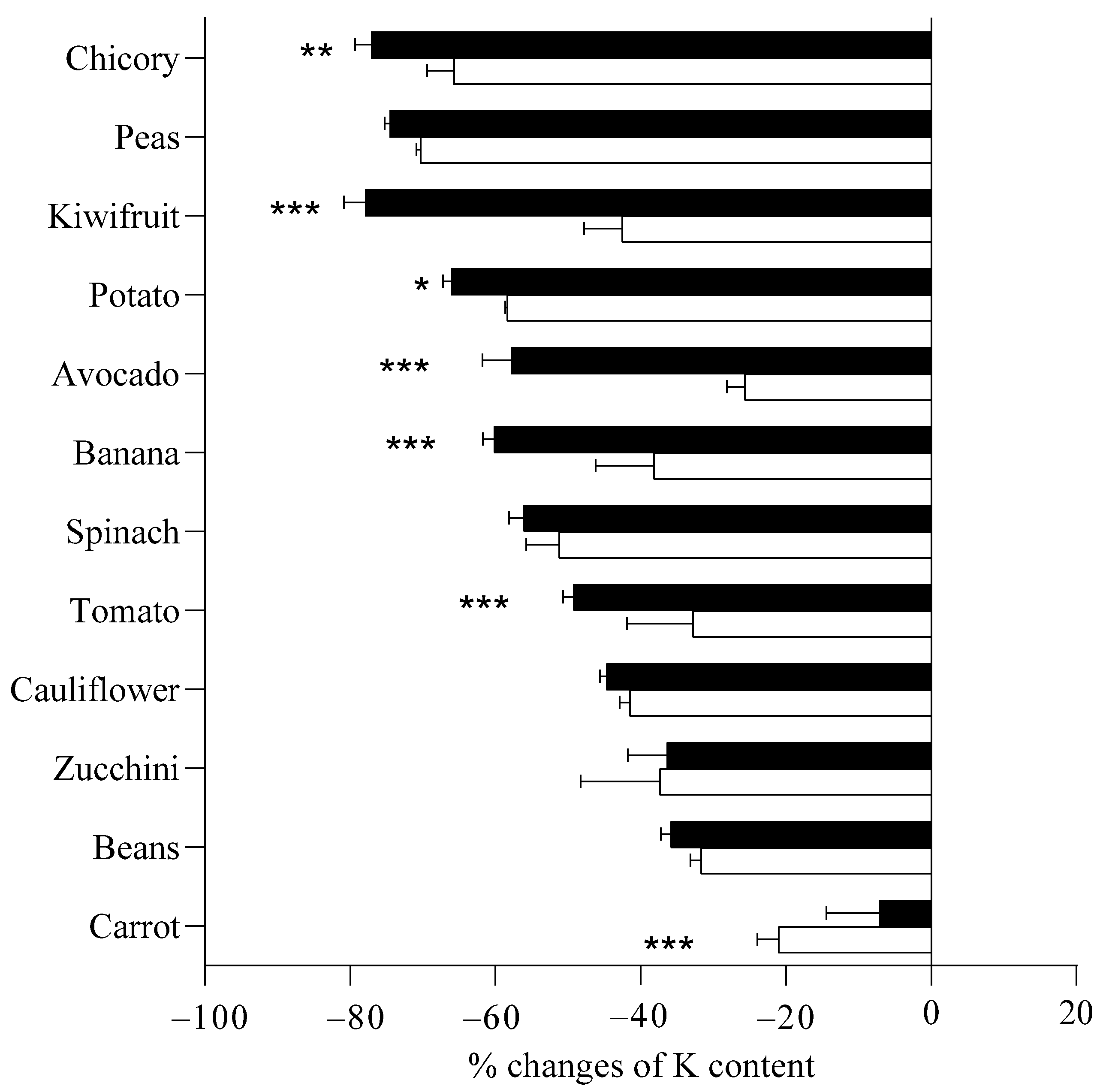
2.2. Effect of Boiling Procedures
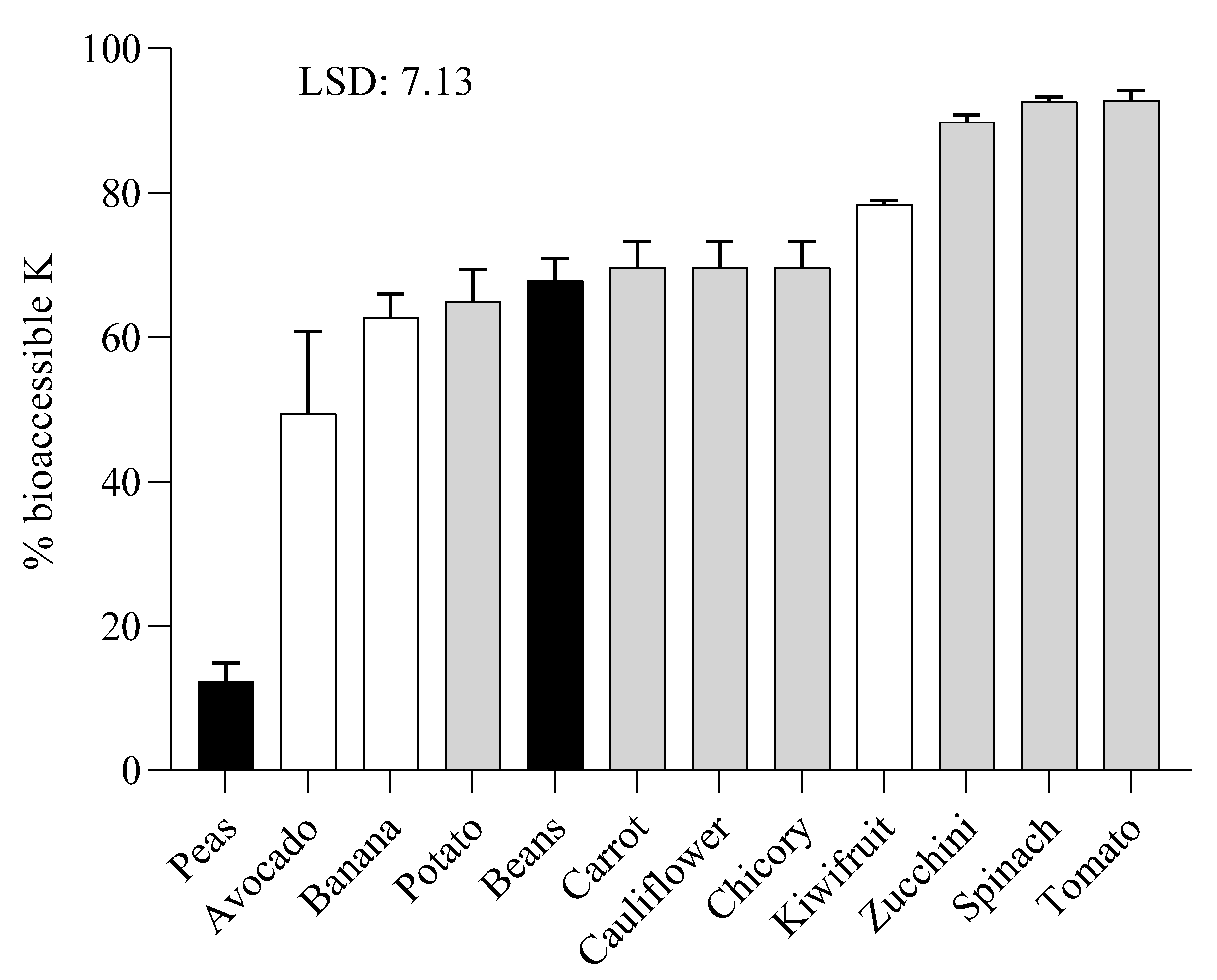
2.3. Potassium Bioaccessibility of Raw Samples
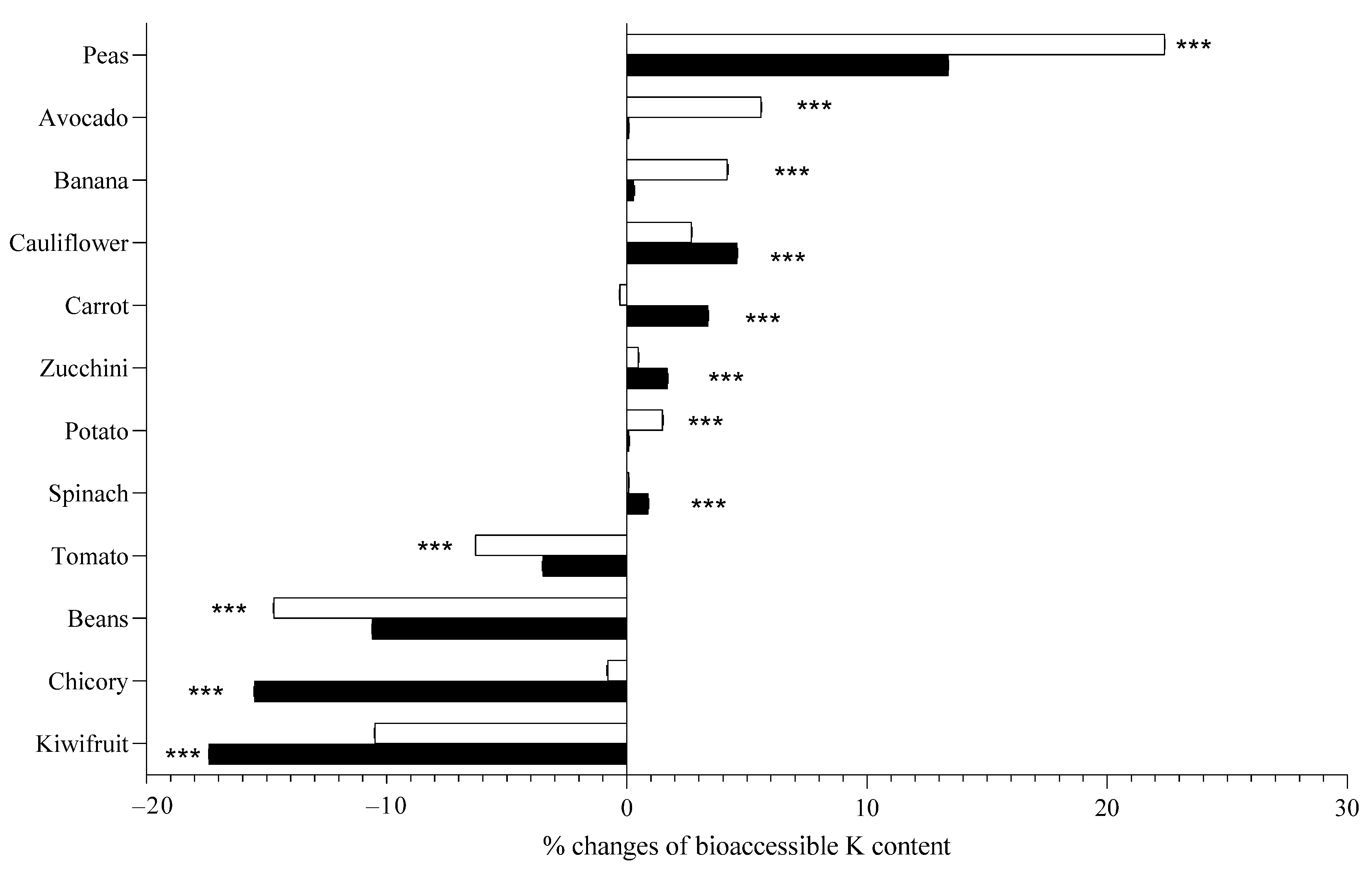
2.4. Effect of Boiling on Potassium Bioaccessibility
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Boiling Procedures
5.3. In Vitro Digestion Protocol
5.4. K Determination
5.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kovesdy, C.P. Management of hyperkalaemia in chronic kidney disease. Nat. Rev. Nephrol. 2014, 10, 653–662. [Google Scholar] [CrossRef]
- Palmer, B.F.; Clegg, D.J. Physiology and pathophysiology of potassium homeostasis: Core curriculum. Am. J. Kidney Dis. 2019, 74, 682–695. [Google Scholar] [CrossRef]
- De Nicola, L.; Di Lullo, L.; Paoletti, E.; Cupisti, A.; Bianchi, S. Chronic hyperkalemia in non-dialysis CKD: Controversial issues in nephrology practice. J. Nephrol. 2018, 31, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; D’Alessandro, C.; Gesualdo, L.; Cosola, C.; Gallieni, M.; Egidi, M.F.; Fusaro, M. Non-Traditional Aspects of Renal Diets: Focus on Fiber, Alkali and Vitamin K1 Intake. Nutrients 2017, 29, 444. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, C.; Pezzica, J.; Bolli, C.; Di Nicola, A.; Falai, A.; Giannese, D.; Cupisti, A. Processed Plant-Based Foods for CKD Patients: Good Choice, but Be Aware. Int. J. Environ. Res. Public Health 2022, 19, 6653. [Google Scholar] [CrossRef] [PubMed]
- Babich, J.S.; Kalantar-Zadeh, K.; Joshi, S. Taking the kale out of hyperkalemia: Plant foods and serum potassium in patients with kidney disease. J. Ren. Nutr. 2022, in press. [CrossRef] [PubMed]
