Body Composition of Healthy Cats and Cats with Chronic Kidney Disease Fed on a Dry Diet Low in Phosphorus with Maintenance Protein
Abstract
1. Introduction
2. Results
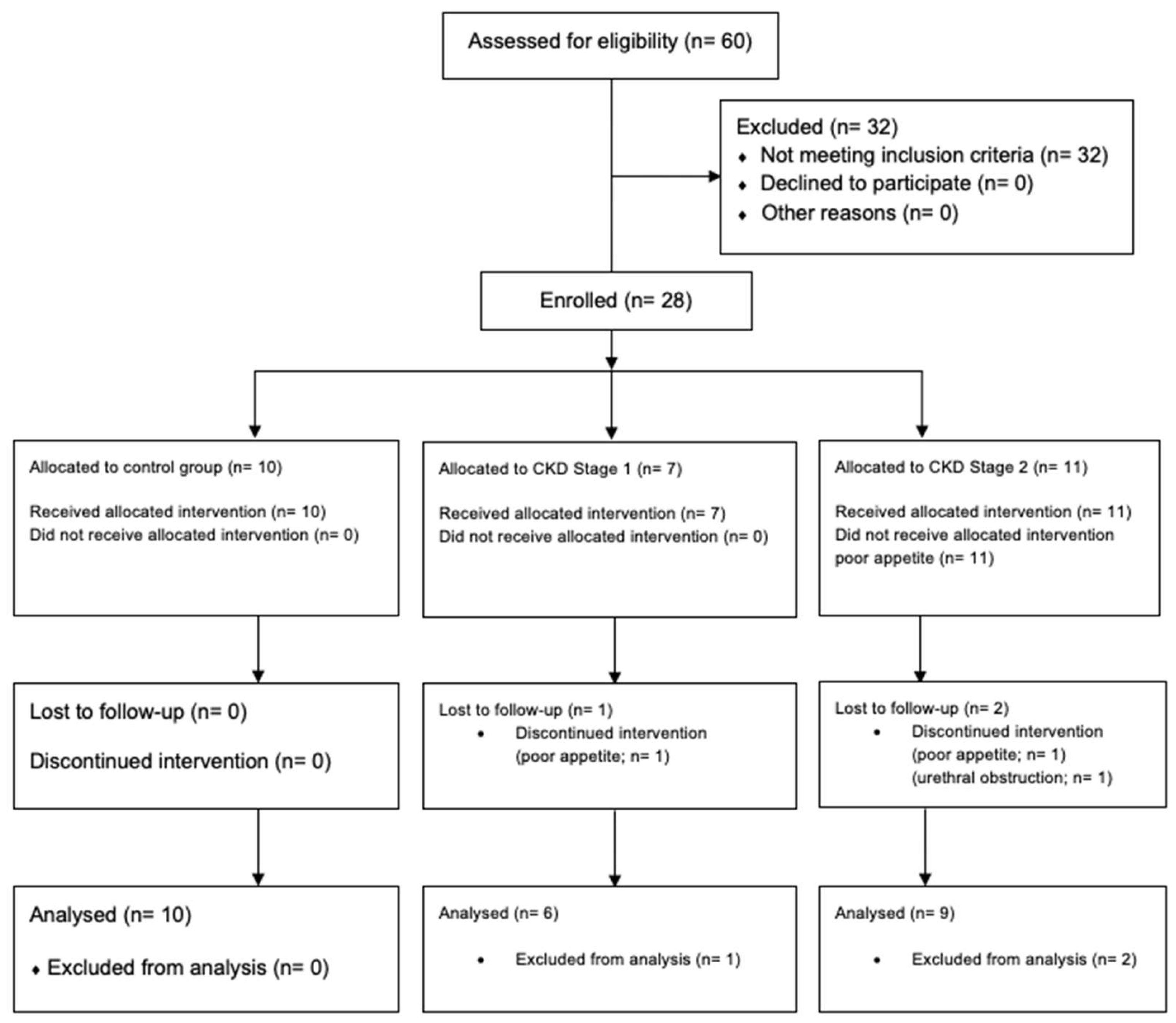
2.1. Animals and Food Consumption
2.2. Body Composition
2.3. Blood Count and Biochemical Profile
2.4. Parathyroid Hormone and Ionized Calcium
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Location and Facilities
5.2. Diets
5.3. Animals and Feed Protocol
5.4. Body Composition (BC)
5.5. Complete Blood Count and Biochemical Profile
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Polzin, D.J. Chronic Kidney Disease. In Textbook of Veterinary Internal Medicine: Diseases of the Dog and the Cat; Ettinger, S.J., Feldman, E.C., Côté, E., Eds.; Sauders Elsevier: St Louis, MO, USA, 2017; pp. 4693–4775. [Google Scholar]
- Forrester, S.D.; Adams, L.G.; Allen, T.A. Chronic Kidney Disease. In Small Animal Clinical Nutrition; Hand, M.S., Thatcher, C.D., Remillard, R.L., Roudebush, P., Novotny, B.J., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 766–810. [Google Scholar] [CrossRef]
- Polzin, D.J. Chronic Kidney Disease. In Nephrology and Urology of Small Animals; Bartges, J., Polzin, D.J., Eds.; Wiley-Blackwell: Chichester, UK, 2011; pp. 433–471. [Google Scholar]
- Ross, S.J. Azotemia and Uremia. In Nephrology and Urology of Small Animals; Bartges, J., Polzin, D.J., Eds.; Blackwell Publishing: Chichester, UK, 2011; pp. 393–399. [Google Scholar]
- Segev, G. Chronic Kidney Disease. In Chronic Disease Management for Small Animals; Gram, W.D., Milner, R.J., Lobetti, R., Eds.; Wiley-Blackwell: Chichester, UK, 2018; pp. 255–259. [Google Scholar]
- Jacob, F.; Polzin, D.J.; Osborne, C.A.; Allen, T.A.; Kirk, C.A.; Neaton, J.D.; Lekcharoensuk, C.; Swanson, L.L. Clinical Evaluation of Dietary Modification for Treatment of Spontaneous Chronic Renal Failure in Dogs. J. Am. Vet. Med. Assoc. 2002, 220, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, E.A.; Everts, H.; Kastelein, A.M.C.; Beynen, A.C. Retrospective Study of the Survival of Cats with Acquired Chronic Renal Insufficiency Offered Different Commercial Diets. Vet. Rec. 2005, 157, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.; Rawlings, J.M.; Markwell, P.J.; Barber, P.J. Survival of Cats with Naturally Occurring Chronic Renal Failure: Effect of Dietary Management. J. Small Anim. Pract. 2000, 41, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Scherk, M.A.; Laflamme, D.P. Controversies in Veterinary Nephrology: Renal Diets Are Indicated for Cats with International Renal Interest Society Chronic Kidney Disease Stages 2 to 4: The Con View. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 1067–1094. [Google Scholar] [CrossRef] [PubMed]
