sPLA2 Wobbles on the Lipid Bilayer between Three Positions, Each Involved in the Hydrolysis Process
Abstract
1. Introduction
2. Results
2.1. Atomic Force Microscopy Results
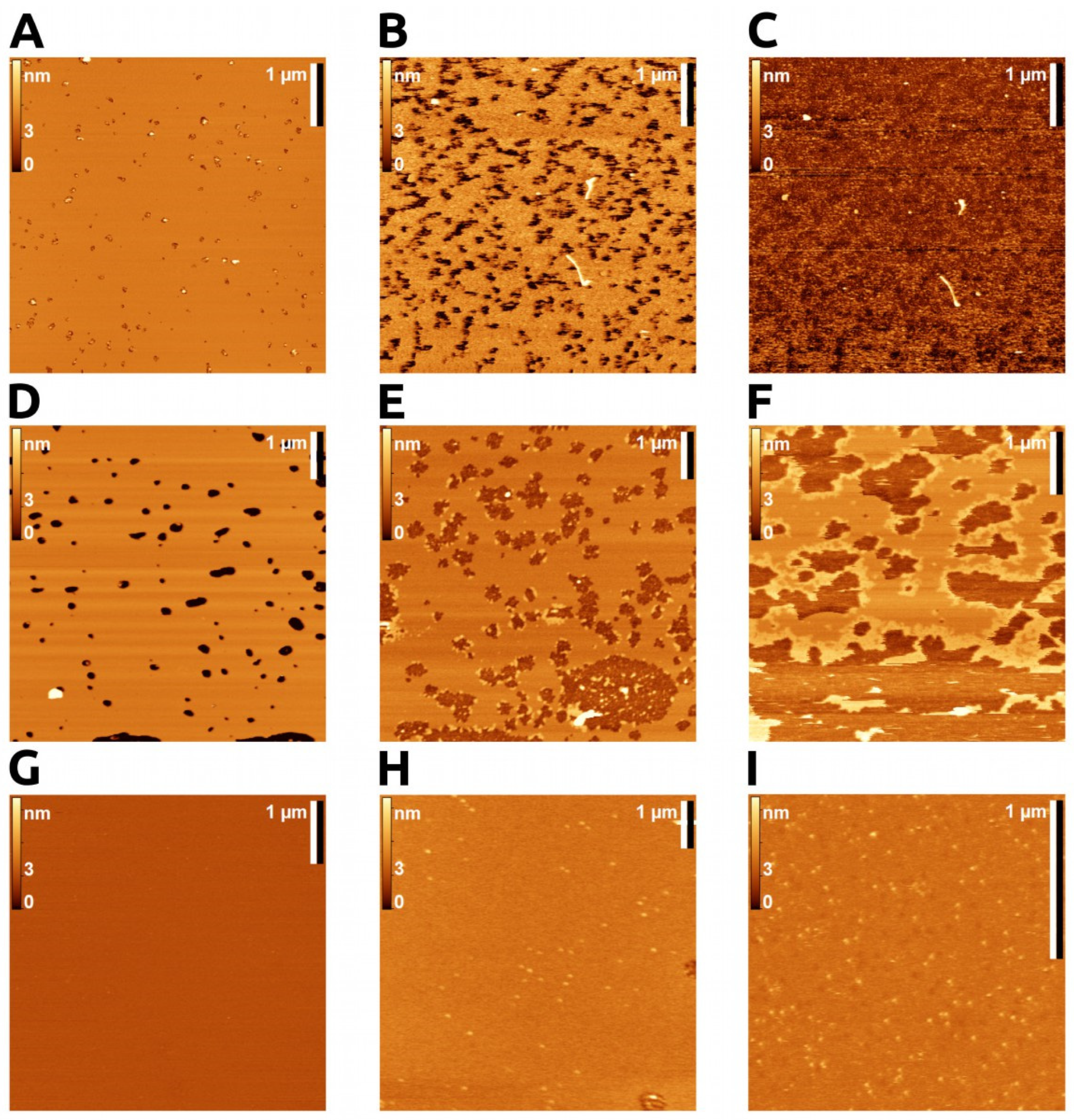
2.1.1. Supported Lipid Bilayer (SLB)
2.1.2. Enzyme Action on SLB
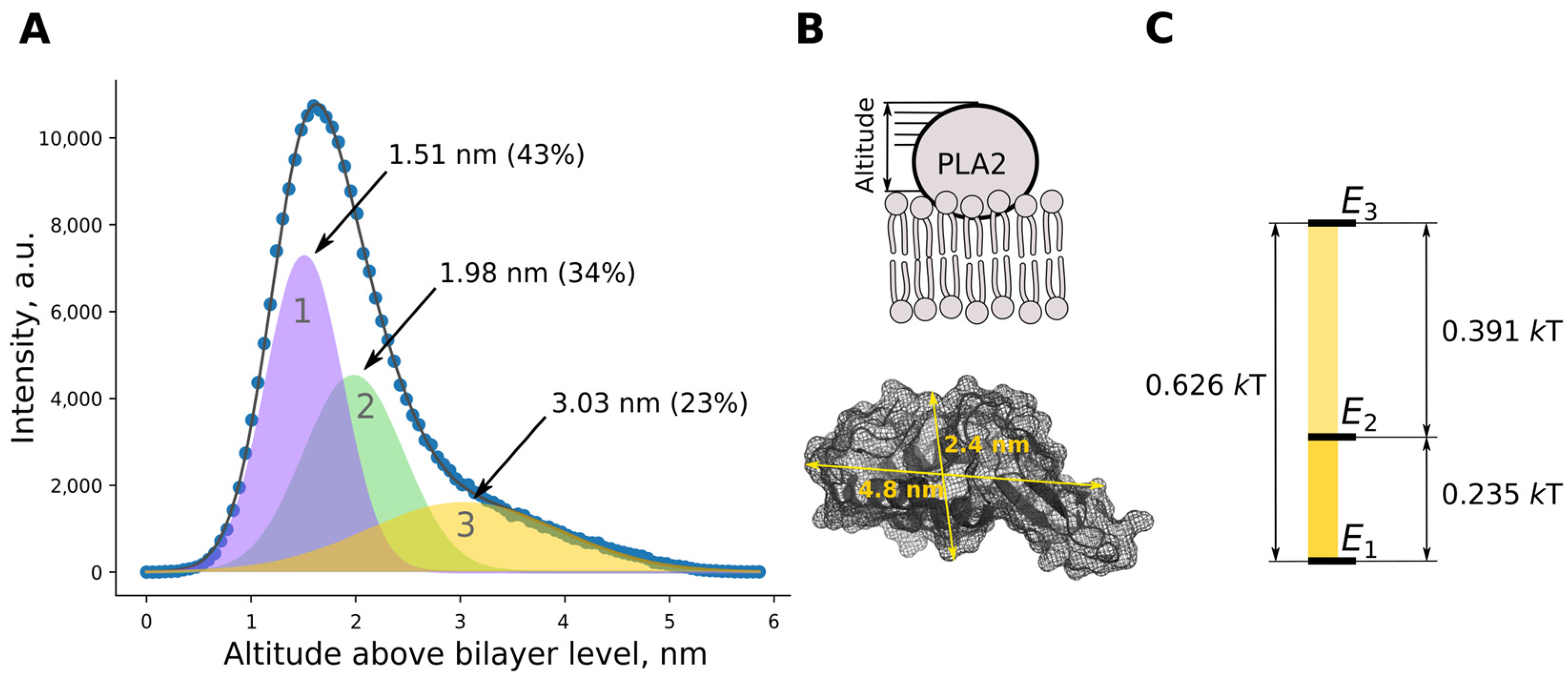
2.1.3. Visualization of Enzyme Bound to the Bilayer
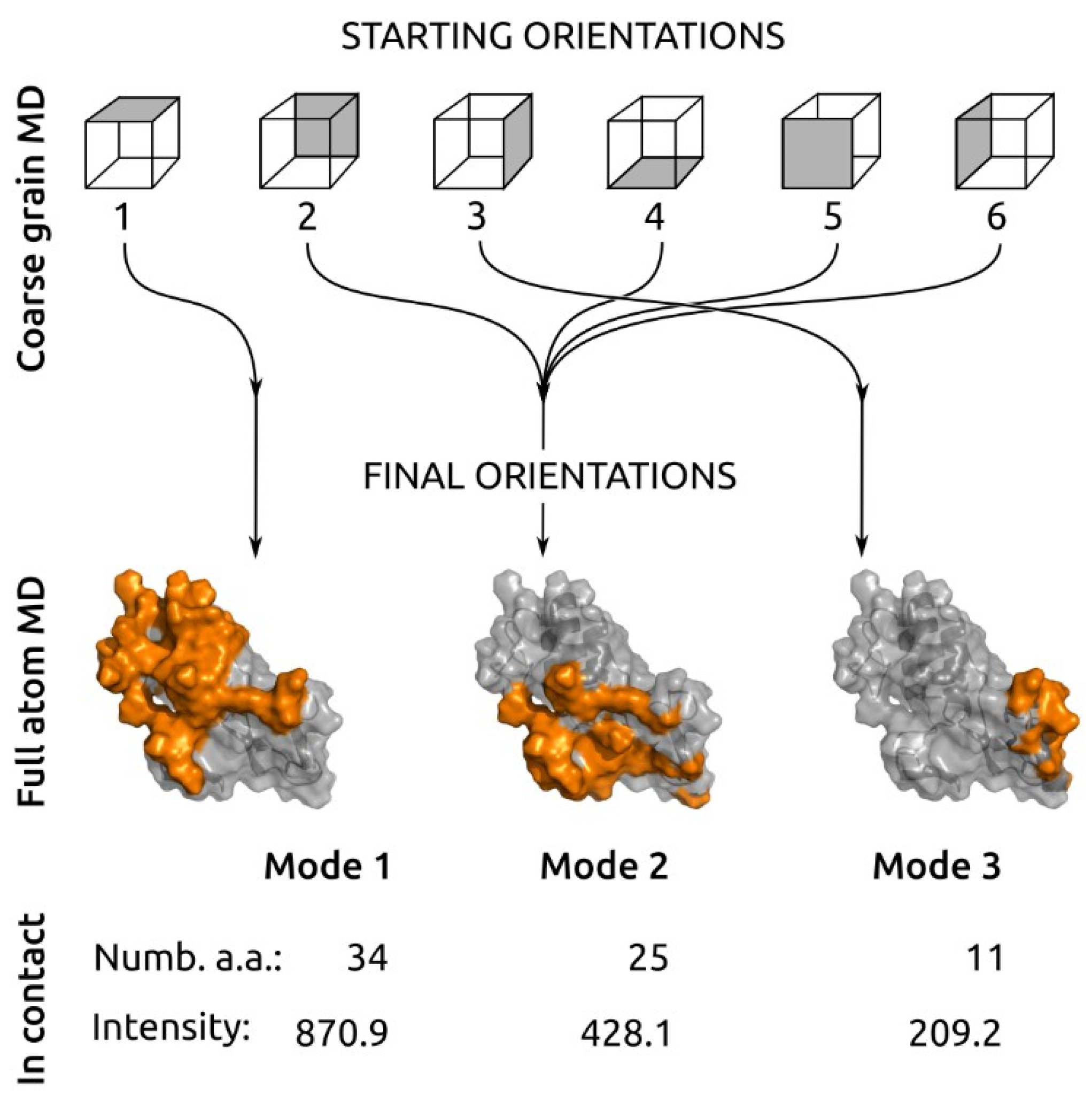
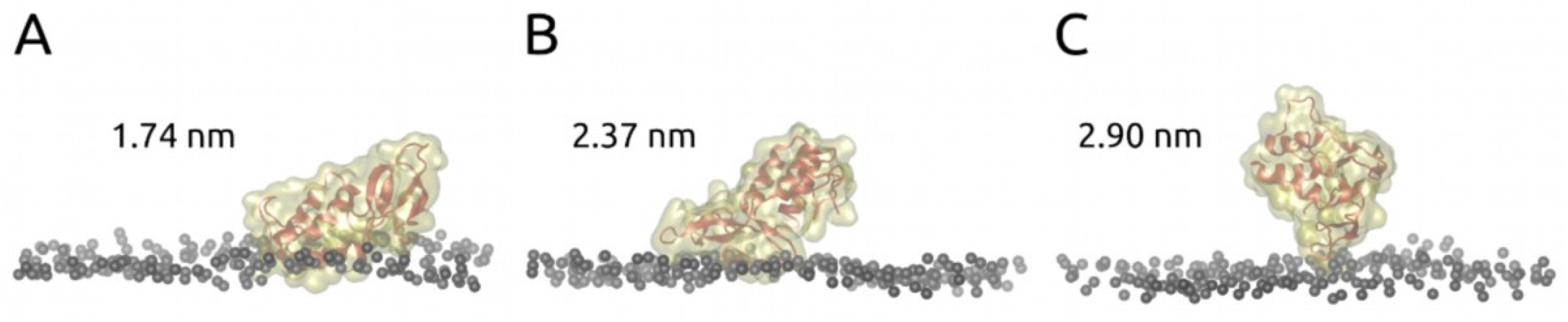
2.2. Molecular Dynamics Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Liposome Preparation
5.2. Atomic Force Microscopy (AFM) Study
5.2.1. Bilayer Formation on the Mica Surface
5.2.2. bvPLA2 Absorption
5.2.3. Data Analysis
5.3. Molecular Dynamics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, O.G.; Gelb, M.H.; Tsai, M.-D.; Jain, M.K. Interfacial Enzymology: The Secreted Phospholipase A 2 -Paradigm. Chem. Rev. 2001, 101, 2613–2654. [Google Scholar] [CrossRef] [PubMed]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating Phospholipase A2 Biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.-H.; Magrioti, V.; Kokotos, G. Phospholipase A 2 Enzymes: Physical Structure, Biological Function, Disease Implication, Chemical Inhibition, and Therapeutic Intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef]
- Šribar, J.; Križaj, I. Secreted Phospholipases A2-Not Just Enzymes. Acta Chim. Slov. 2011, 58, 678–688. [Google Scholar]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Selectivity of Phospholipid Hydrolysis by Phospholipase A2 Enzymes in Activated Cells Leading to Polyunsaturated Fatty Acid Mobilization. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Dozono, N.; Neyama, H.; Nagai, J.; Tsukahara, R.; Nagayasu, K.; Kaneko, S.; Ueda, H. Secreted PLA2-III Is a Possible Therapeutic Target to Treat Neuropathic Pain. Biochem. Biophys. Res. Commun. 