Botulinum Toxin, a Drug with Potential Interest for Dentists—An Introduction
Abstract
1. Effects of Botulinum Toxin
2. Oromandibular Dystonia (OMD) and Other Movement Disorders in the Oromandibular Area
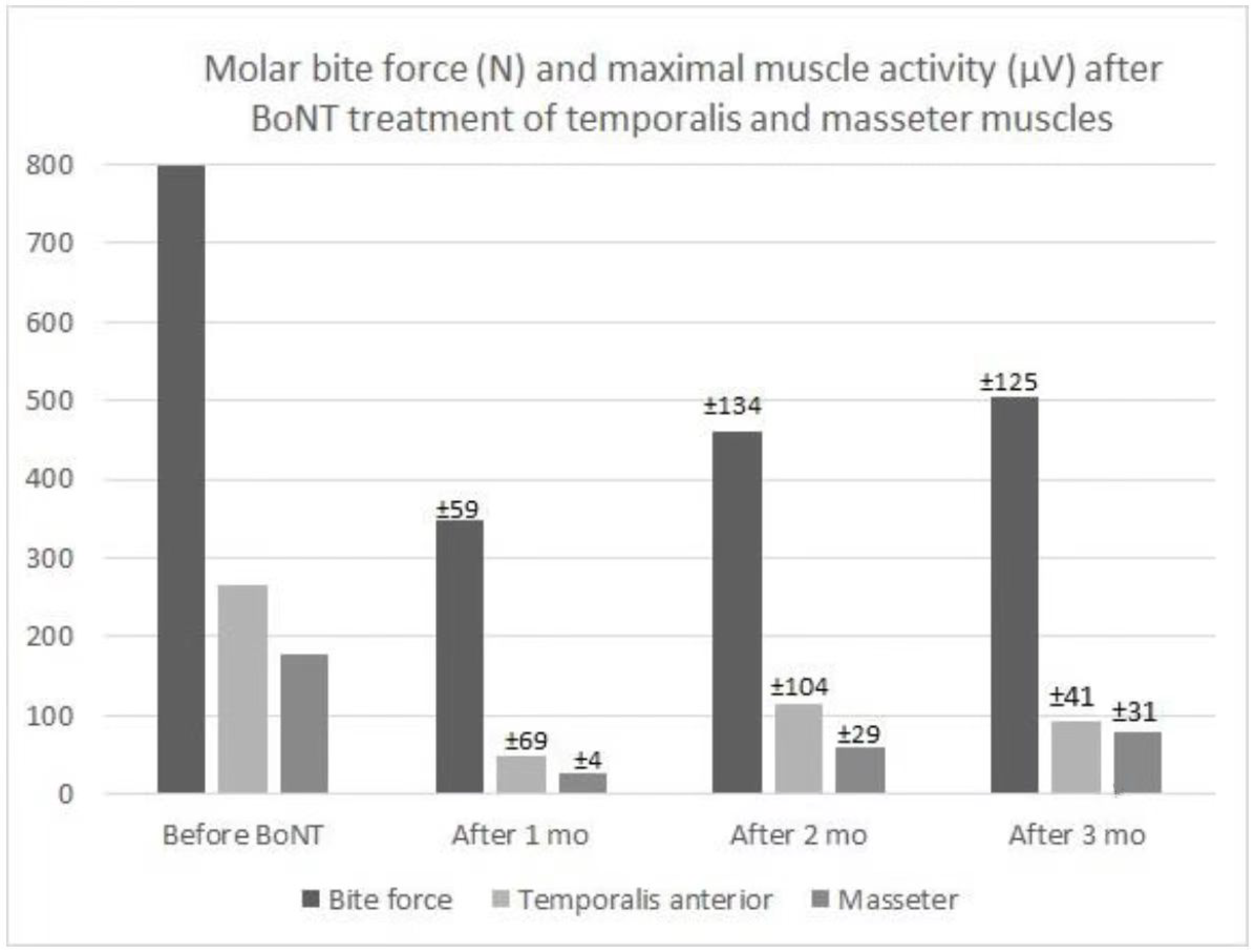
3. Injections of BoNT in the Oromandibular Muscles
4. Saliva Secretion and Sialorrhea
5. Treatment of Sialorrhea with BoNT
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BoNT | botulinum toxin |
| Botox | onabotulinumtoxinA |
| CP | cerebral palsy |
| EMG | electromyography |
| Myobloc/neurobloc | botulinumtoxinB |
| OMD | oromandibular disorders |
| Xenon | incobotulinumtoxinA |
| PD | Parkinson’s disease |
References
- Bakke, M.; Møller, E.; Dalager, T. Botulinum toxin—Et lægemiddel af potentiel interesse for tandlæger. Tandlaegebladet 2013, 117, 894–899. [Google Scholar]
- Bakke, M.; Dalager, T.; Møller, E. What clinical strategies are applied for botulinum toxin injection in the oromandibular region? In Botulinum Toxin Therapy Manual for Dystonia and Spasticity; Rosales, R., Dressler, D., Eds.; InTech: Rijeka, Croatia, 2016; pp. 79–95. [Google Scholar]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Park, J.; Park, J.P. Botulinum toxin for the treatment of neuropathic pain. Toxins 2017, 9, 260. [Google Scholar] [CrossRef]
- Sandrini, G.; De Icco, R.; Tassorelli, C.; Smania, N.; Tamburin, S. Botulinum neurotoxin type A for the treatment of pain: Not just in migraine and trigeminal neuralgia. J. Headache Pain 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- Mor, N.; Tang, C.; Blitzer, A. Temporomandibular myofacial pain treated with botulinum toxin injection. Toxins 2015, 7, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.; Smith, H.S. Botulinum toxins: Mechanisms of action, antinociception and clinical applications. Toxicology 2013, 306, 124–146. [Google Scholar] [CrossRef]
- Waskitho, A.; Yamamoto, Y.; Raman, S.; Kano, F.; Yan, H.; Raju, R.; Afroz, S.; Morita, T.; Ikutame, D.; Okura, K.; et al. Peripherally administered botulinum toxin type A localizes bilaterally in trigeminal ganglia of animal model. Toxins 2021, 13, 704. [Google Scholar] [CrossRef]
- Matak, I.; Lackovic, Z. Botulinum toxin A, brain and pain. Prog. Neurobiol. 2014, 119–120, 39–59. [Google Scholar] [CrossRef]
- Ernberg, M.; Hedenberg-Magnusson, B.; List, T.; Svensson, P. Efficacy of botulinum toxin type A for treatment of persistent myofascial TMD pain: A randomized, controlled, double-blind multicenter study. Pain 2011, 152, 1988–1996. [Google Scholar] [CrossRef]
- Machado, D.; Martimbianco, A.L.C.; Bussadori, S.K.; Pacheco, R.L.; Riera, R.; Santos, E.M. Botulinum Toxin Type A for painful temporomandibular disorders: Systematic review and meta-analysis. J. Pain 2020, 21, 281–293. [Google Scholar] [CrossRef]
- De la Torre Canales, G.; Alvarez-Pinzon, N.; Muñoz-Lora, V.R.M.; Peroni, L.V.; Gomes, A.F.; Sánchez-Ayala, A.; Haiter-Neto, F.; Manfredini, D.; Rizzatti-Barbosa, C.M. Efficacy and safety of botulinum toxin type A on persistent myofascial pain: A randomized clinical trial. Toxins 2020, 12, 395. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Kharbanda, S.; Pal, U.S.; Shah, V. Application of botulinum toxin in dentistry: A comprehensive review. Natl. J. Maxillofac. Surg. 2015, 6, 152–159. [Google Scholar]