- Chacar, F.C.; Kogika, M.M.; Zafalon, R.V.A.; Brunetto, M.A. Vitamin d Metabolism and Its Role in Mineral and Bone Disorders in Chronic Kidney Disease in Humans, Dogs and Cats. Metabolites 2020, 10, 499. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J.; Osborne, C.A.; Adams, L.G. Effect of Modified Protein Diets in Dogs and Cats with Chronic Renal Failure: Current Status. J. Nutr. 1991, 121 (Suppl. 1), 140–144. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J.; Osborne, C.A.; Ross, S.; Jacob, F. Where Are We Now? In What Direction Are We Headed? Clin. Sci. 2000, 2, 75–82. [Google Scholar]
- IRIS. Staging of CKD; International Renal Interest Society: London, UK, 2019; pp. 1–8. [Google Scholar]
- Cupp, C.J.; Kerr, W. Effect of Diet and Body Composition on Life Span in Aging Cats. In Companion Animal Nutrition Summit: Focus on Gerontology; Clearwater Beach: Clearwater, FL, USA, 2010; pp. 36–42. [Google Scholar]
- Freeman, L.M.; Lachaud, M.P.; Matthews, S.; Rhodes, L.; Zollers, B. Evaluation of Weight Loss over Time in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2016, 30, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Fritsch, D.A.; Jewell, D.E.; Burris, P.A.; Gross, K.L. Cats with IRIS Stage 1 and 2 Chronic Kidney Disease Maintain Body Weight and Lean Muscle Mass When Fed Food Having Increased Caloric Density, and Enhanced Concentrations of Carnitine and Essential Amino Acids. Vet. Rec. 2019, 184, 190. [Google Scholar] [CrossRef] [PubMed]
- Boyd, L.M.; Langston, C.; Thompson, K.; Zivin, K.; Imanishi, M. Survival in Cats with Naturally Occurring Chronic Kidney Disease (2000–2002). J. Vet. Intern. Med. 2008, 22, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, S.; Syme, H.M.; Elliott, J. Clinicopathological Variables Predicting Progression of Azotemia in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2012, 26, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, D.P. Development and Validation of a Body Condition Score System for Cats: A Clinical Tool. Feline Pract. 1997, 25, 13–18. [Google Scholar]
- FEDIAF. Nutritional Guidelines for Complete and Complementary Pet Food for Cats and Dogs; Federation Europeene de I’Industrie des Aliments pour Animaux Familiers: Brussels, Belgium, 2021. [Google Scholar] [CrossRef]
- Tom, A.; Nair, K.S. Assessment of Branched-Chain Amino Acid Status and Potential for Biomarkers. J. Nutr. 2006, 136, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Ephraim, E.; Jewell, D.E. High Protein Consumption with Controlled Phosphorus Level Increases Plasma Concentrations of Uremic Toxins in Cats with Early Chronic Kidney Disease. J. Food Sci. Nutr. 2021, 7, 096. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirement of Dogs and Cats; National Research Council; National Academy Press: Washington, DC, USA, 2006. [Google Scholar]