2021, 568, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Mallat, Z.; Lambeau, G.; Tedgui, A. Lipoprotein-Associated and Secreted Phospholipases A 2 in Cardiovascular Disease. Circulation 2010, 122, 2183–2200. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kokotou, M.G.; Vasilakaki, S.; Kokotos, G. Small-Molecule Inhibitors as Potential Therapeutics and as Tools to Understand the Role of Phospholipases A2. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2019, 1864, 941–956. [Google Scholar] [CrossRef]
- Alekseeva, A.S.; Korotaeva, A.A.; Samoilova, E.V.; Volynsky, P.E.; Vodovozova, E.L.; Boldyrev, I. A Secretory Phospholipase A2 Activity in Blood Serum: The Challenge to Sense. Biochem. Biophys. Res. Commun. 2014, 454, 178–182. [Google Scholar] [CrossRef]
- Alekseeva, A.S.; Boldyrev, I.A. Phospholipase A2. Methods for Activity Monitoring. Biochem. Suppl. Ser. A Membr. Cell Biol. 2020, 14, 267–278. [Google Scholar] [CrossRef]
- Winget, J.M.; Pan, Y.H.; Bahnson, B.J. The Interfacial Binding Surface of Phospholipase A2s. Biochim. Biophys. Acta 2006, 1761, 1260–1269. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Chen, Y.; Andrew McCammon, J.; Dennis, E.A. Membrane Allostery and Unique Hydrophobic Sites Promote Enzyme Substrate Specificity. J. Am. Chem. Soc. 2018, 140, 3285–3291. [Google Scholar] [CrossRef]
- Hayashi, D.; Mouchlis, V.D.; Dennis, E.A. Each Phospholipase A2 Type Exhibits Distinct Selectivity Toward Sn-1 Ester, Alkyl Ether, and Vinyl Ether Phospholipids. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2021, 1867, 159067. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Hayashi, D.; Vasquez, A.M.; Cao, J.; McCammon, J.A.; Dennis, E.A. Lipoprotein-Associated Phospholipase A 2: A Paradigm for Allosteric Regulation by Membranes. Proc. Natl. Acad. Sci. USA 2022, 119, e2102953118. [Google Scholar] [CrossRef]
