Real-World Evaluation of the Tolerability to Onabotulinum Toxin A: The RETO Study
Abstract
1. Introduction
2. Results
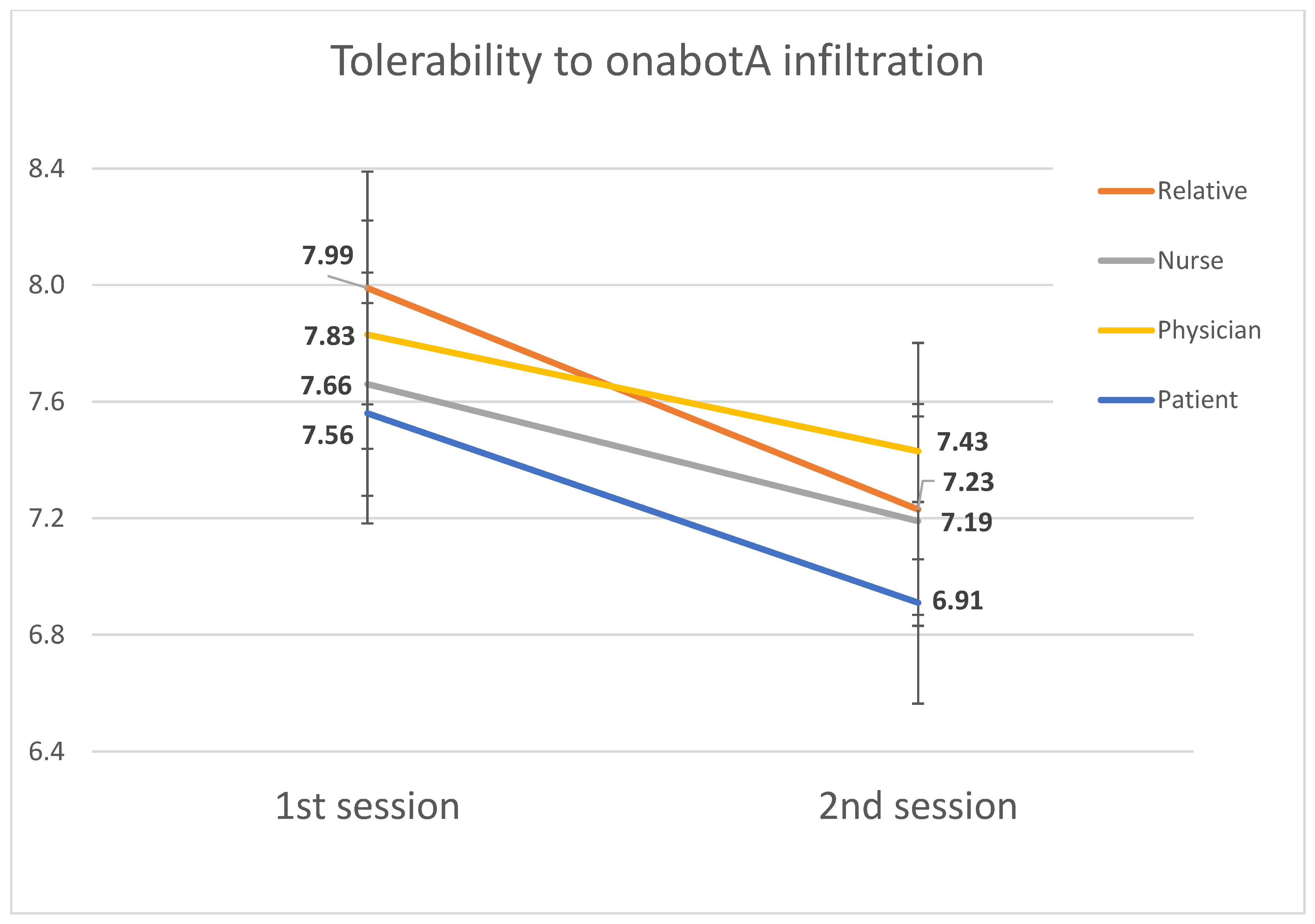
2.1. Tolerability
2.2. Adverse Event Occurrence
2.3. OnabotA Response
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Design and Setting
5.2. Eligibility Criteria
5.3. Sources and Methods of Selection
5.4. Study Endpoints
5.5. Variables
5.6. Intervention
5.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buse, D.C.; Manack, A.N.; Fanning, K.M.; Serrano, D.; Reed, M.L.; Turkel, C.C.; Lipton, R.B. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache 2012, 52, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Abajobir, A.A.; Abate, K.H.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abdulkader, R.S.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: A systematic analysis for the global burden of disease study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.M.; Kaur, G.; Mahajan, A.; Shukla, H.; Sommer, K.; Tung, A.; Knievel, K.L. Effectiveness and safety of chronic migraine preventive treatments: A systematic literature review. Pain Ther. 2022. [Google Scholar] [CrossRef]
- Dodick, D.W. Clinical practice. Chronic daily headache. N. Engl. J. Med. 2006, 354, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Serrano, D.; Reed, M.; Lipton, R.B. Chronic migraine in the population: Burden, diagnosis, and satisfaction with treatment. Neurology 2008, 71, 559–566. [Google Scholar] [CrossRef]
- Lipton, R.B.; Stewart, W.F.; Diamond, S.; Diamond, M.L.; Reed, M. Prevalence and burden of migraine in the United States: Data from the American Migraine Study II. Headache 2001, 41, 646–657. [Google Scholar] [CrossRef]
- Murray, C.J.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef]
- Straube, A.; Pfaffenrath, V.; Ladwig, K.H.; Meisinger, C.; Hoffmann, W.; Fendrich, K.; Vennemann, M.; Berger, K. Prevalence of chronic migraine and medication overuse headache in Germany—The German DMKG headache study. Cephalalgia 2010, 30, 207–213. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Lipton, R.B.; Dodick, D.W.; Freitag, F.G.; Ramadan, N.; Mathew, N.; Brandes, J.L.; Bigal, M.; Saper, J.; Ascher, S.; et al. Efficacy and safety of topiramate for the treatment of chronic migraine: A randomized, double-blind, placebo-controlled trial. Headache 2007, 47, 170–180. [Google Scholar] [CrossRef]
- Diener, H.C.; Bussone, G.; Oene, J.V.; Lahaye, M.; Schwalen, S.; Goadsby, P.J. Topiramate Reduces Headache Days in Chronic Migraine: A Randomized, Double-Blind, Placebo-Controlled Study. Cephalalgia 2007, 27, 814–823. [Google Scholar] [CrossRef]
- Silberstein, S.; Lipton, R.; Dodick, D.; Freitag, F.; Mathew, N.; Brandes, J.; Bigal, M.; Ascher, S.; Morein, J.; Wright, P.; et al. Topiramate Treatment of Chronic Migraine: A Randomized, Placebo-Controlled Trial of Quality of Life and Other Efficacy Measures. Headache J. Head Face Pain 2009, 49, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.K.; Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 2010, 50, 921–936. [Google Scholar] [CrossRef]