- King, J.N.; Tasker, S.; Gunn-Moore, D.A.; Strehlau, G. Prognostic factors in cats with chronic kidney disease. J. Vet. Intern. Med. 2007, 21, 906–916. [Google Scholar] [CrossRef] [PubMed]
- Polzin, D.J. Chronic Kidney Disease in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Michel, K.E.; Anderson, W.; Cupp, C.; Laflamme, D.P. Correlation of a feline muscle mass score with body composition determined by dual-energy X-ray absorptiometry. Br. J. Nutr. 2011, 106, 57–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barber, P.J.; Elliott, J. Feline Chronic Renal Failure: Calcium Homeostasis in 80 Cases Diagnosed between 1992 and 1995. J. Small Anim. Pract. 1998, 39, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Akademiai Kiado: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
| Treatment | Time Point | Mean | p-Value | ||||
|---|---|---|---|---|---|---|---|
| T0 | T30 | T60 | Treatment | Time Point | Treatment × Time | ||
| Energy (kcal/BW0.67) | |||||||
| Control group | 67.18 ± 6.23 | 73.26 ± 6.24 | 74.41 ± 6.24 | 71.62 ± 6.24 | 0.4487 | 0.0069 | 0.4632 |
| IRIS stage 1 | 61.11 ± 8.00 | 64.50 ± 8.00 | 67.98 ± 8.00 | 64.53 ± 8.00 | |||
| IRIS stage 2 | 63.90 ± 6.16 | 63.87 ± 6.16 | 67.04 ± 6.16 | 64.94 ± 6.16 | |||
| Mean | 64.06 ± 5.76 b | 67.21 ± 5.76 a | 69.81 ± 5.76 a | - | |||
| Crude protein (g/BW0.67) | |||||||
| Control group | 6.70 ± 0.56 | 6.02 ± 0.56 | 6.23 ± 0.56 | 6.32 ± 0.55 | 0.4467 | <0.0001 | 0.8145 |
| IRIS stage 1 | 5.98 ± 0.71 | 5.38 ± 0.71 | 5.68 ± 0.71 | 5.68 ± 0.69 | |||
| IRIS stage 2 | 6.25 ± 0.56 | 5.33 ± 0.56 | 5.60 ± 0.56 | 5.73 ± 0.55 | |||
| Mean | 6.31 ± 0.56 a | 5.58 ± 0.56 b | 5.84 ± 0.56 b | - | |||
| Phosphorus (g/BW0.67) | |||||||
| Control group | 0.19 ± 0.01 a,A | 0.08 ± 0.01 b,A | 0.08 ± 0.01 b,A | 0.12 ± 0.01 | 0.4330 | <0.0001 | 0.0009 |
| IRIS stage 1 | 0.16 ± 0.01 a,B | 0.07 ± 0.01 b,A | 0.08 ± 0.01 b,A | 0.10 ± 0.01 | |||
| IRIS stage 2 | 0.17 ± 0.01 a,B | 0.07 ± 0.01 b,A | 0.08 ± 0.01 b,A | 0.11 ± 0.01 | |||
| Mean | 0.17 ± 0.01 | 0.08 ± 0.01 | 0.08 ± 0.01 | - | |||
| Treatment | Time Point | Mean | p-Value | ||||
|---|---|---|---|---|---|---|---|
| T0 | T30 | T60 | Treatment | Time Point | Treatment × Time | ||
| Body weight (kg) | |||||||
| Control group | 4.92 ± 0.53 a,A | 4.89 ± 0.53 a,A | 4.90 ± 0.53 a,A,B | 4.90 ± 0.53 | 0.1927 | 0.0220 | 0.0106 |
| IRIS stage 1 | 5.44 ± 0.63 a,A | 5.39 ± 0.63 a,A | 5.43 ± 0.63 a,A | 5.42 ± 0.63 | |||