- Bakke, M.; Baram, S.; Dalager, T.; Biernat, H.B.; Møller, E. Oromandibular dystonia, mental distress and oro-facial dysfunction—A follow-up 8–10 years after start of treatment with botulinum toxin. J. Oral Rehabil. 2019, 46, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Møller, E.; Werdelin, L.M.; Dalager, T.; Kitai, N.; Kreiborg, S. Treatment of severe temporomandibular joint clicking with botulinum toxin in the lateral pterygoid muscle in two cases of anterior disc displacement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 693–700. [Google Scholar] [CrossRef]
- Møller, E.; Bakke, M.; Dalager, T.; Werdelin, L.M. Oromandibular dystonia involving the lateral pterygoid muscles: Four cases with different complexity. Mov. Disord. 2007, 22, 785–790. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum neurotoxin injection for the treatment of recurrent temporomandibular joint dislocation with and without neurogenic muscular hyperactivity. Toxins 2018, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K. Effects of botulinum toxin type A on pain among trigeminal neuralgia, myofascial temporomandibular disorders, and oromandibular dystonia. Toxins 2021, 13, 605. [Google Scholar] [CrossRef]
- Long, H.; Liao, Z.; Wang, Y.; Liao, L.; Lai, W. Efficacy of botulinum toxins on bruxism: An evidence-based review. Efficacy of botulinum toxins on bruxism: An evidence-based review. Int. Dent. J. 2012, 62, 1–5. [Google Scholar] [CrossRef]
- Bakke, M.; Larsen, B.M.; Dalager, T.; Møller, E. Oromandibular dystonia—Functional and clinical characteristics: A report on 21 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e21–e26. [Google Scholar] [CrossRef]
- De Meyer, M.; Vereecke, L.; Bottenberg, P.; Jacquet, W.; Sims, A.B.; Santens, P. Oral appliances in the treatment of oromandibular dystonia: A systematic review. Acta Neurol. Belg. 2020, 120, 831–836. [Google Scholar] [CrossRef]
- Kjelde, A.B.T.; Rasmussen, H.S.; Bakke, M. Oromandibulær dystoni. Tandlaegebladet 2021, 125, 658–663. [Google Scholar]
- Tan, E.K.; Jankovic, J. Botulinum toxin A in patients with oromandibular dystonia: Long-term follow-up. Neurology 1999, 53, 2102–2107. [Google Scholar] [CrossRef]
- Yoshida, K. Botulinum toxin therapy for oromandibular dystonia and other movement disorders in the stomatognathic system. Toxins 2022, 14, 282. [Google Scholar] [CrossRef]
- Dadgardoust, P.D.; Rosales, R.L.; Asuncion, R.M.; Dressler, D. Botulinum neurotoxin a therapy efficacy and safety for oromandibular dystonia: A meta-analysis. J. Neural Transm. 2019, 126, 141–148. [Google Scholar] [CrossRef]
- Comella, C.L. Systematic review of botulinum toxin treatment for oromandibular dystonia. Toxicon 2018, 147, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Baker, M.R.; Chatterjee, S.; Hrishikesh, K. Botulinum toxin: An update on pharmacology and newer products in development. Toxins 2021, 13, 58. [Google Scholar] [CrossRef]
- Balanta-Melo, J.; Toto-Ibacache, V.; Kupczik, K.; Buvinic, S. Mandibular bone loss after masticatory muscles intervention with botulinum toxin: An approach from basic research to clinical findings. Toxins 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Kahn, A.; Kün-Darbois, J.D.; Bertin, H.; Corre, P.; Chappard, D. Mandibular bone effects of botulinum toxin injections in masticatory muscles in adult. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 129, 100–108. [Google Scholar] [CrossRef]