- Peijnenburg, W.J.G.M.; Jager, T. Monitoring approaches to assess bioaccessibility and bioavailability of metals: Matrix issues. Ecotoxicol. Environ. Saf. 2003, 56, 63–77. [Google Scholar] [CrossRef]
- Cupisti, A.; Kovesdy, C.P.; D’Alessandro, C.; Kalantar-Zadeh, K. Dietary Approach to Recurrent or Chronic Hyperkalaemia in Patients with Decreased Kidney Function. Nutrients 2018, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.H.; McGlasson, W.B.; Graham, D.; Joyce, D.C. Structure and composition. In Postharvest: An Introduction to the Physiology and Handling of Fruit, Vegetables and Ornamentals, 5th ed.; CABI: Wallingford, UK, 2007; pp. 15–27. [Google Scholar]
- Martin, C.; Zhang, Y.; Tonelli, C.; Petroni, K. Plants, Diet, and Health. Annu. Rev. Plant Biol. 2013, 64, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Waldron, K.; Parker, M.L.; Smith, A. Plant Cell Walls and Food Quality. Compr. Rev. Food Sci. Food Saf. 2003, 2, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gidley, M.J.; Dhital, S. Wall porosity in isolated cells from food plants: Implications for nutritional functionality. Food Chem. 2019, 279, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Lentle, R.G.; Janssen, P.W.M. The Physical Processes of Digestion; Springer: New York, NY, USA, 2011. [Google Scholar]
- Holland, C.; Ryden, P.; Edwards, C.H.; Grundy, M.M.L. Plant Cell Walls: Impact on Nutrient Bioaccessibility and Digestibility. Foods 2020, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; González-Ortiz, A.; Avesani, C.M.; Bakker, S.J.L.; Bellizzi, V.; Chauveau, P.; Clase, C.M.; Cupisti, A.; Espinosa-Cuevas, A.; Molina, P.; et al. Plant-based diets to manage the risks and complications of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 525–542. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Clarke, C.J.; Martin, B.R.; McCabe, L.D.; McCabe, G.P.; Lachcik, P.J.; Wastney, M.; Weaver, C.M. Bioavailability of potassium from potatoes and potassium gluconate: A randomized dose response trial. Am. J. Clin. Nutr. 2016, 104, 346–353. [Google Scholar] [CrossRef]
- Naismith, D.J.; Braschi, A. An investigation into the bioaccessibility of potassium in unprocessed fruits and vegetables. Int. J. Food Sci. Nutr. 2008, 59, 438–450. [Google Scholar] [CrossRef]
- Mickelsen, O.D.J.R.; Makdani, D.; Gill, J.L.; Frank, R.L. Sodium and potassium intakes and excretions of normal men consuming sodium chloride or a 1: 1 mixture of sodium and potassium chlorides. Am. J. Clin. Nutr. 1977, 30, 2033–2040. [Google Scholar] [CrossRef]
- Holbrook, J.T.; Patterson, K.Y.; Bodner, J.E.; Douglas, L.W.; Veillon, C.; Kelsay, J.L.; Smith, J.C., Jr. Sodium and potassium intake and balance in adults consuming self-selected diets. Am. J. Clin. Nutr. 1984, 40, 786–793. [Google Scholar] [CrossRef]
- Krogholm, K.S.; Haraldsdóttir, J.; Knuthsen, P.; Rasmussen, S.E. Urinary total flavonoid excretion but not 4-pyridoxic acid or potassium can be used as a biomarker for the intake of fruits and vegetables. J. Nutr. 2004, 134, 445–451. [Google Scholar] [CrossRef]