| IRIS stage 2 | 4.76 ± 0.57 a,A | 4.63 ± 0.57 b,A | 4.47 ± 0.57 c,B | 4.62 ± 0.57 | |||
| Mean | 5.04 ± 0.50 | 4.97 ± 0.50 | 4.94 ± 0.50 | - | |||
| Body condition score | |||||||
| Control group | 5.59 ± 0.37 a,A | 5.57 ± 0.37 a,A | 5.47 ± 0.37 a,A | 5.54 ± 0.37 | 0.4964 | 0.0012 | 0.0016 |
| IRIS stage 1 | 6.15 ± 0.47 a,A | 6.14 ± 0.47 a,A | 6.15 ± 0.47 a,A | 6.14 ± 0.47 | |||
| IRIS stage 2 | 5.75 ± 0.42 a,A | 5.64 ± 0.42 a,A | 5.09 ± 0.42 b,A | 5.50 ± 0.42 | |||
| Mean | 5.83 ± 0.26 | 5.79 ± 0.26 | 5.57 ± 0.26 | - | |||
| Treatment | Time Point | Mean | p-Value | ||||
|---|---|---|---|---|---|---|---|
| T0 | T30 | T60 | Treatment | Time Point | Treatment × Time | ||
| Muscle mass score | |||||||
| Control group | 2.83 ± 0.14 | 2.84 ± 0.14 | 2.84 ± 0.14 | 2.84 ± 0.14 A | 0.0008 | 0.2131 | 0.1341 |
| IRIS stage 1 | 3.00 ± 0.18 | 3.00 ± 0.18 | 3.00 ± 0.18 | 3.00 ± 0.18 A | |||
| IRIS stage 2 | 2.38 ± 0.15 | 2.38 ± 0.15 | 2.16 ± 0.15 | 2.31 ± 0.15 B | |||
| Mean | 2.75 ± 0.12 | 2.75 ± 0.12 | 2.6 ± 0.12 | - | |||
| Lean mass(kg) | |||||||
| Control group | 3.98 ± 0.41 | NM | 3.91 ± 0.41 | 3.95 ± 0.40 | 0.1352 | 0.4559 | 0.2823 |
| IRIS stage 1 | 3.94 ± 0.46 | NM | 4.04 ± 0.46 | 3.99 ± 0.46 | |||
| IRIS stage 2 | 3.50 ± 0.41 | NM | 3.26 ± 0.41 | 3.38 ± 0.41 | |||
| Mean | 3.81 ± 0.36 | NM | 3.74 ± 0.36 | - | |||
| Fat mass (kg) | |||||||
| Control group | 1.42 ± 0.26 | NM | 1.13 ± 0.26 | 1.27 ± 0.24 | 0.1409 | 0.1887 | 0.5326 |
| IRIS stage 1 | 1.90 ± 0.31 | NM | 1.81 ± 0.31 | 1.86 ± 0.30 | |||
| IRIS stage 2 | 1.30 ± 0.25 | NM | 1.28 ± 0.25 | 1.29 ± 0.24 | |||
| Mean | 1.54 ± 0.20 | NM | 1.41 ± 0.20 | - | |||
| Variables | Reference Range | Control Group (n = 10) | IRIS Stage 1 (n = 6) | IRIS Stage 2 (n = 9) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Treatment | Time Point | Treatment × Time | |||||
| Total protein (g/dL) | 5.7–7.8 | 7.92 ± 0.22 | 8.03 ± 0.25 | 7.95 ± 0.23 | 0.9185 | 0.0026 | 0.9368 |
| Creatinine (mg/dL) | <1.6 | 1.30 ± 0.13 b | 1.21 ± 0.16 b | 1.87 ± 0.14 a | 0.0012 | 0.1284 | 0.1848 |
| BUN (mg/dL) | 17.6–32.8 | 24.00 ± 2.18 b | 22.67 ± 2.48 b | 34.18 ± 2.31 a | 0.0002 | 0.0811 | 0.4588 |
| Ca (mg/dL) | 8.8–11.9 | 9.69 ± 0.12 b | 10.25 ± 0.17 a | 10.18 ± 0.14 a | 0.0149 | 0.0024 | 0.3613 |
| iCa (mmol/L) | 1.0–1.4 | 1.27 ± 0.03 | 1.30 ± 0.02 | 1.30 ± 0.02 | 0.7569 | 0.3136 | 0.6607 |
| Phosphorus (mg/dL) | 2.6–6.0 | 5.76 ± 0.22 a | 4.88 ± 0.26 b | 5.50 ± 0.23 a,b | 0.0210 | 0.1253 | 0.5378 |
| Sodium (mEq/L) | 147–156 | 153.82± 0.68 | 154.24 ± 0.90 | 154.64 ± 0.75 | 0.5862 | <0.0001 | 0.3073 |
| Chlorine (mEq/L) | 107–120 | 117.82 ± 0.98 | 117.87 ± 1.25 | 119.28 ± 1.08 | 0.2305 | <0.0001 | 0.5265 |