- Møller, E.; Werdelin, L.M.; Bakke, M.; Dalager, T.; Prytz, S.; Regeur, L. Treatment of perioral dystonia with botulinum toxin in 4 cases of Meige’s syndrome. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 544–549. [Google Scholar] [CrossRef]
- Sinclair, C.F.; Gurey, L.E.; Blitzer, A. Oromandibular dystonia: Long-term management with botulinum toxin. Laryngoscope 2013, 123, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Baümer, T.; Laskaw, R.; Slawek, J.; Spittau, B.; Steffen, A.; Winterholler, M.; Bavikatte, G. Therapy of sialorrhea with botulinum neurotoxin. Neurol. Ther. 2019, 8, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Møller, E.; Karlsborg, M.; Bardow, A.; Lykkeaa, J.; Nissen, F.H.; Bakke, M. Treatment of severe drooling with botulinum toxin in amyotrophic lateral sclerosis and Parkinson’s disease: Efficacy and possible mechanisms. Acta Odont. Scand. 2011, 69, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Bakke, M.; Bardow, A.; Møller, E. Severe drooling and treatment with botulinum toxin. Perspect. Swallowing Swallowing Disord. (Dysphagia) 2012, 21, 15–21. [Google Scholar] [CrossRef]
- Jost, W.H. The option of sonographic guidance in botulinum toxin injection for drooling in Parkinson’s disease. J. Neural. Transm. 2016, 123, 51–55. [Google Scholar] [CrossRef]
- Thomas-Stonell, N.; Greenberg, S.J. Three treatment approaches and clinical factors in the reduction of drooling. Dysphagia 1988, 3, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Jost, W.H.; Friedman, A.; Michel, O.; Oehlwein, C.; Slawek, J.; Bogucki, A.; Ochudlo, S.; Banach, M.; Pagan, F.; Flatau-Baqué, B.; et al. Placebo-controlled, randomized, double-blind study of incobotulinumtoxin A for sialorrhea. Neurology 2019, 92, e1982–e1991. [Google Scholar] [CrossRef]
- Ruiz-Roca, J.A.; Pons-Fuster, E.; Lopez-Jorne, P. Effectiveness of the botulinum toxin for treating sialorrhea in patients with Parkinson’s disease: A systematic review. J. Clin. Med. 2019, 8, 317. [Google Scholar] [CrossRef]
- Hung, S.A.; Liao, C.L.; Lin, W.P.; Hsu, J.C.; Guo, Y.H.; Lin, Y.C. Botulinum toxin injections for treatment of drooling in children with cerebral palsy: A systematic review and meta-analysis. Children 2021, 8, 1089. [Google Scholar] [CrossRef]
- Tiigimäe-Saar, J.; Taba, P.; Tamme, T. Does the botulinum neuro-toxin type A treatment for sialorrhea change oral health? Clin. Oral Investig. 2017, 21, 795–800. [Google Scholar] [CrossRef]
- Lynge Pedersen, A.M.; Belstrøm, D. The role of natural salivary defences in maintaining a healthy oral microbiota. J. Dent. 2019, 80 (Suppl. S1), S3–S12. [Google Scholar] [CrossRef]
- Correa, L.B.; Basso, M.B.; Tiigimäe-Saar Sousa-Pinto, B.; Leal, S.C. Oral health effects of botulinum toxin treatment for drooling: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2021, 26, e172–e180. [Google Scholar] [CrossRef] [PubMed]
- Tiigimäe-Saar, J.; Tamme, T.; Rosenthal, M.; Kadastik-Eerme, L.; Taba, P. Saliva changes in Parkinson’s disease patients after injection of botulinum neurotoxin type A. Neurol. Sci. 2018, 39, 871–877. [Google Scholar] [CrossRef] [PubMed]

| Area | Diagnosis | Description |
|---|---|---|