- Aurora, S.K.; Winner, P.; Freeman, M.C.; Spierings, E.L.; Heiring, J.O.; DeGryse, R.E.; VanDenburgh, A.M.; Nolan, M.E.; Turkel, C.C. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56-week PREEMPT clinical program. Headache 2011, 51, 1358–1373. [Google Scholar] [CrossRef]
- Diener, H.C.; Dodick, D.W.; Turkel, C.C.; Demos, G.; Degryse, R.E.; Earl, N.L.; Brin, M.F. Pooled analysis of the safety and tolerability of onabotulinumtoxinA in the treatment of chronic migraine. Eur. J. Neurol. 2014, 21, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Tepper, S.; Ashina, M.; Reuter, U.; Brandes, J.L.; Doležil, D.; Silberstein, S.; Winner, P.; Leonardi, D.; Mikol, D.; Lenz, R. Safety and Efficacy of Erenumab for Preventive Treatment of Chronic Migraine: A Randomised, Double-Blind, Placebo-Controlled Phase 2 Trial. Lancet Neurol. 2017, 16, 425–434. [Google Scholar] [CrossRef]
- Detke, H.C.; Goadsby, P.J.; Wang, S.; Friedman, D.I.; Selzler, K.J.; Aurora, S.K. Galcanezumab in Chronic Migraine: The Randomized, Double-Blind, Placebo-Controlled REGAIN Study. Neurology 2018, 91, 2211–2221. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Dodick, D.W.; Bigal, M.E.; Yeung, P.P.; Goadsby, P.J.; Blankenbiller, T.; Grozinski-Wolff, M.; Yang, R.; Ma, Y.; Aycardi, E. Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 2017, 377, 2113–2122. [Google Scholar] [CrossRef]
- Lipton, R.B.; Goadsby, P.J.; Smith, J.; Schaeffler, B.A.; Biondi, D.M.; Hirman, J.; Pederson, S.; Allan, B.; Cady, R. Efficacy and Safety of Eptinezumab in Patients with Chronic Migraine: PROMISE-2. Neurology 2020, 94, e1365–e1377. [Google Scholar] [CrossRef]
- Khanal, S.; Underwwod, M.; Naghdi, S.; Brown, A.; Duncan, C.; Matharu, M.; Mistry, H. A systematic review of economic evaluations of pharmacological treatment for adults with chronic migraine. J. Headache Pain 2022, 23, 122. [Google Scholar] [CrossRef] [PubMed]
- Gago-Veiga, A.B.; Santos-Lasaosa, S.; Cuadrado, M.L.; Guerrero, Á.L.; Irimia, P.; Láinez, J.M.; Leira, R.; Pascual, J.; Del Río, M.S.; Viguera, J.; et al. Evidence and experience with onabotulinumtoxinA in chronic migraine: Recommendations for daily clinical practice. Neurología (Engl. Ed.) 2019, 34, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Raciti, L.; Raciti, G.; Militi, D.; Casella, C.; Calabrò, R.S. Chronic migraine: A narrative review on the use of botulinum toxin with clinical indications and future directions. J. Integr. Neurosci. 2022, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Lanteri-Minet, M.; Ducros, A.; Francois, C.; Olewinska, E.; Nikodem, M.; Dupont-Benjamin, L. Effectiveness of onabotulinumtoxinA (BOTOX®) for the preventive treatment of chronic migraine: A meta-analysis on 10 years of real-world data. Cephalalgia 2022, 42. [Google Scholar] [CrossRef]
- Altamura, C.; Ornello, R.; Ahmed, F.; Negro, A.; Miscio, A.M.; Santoro, A.; Alpuente, A.; Russo, A.; Silvestro, M.; Cevoli, S.; et al. OnabotulinumtoxinA in elderly patients with chronic migraine: Insights from a real-life european multicenter study. J. Neurol. 2022. [Google Scholar] [CrossRef]