- Wang, X.; Horisberger, J.D. A conformation of Na(+)-K+ pump is permeable to proton. Am. J. Physiol.-Cell Physiol. 1995, 268, C590–C595. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Palmer, B.F.; Colbert, G.; Clegg, D.J. Potassium Homeostasis, Chronic Kidney Disease, and the Plant-Enriched Diets. Kidney360 2020, 1, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wolters, M.G.E.; Diepenmaat, H.B.; Hermus, R.J.J.; Voragen, A.G.J. Relation Between in vitro Availability of Minerals and Food Composition: A Mathematical Model. J. Food Sci. 1993, 58, 1349–1355. [Google Scholar] [CrossRef]
- Gupta, S.; Lakshmi, J.A.; Prakash, J. In vitro bioavailability of calcium and iron from selected green leafy vegetables. J. Sci. Food Agric. 2006, 86, 2147–2152. [Google Scholar] [CrossRef]
- Hemalatha, S.; Platel, K.; Srinivasan, K. Influence of heat processing on the bioaccessibility of zinc and iron from cereals and pulses consumed in India. J. Trace Elem. Med. Biol. 2007, 21, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Thakura, N.; Raigond, P.; Singh, Y.; Mishra, T.; Brajesh Singha, B.; Kumar Lal, M.; Dutta, S. Recent updates on bioaccessibility of phytonutrients. Trends Food Sci. Technol. 2020, 97, 366–380. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Tsaltas, T.T. Dietetic management of uremic patients. Am. J. Clin. Nutr. 1969, 22, 490–493. [Google Scholar] [CrossRef]
- Bethke, P.C.; Jansky, S.H. The effects of boiling and leaching on the content of potassium and other minerals in potatoes. J. Food Sci. 2008, 73, H80–H85. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardized static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]

| Boiling Procedures | ||
|---|---|---|
| Cold Water | Hot Water | |
| Chicory | 215.8 | 322.7 |