| ALP (U/L) | 9–53 | 26.99 ± 5.11 | 32.94 ± 6.28 | 36.58 ± 5.69 | 0.4060 | 0.0099 | 0.2783 |
| ALT (U/L) | 22–84 | 63.03 ± 10.25 | 56.68 ± 13.41 | 77.38 ± 11.79 | 0.4888 | 0.0096 | 0.5270 |
| Cholesterol (mg/dL) | 89–176 | 146.39 ± 17.24 | 205.58 ± 20.18 | 176.90 ± 18.63 | 0.0541 | <0.0001 | 0.5688 |
| PTH (Mmol/L) | 0.4–2.5 | 0.78 ± 0.16 | 0.79 ± 0.15 | 0.58 ± 0.15 | 0.4864 | 0.0151 | 0.0830 |
| SDMA (µg/dL) | <18 | 10.16 ± 1.49 | 7.91 ± 1.96 | 12.26 ± 1.61 | 0.0594 | 0.0103 | 0.3137 |
| Nutrients (g/100 kcal) | Senior Diet 2 | Renal Test Diet 3 |
|---|---|---|
| Protein | 10.34 | 8.68 |
| Fat | 5.23 | 3.94 |
| Crude fiber | 0.62 | 0.41 |
| Ash | 1.87 | 1.08 |
| Calcium | 0.32 | 0.13 |
| Phosphorus | 0.29 | 0.12 |
| Ca/P ratio | 1.12 | 1.08 |
| Potassium | 0.17 | 0.22 |
| Sodium | 0.20 | 0.08 |
| Omega-3 | 0.10 | 0.28 |
| Metabolizable energy (kcal/kg) | 3.920 | 4.353 * |
| Essential amino acids | ||
| Arginine | 0.70 | 0.48 |
| Phenylalanine | 0.49 | 0.36 |
| Histidine | 0.26 | 0.18 |
| Isoleucine | 0.42 | 0.37 |
| Leucine | 0.94 | 0.79 |
| Lysine | 0.59 | 0.49 |
| Methionine | 0.28 | 0.17 |
| Taurine | 0.07 | 0.05 |
| Threonine | 0.41 | 0.35 |
| Tryptophan | 0.08 | 0.09 |
| Valine | 0.52 | 0.46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, D.P.; Ruberti, B.; Teixeira, F.A.; Vendramini, T.H.A.; Pfrimer, K.; Chacar, F.C.; Balieiro, J.C.C.; Pontieri, C.F.F.; Brunetto, M.A. Body Composition of Healthy Cats and Cats with Chronic Kidney Disease Fed on a Dry Diet Low in Phosphorus with Maintenance Protein. Toxins 2022, 14, 865. https://doi.org/10.3390/toxins14120865
Machado DP, Ruberti B, Teixeira FA, Vendramini THA, Pfrimer K, Chacar FC, Balieiro JCC, Pontieri CFF, Brunetto MA. Body Composition of Healthy Cats and Cats with Chronic Kidney Disease Fed on a Dry Diet Low in Phosphorus with Maintenance Protein. Toxins. 2022; 14(12):865. https://doi.org/10.3390/toxins14120865
Chicago/Turabian StyleMachado, Daniela P., Bruna Ruberti, Fabio A. Teixeira, Thiago H. A. Vendramini, Karina Pfrimer, Fernanda C. Chacar, Julio C. C. Balieiro, Cristiana F. F. Pontieri, and Marcio A. Brunetto. 2022. "Body Composition of Healthy Cats and Cats with Chronic Kidney Disease Fed on a Dry Diet Low in Phosphorus with Maintenance Protein" Toxins 14, no. 12: 865. https://doi.org/10.3390/toxins14120865
APA StyleMachado, D. P., Ruberti, B., Teixeira, F. A., Vendramini, T. H. A., Pfrimer, K., Chacar, F. C., Balieiro, J. C. C., Pontieri, C. F. F., & Brunetto, M. A. (2022). Body Composition of Healthy Cats and Cats with Chronic Kidney Disease Fed on a Dry Diet Low in Phosphorus with Maintenance Protein. Toxins, 14(12), 865. https://doi.org/10.3390/toxins14120865