- Becker, W.J.; Boudreau, G.; Finkelstein, I.; Graboski, C.; Ong, M.; Christie, S.; Sommer, K.; Bhogal, M.; Davidovic, G. OnabotulinumtoxinA reduces health resource utilization in chronic migraine: PREDICT study. Can. J. Neurol. Sci. 2022, 1–10. [Google Scholar] [CrossRef]
- Barbanti, P.; Ferroni, P. Onabotulinum toxin A in the treatment of chronic migraine: Patient selection and special considerations. J. Pain Res. 2017, 10, 2319–2329. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Dermitzakis, E.V.; Vlachos, G.S.; Vikelis, M. Long-term adherence, safety, and efficacy of repeated onabotulinumtoxinA over five years in chronic migraine prophylaxis. Acta Neurol. Scand. 2022, 145, 676–683. [Google Scholar] [CrossRef]
- Matharu, M.; Pascual, J.; Nilsson Remahl, I.; Straube, A.; Lum, A.; Davar, G.; Odom, D.; Bennett, L.; Proctor, C.; Gutierrez, L.; et al. Utilization and safety of onabotulinumtoxinA for the prophylactic treatment of chronic migraine from an observational study in Europe. Cephalalgia 2017, 37, 1384–1397. [Google Scholar] [CrossRef]
- Schiano di Cola, F.; Caratozzolo, S.; Liberini, P.; Rao, R.; Padovani, A. Response Predictors in Chronic Migraine: Medication Overuse and Depressive Symptoms Negatively Impact Onabotulinumtoxin-A Treatment. Front. Neurol. 2019, 10, 678. [Google Scholar] [CrossRef]
- Ornello, R.; Ahmed, F.; Negro, A.; Miscio, A.M.; Santoro, A.; Alpuente, A.; Russo, A.; Silvestro, M.; Cevoli, S.; Brunelli, N.; et al. Is There a Gender Difference in the Response to onabotulinumtoxinA in Chronic Migraine? Insights from a Real-Life European Multicenter Study on 2879 Patients. Pain Ther. 2021, 10, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Ornello, R.; Baraldi, C.; Ahmed, F.; Negro, A.; Miscio, A.M.; Santoro, A.; Alpuente, A.; Russo, A.; Silvestro, M.; Cevoli, S.; et al. Excellent Response to OnabotulinumtoxinA: Different Definitions, Different Predictors. Int. J. Environ. Res. Public Health 2022, 19, 10975. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.; Pozo-Rosich, P.; Torres-Ferrus, M.; Hernández-Beltrán, N.; JuradoCobo, C.; González-Oria, C. Onabotulinumtoxin A in chronic migraine: Predictors of response. A prospective multicentre descriptive study. Eur. J. Neurol. 2018, 25, 411–416. [Google Scholar] [CrossRef]
- DDermitzakis, E.V.; Vikelis, M.; Vlachos, G.S.; Argyriou, A.A. Assessing the Significance of the Circadian Time of Administration on the Effectiveness and Tolerability of OnabotulinumtoxinA for Chronic Migraine Prophylaxis. Toxins 2022, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Dermitzakis, E.V.; Vikelis, M.; Vlachos, G.S.; Argyriou, A.A. Prospective Comparison of Longer Needle Lengths to Assess the Risk of OnabotulinumtoxinA-Associated Neck Pain in Patients with Chronic Migraine. Toxins 2022, 14, 434. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, A.M.; Stark, R.J.; Freeman, M.C.; Orejudos, A.; Manack Adams, A. Long-term study of the efficacy and safety of OnabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J. Headache Pain 2018, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Kokoti, L.; Drellia, K.; Papadopoulos, D.; Mitsikostas, D.D. Placebo and nocebo phenomena in anti- CGRP monoclonal antibody trials for migraine prevention: A meta-analysis. J. Neurol. 2020, 267, 1158–1170. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. PLoS Med. 2007, 4, e296. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef]