- Alekseeva, A.S.; Volynsky, P.E.; Krylov, N.A.; Chernikov, V.P.; Vodovozova, E.L.; Boldyrev, I.A. Phospholipase A2 Way to Hydrolysis: Dint Formation, Hydrophobic Mismatch, and Lipid Exclusion. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183481. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Risbo, J.; Callisen, T.H.; Bjørnholm, T. Lag-Burst Kinetics in Phospholipase A2 Hydrolysis of DPPC Bilayers Visualized by Atomic Force Microscopy. Biochim. Biophys. Acta-Biomembr. 1999, 1420, 266–271. [Google Scholar] [CrossRef]
- Grandbois, M.; Clausen-Schaumann, H.; Gaub, H. Atomic Force Microscope Imaging of Phospholipid Bilayer Degradation by Phospholipase A2. Biophys. J. 1998, 74, 2398–2404. [Google Scholar] [CrossRef]
- Jensen, T.R.; Balashev, K.; Bjørnholm, T.; Kjaer, K. Novel Methods for Studying Lipids and Lipases and Their Mutual Interaction at Interfaces. Part II. Surface Sensitive Synchrotron X-Ray Scattering. Biochimie 2001, 83, 399–408. [Google Scholar] [CrossRef]
- Panaiotov, I.; Ivanova, M.; Verger, R. Interfacial and Temporal Organization of Enzymatic Lipolysis. Curr. Opin. Colloid Interface Sci. 1997, 2, 517–525. [Google Scholar] [CrossRef]
- Jain, M.K.; Yu, B.Z.; Berg, O.G. Relationship of Interfacial Equilibria to Interfacial Activation of Phospholipase A2. Biochemistry 1993, 32, 11319–11329. [Google Scholar] [CrossRef]
- Speijer, H.; Giesen, P.L.; Zwaal, R.F.; Hack, C.E.; Hermens, W.T. Critical Micelle Concentrations and Stirring Are Rate Limiting in the Loss of Lipid Mass during Membrane Degradation by Phospholipase A2. Biophys. J. 1996, 70, 2239–2247. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Yu, L.; Tong, Y.; Ge, A.; Yau, S.; Osawa, M.; Ye, S. Enzyme-Catalyzed Hydrolysis of the Supported Phospholipid Bilayers Studied by Atomic Force Microscopy. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.M.; Grandbois, M.; Grainger, D.W.; Salesse, C.; Lewis, K.A.; Roberts, M.F. Phospholipase A2 Domain Formation in Hydrolyzed Asymmetric Phospholipid Monolayers at the Air/Water Interface. Biochim. Biophys. Acta-Biomembr. 1995, 1235, 395–405. [Google Scholar] [CrossRef]
- Nielsen, L.K.; Balashev, K.; Callisen, T.H.; Bjørnholm, T. Influence of Product Phase Separation on Phospholipase A2 Hydrolysis of Supported Phospholipid Bilayers Studied by Force Microscopy. Biophys. J. 2002, 83, 2617–2624. [Google Scholar] [CrossRef]
- Prachayasittikul, V.; Isarankura-Na-Ayudhya, C.; Tantimongcolwat, T.; Nantasenamat, C.; Galla, H.-J. EDTA-Induced Membrane Fluidization and Destabilization: Biophysical Studies on Artificial Lipid Membranes. Acta Biochim. Biophys. Sin. 2007, 39, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Leidy, C.; Mouritsen, O.G.; Jørgensen, K.; Peters, G.H. Evolution of a Rippled Membrane during Phospholipase A2 Hydrolysis Studied by Time-Resolved AFM. Biophys. J. 2004, 87, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Gelb, M.; Cho, W.; Wilton, D. Interfacial Binding of Secreted Phospholipases A2: More than Electrostatics and a Major Role for Tryptophan. Curr. Opin. Struct. Biol. 1999, 9, 428–432. [Google Scholar] [CrossRef]
- Stahelin, R.V.; Cho, W. Differential Roles of Ionic, Aliphatic, and Aromatic Residues in Membrane−Protein Interactions: A Surface Plasmon Resonance Study on Phospholipases A2. Biochemistry 2001, 40, 4672–4678. [Google Scholar] [CrossRef]
- Lin, Y.; Nielsen, R.; Murray, D.; Hubbell, W.L.; Mailer, C.; Robinson, B.H.; Gelb, M.H. Docking Phospholipase A 2 on Membranes Using Electrostatic Potential-Modulated Spin Relaxation Magnetic Resonance. Science 1998, 279, 1925–1929. [Google Scholar] [CrossRef]