| Peas | 529.5 | 616.3 |
| Kiwifruit | 142.1 | 369.8 |
| Potato | 251.5 | 308.3 |
| Avocado | 510.9 | 899.9 |
| Banana | 288.9 | 447.8 |
| Spinach | 823.2 | 914.5 |
| Tomato | 300.9 | 398.4 |
| Cauliflower | 168.8 | 178.5 |
| Zucchini | 314.3 | 309.4 |
| Beans | 746.9 | 794.9 |
| Carrot | 374.9 | 311.3 |
| Raw | Cold Water | Hot Water | |
|---|---|---|---|
| Peas | 153.7 | 46.5 | 41.2 |
| Avocado | 91.2 | 39.5 | 65.7 |
| Banana | 40.2 | 32.7 | 38.2 |
| Cauliflower | 49.8 | 18.8 | 30.0 |
| Carrot | 52.5 | 32.2 | 47.2 |
| Zucchini | 54.3 | 16.9 | 29.3 |
| Potato | 66.1 | 8.7 | 13.6 |
| Spinach | 117.4 | 39.4 | 48.7 |
| Tomato | 30.4 | 29.1 | 95.8 |
| Beans | 31.5 | 26.9 | 46.4 |
| Chicory | 95.3 | 54.7 | 28.3 |
| Kiwifruit | 39.9 | 12.8 | 33.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceccanti, C.; Guidi, L.; D’Alessandro, C.; Cupisti, A. Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology. Toxins 2022, 14, 668. https://doi.org/10.3390/toxins14100668
Ceccanti C, Guidi L, D’Alessandro C, Cupisti A. Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology. Toxins. 2022; 14(10):668. https://doi.org/10.3390/toxins14100668
Chicago/Turabian StyleCeccanti, Costanza, Lucia Guidi, Claudia D’Alessandro, and Adamasco Cupisti. 2022. "Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology" Toxins 14, no. 10: 668. https://doi.org/10.3390/toxins14100668
APA StyleCeccanti, C., Guidi, L., D’Alessandro, C., & Cupisti, A. (2022). Potassium Bioaccessibility in Uncooked and Cooked Plant Foods: Results from a Static In Vitro Digestion Methodology. Toxins, 14(10), 668. https://doi.org/10.3390/toxins14100668