- Sociedad Española de Neurología. Guías Diagnósticas y Terapéuticas de la Sociedad Española de Neurología 2015. Guía Official de Práctica Clínica en Cefaleas 2015; Sociedad Española de Neurología: Barcelona, Spain, 2015; ISBN 978-84-15198-99-4. [Google Scholar]
- Tassorelli, C.; Diener, H.C.; Dodick, D.W.; Silberstein, S.D.; Lipton, R.B.; Ashina, M.; Becker, W.J.; Ferrari, M.D.; Goadsby, P.J.; Pozo-Rosich, P.; et al. Guidelines of the International Headache Society for controlled trials of preventive treatment of chronic migraine in adults. Cephalalgia 2018, 38, 815–832. [Google Scholar] [CrossRef]



| Item | Analysis | Standardized Beta Coefficient | 95% CI | p-Value |
|---|---|---|---|---|
| Female sex | Univariate | −0.033 | −1.393–0.988 | 0.737 |
| Multivariate | −0.063 | −1.558–0.796 | 0.522 | |
| Age (years) | Univariate | 0.222 | 0.006–0.078 | 0.023 |
| Multivariate | 0.196 | −0.002–0.075 | 0.061 | |
| Months of CM | Univariate | 0.153 | −0.002–0.016 | 0.118 |
| Multivariate | 0.414 | −0.008–0.012 | 0.680 | |
| Intensity of headache (0–10 NRS) | Univariate | −0.083 | −0.196–0.079 | 0.400 |
| Multivariate | −0.135 | −0.237–0.046 | 0.182 | |
| Frequency of headache (days) | Univariate | 0.166 | −0.010–0.137 | 0.090 |
| Multivariate | 0.138 | −0.027–0.132 | 0.196 |
| Item | Adverse Events (n = 75) | No Adverse Events (n = 30) | p-Value |
|---|---|---|---|
| Mean age | 43.6 | 44.6 | 0.67 |
| Months of CM | 40.3 | 23.8 | 0.023 |
| Mean intensity | 3.5 | 3.3 | 0.62 |
| Mean tolerability | 7.6 | 7.5 | 0.92 |
| Headache days per month at baseline | 24.7 | 22.1 | 0.026 |
| Intense headache days per month at baseline | 12.8 | 10.6 | 0.11 |
| Acute medication days per month at baseline | 18.3 | 17.7 | 0.75 |
| Triptan days per month at baseline | 7.3 | 5.9 | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Azorín, D.; Martínez, B.; Gutiérrez, M.; Ruiz-Piñero, M.; Echavarría, A.; Sierra, Á.; Guerrero, Á.L. Real-World Evaluation of the Tolerability to Onabotulinum Toxin A: The RETO Study. Toxins 2022, 14, 850. https://doi.org/10.3390/toxins14120850
García-Azorín D, Martínez B, Gutiérrez M, Ruiz-Piñero M, Echavarría A, Sierra Á, Guerrero ÁL. Real-World Evaluation of the Tolerability to Onabotulinum Toxin A: The RETO Study. Toxins. 2022; 14(12):850. https://doi.org/10.3390/toxins14120850
Chicago/Turabian StyleGarcía-Azorín, David, Blanca Martínez, María Gutiérrez, Marina Ruiz-Piñero, Ana Echavarría, Álvaro Sierra, and Ángel L. Guerrero. 2022. "Real-World Evaluation of the Tolerability to Onabotulinum Toxin A: The RETO Study" Toxins 14, no. 12: 850. https://doi.org/10.3390/toxins14120850
APA StyleGarcía-Azorín, D., Martínez, B., Gutiérrez, M., Ruiz-Piñero, M., Echavarría, A., Sierra, Á., & Guerrero, Á. L. (2022). Real-World Evaluation of the Tolerability to Onabotulinum Toxin A: The RETO Study. Toxins, 14(12), 850. https://doi.org/10.3390/toxins14120850