- Gudmand, M.; Rocha, S.; Hatzakis, N.S.; Peneva, K.; Müllen, K.; Stamou, D.; Uji-I, H.; Hofkens, J.; Bjørnholm, T.; Heimburg, T. Influence of Lipid Heterogeneity and Phase Behavior on Phospholipase A2 Action at the Single Molecule Level. Biophys. J. 2010, 98, 1873–1882. [Google Scholar] [CrossRef]
- Scott, D.L.; Otwinowski, Z.; Gelb, M.H.; Sigler, P.B. Crystal Structure of Bee-Venom Phospholipase A 2 in a Complex with a Transition-State Analogue. Science 1990, 250, 1563–1566. [Google Scholar] [CrossRef]
- Ghomashchi, F.; Lin, Y.; Hixon, M.S.; Yu, B.-Z.; Annand, R.; Jain, M.K.; Gelb, M.H. Interfacial Recognition by Bee Venom Phospholipase A 2: Insights into Nonelectrostatic Molecular Determinants by Charge Reversal Mutagenesis. Biochemistry 1998, 37, 6697–6710. [Google Scholar] [CrossRef]
- Bollinger, J.G.; Diraviyam, K.; Ghomashchi, F.; Murray, D.; Gelb, M.H. Interfacial Binding of Bee Venom Secreted Phospholipase A 2 to Membranes Occurs Predominantly by a Nonelectrostatic Mechanism. Biochemistry 2004, 43, 13293–13304. [Google Scholar] [CrossRef]
- Bezzine, S.; Bollinger, J.G.; Singer, A.G.; Veatch, S.L.; Keller, S.L.; Gelb, M.H. On the Binding Preference of Human Groups IIA and X Phospholipases A2 for Membranes with Anionic Phospholipids. J. Biol. Chem. 2002, 277, 48523–48534. [Google Scholar] [CrossRef]
- Baker, S.F.; Othman, R.; Wilton, D.C. Tryptophan-Containing Mutant of Human (Group IIa) Secreted Phospholipase A 2 Has a Dramatically Increased Ability To Hydrolyze Phosphatidylcholine Vesicles and Cell Membranes. Biochemistry 1998, 37, 13203–13211. [Google Scholar] [CrossRef]