| Face | Hemifacial spasm | Irregular, involuntary muscle contractions on one side of the face |
| Frey’s syndrome (gustatory sweating) | Sweating on the cheek area resulting from damage to or near the parotid gland | |
| Mouth and jaws | Oromandibular dystonia | Involuntary tension or spasm with abnormal movements of the jaw and mouth |
| Hypersalivation/sialorrhea | Drooling caused by increased saliva secretion and/or reduced swallowing function |
| OMD (ICD-11: 8A02—Dystonic Disorders) | Bruxism (ICD-11: 13DA0E.7—Dentofacial Anomalies) | |
|---|---|---|
| Main responsible discipline | Neurology | Dentistry |
| Clinical characteristics | Awake state | Mainly during sleep |
| Pain | No or only modest tenderness, but fatigue during the day and in the evening | Pain or tenderness of the masticatory muscles and tension headaches, often worst in the morning and on awakening |
| Dental attrition and trauma | Marked or pathological tooth wear Tooth infractions and fractures Wear or damage to dental restorations | |
| Provoking factors | Idiopathic or caused by brain damage or drug treatment | Uncertain or physiological and psychosocial factors |
| Aggravating factors | Psychological stress | |
| Primary treatment | BoNT and drug treatment | Flat plane stabilizing bite splint |
| Additional treatment | Flat plane stabilizing bite splint | BoNT |
| Oromandibular Muscles | Maximal Voluntary Contraction | Units of Botox or Xeomin per Muscle and Side |
|---|---|---|
| Temporalis Masseter Medial pterygoid | Jaw closing—strong biting | 10–45 IU |
| Digastricus venter anterior | Jaw opening—full mouth opening | 5–10 IU |
| Lateral pterygoid | Jaw opening—side movement and protrusion | 10–45 IU |
| Orbicularis oris | Lip pursing | 5–10 IU |
| Authors | Study Type | Patients | Glands (BoNT) | Guidance |
|---|---|---|---|---|
| Jost, Friedman, Michel et al., 2019 [35] | Randomized, placebo-controlled, double-blind study | Adults with PD, parkinsonism, stroke, or traumatic brain injury | Parotid 30 IU bilaterally + submandibular 20 IU bilaterally (all Xeomin) | All with ultrasonic guidance |
| Ruiz-Roca, Pons-Fuster and Lopez-Jorne, 2019 [38] | Systematic review of 21 studies | Mainly elderly with PD | Most cases both parotid + submandibular (mainly BoNT-A, no consensus on doses or type of BoNT) | 43% with ultrasonic guidance, else anatomical landmarks or palpation |
| Hung, Liao, Lin et al., 2021 [39] | Systematic review and meta-analysis of 21 studies | Children with CP | 62% both parotid + submandibular bilaterally (90% Botox, no consensus on doses, but should not exceed 4 IU/kg) | 81% with ultrasonic guidance |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakke, M. Botulinum Toxin, a Drug with Potential Interest for Dentists—An Introduction. Toxins 2022, 14, 667. https://doi.org/10.3390/toxins14100667
Bakke M. Botulinum Toxin, a Drug with Potential Interest for Dentists—An Introduction. Toxins. 2022; 14(10):667. https://doi.org/10.3390/toxins14100667
Chicago/Turabian StyleBakke, Merete. 2022. "Botulinum Toxin, a Drug with Potential Interest for Dentists—An Introduction" Toxins 14, no. 10: 667. https://doi.org/10.3390/toxins14100667
APA StyleBakke, M. (2022). Botulinum Toxin, a Drug with Potential Interest for Dentists—An Introduction. Toxins, 14(10), 667. https://doi.org/10.3390/toxins14100667