- Beers, S.A.; Buckland, A.G.; Giles, N.; Gelb, M.H.; Wilton, D.C. Effect of Tryptophan Insertions on the Properties of the Human Group IIA Phospholipase A 2: Mutagenesis Produces an Enzyme with Characteristics Similar to Those of the Human Group V Phospholipase A 2. Biochemistry 2003, 42, 7326–7338. [Google Scholar] [CrossRef]
- Gaspar, D.; Lúcio, M.; Rocha, S.; Lima, J.L.F.C.; Reis, S. Changes in PLA2 Activity after Interacting with Anti-Inflammatory Drugs and Model Membranes: Evidence for the Involvement of Tryptophan Residues. Chem. Phys. Lipids 2011, 164, 292–299. [Google Scholar] [CrossRef]
- Alekseeva, A.S.; Volynsky, P.E.; Boldyrev, I.A. Estimation of the Phospholipase A2 Selectivity on POPC/POPG Membranes Using the Interaction Map. Biochem. Suppl. Ser. A Membr. Cell Biol. 2021, 15, 329–333. [Google Scholar] [CrossRef]
- Nasri, Z.; Memari, S.; Wenske, S.; Clemen, R.; Martens, U.; Delcea, M.; Bekeschus, S.; Weltmann, K.; Woedtke, T.; Wende, K. Singlet-Oxygen-Induced Phospholipase A 2 Inhibition: A Major Role for Interfacial Tryptophan Dioxidation. Chem.-A Eur. J. 2021, 27, 14702–14710. [Google Scholar] [CrossRef]
- Jain, M.K.; van Echteld, C.J.A.; Ramirez, F.; de Gier, J.; de Haas, G.H.; van Deenen, L.L.M. Association of Lysophosphatidylcholine with Fatty Acids in Aqueous Phase to Form Bilayers. Nature 1980, 284, 486–487. [Google Scholar] [CrossRef]
- Murakami, M.; Koduri, R.S.; Enomoto, A.; Shimbara, S.; Seki, M.; Yoshihara, K.; Singer, A.; Valentin, E.; Ghomashchi, F.; Lambeau, G.; et al. Distinct Arachidonate-Releasing Functions of Mammalian Secreted Phospholipase A2s in Human Embryonic Kidney 293 and Rat Mastocytoma RBL-2H3 Cells through Heparan Sulfate Shuttling and External Plasma Membrane Mechanisms. J. Biol. Chem. 2001, 276, 10083–10096. [Google Scholar] [CrossRef]
- Murakami, M.; Shimbara, S.; Kambe, T.; Kuwata, H.; Winstead, M.V.; Tischfield, J.A.; Kudo, I. The Functions of Five Distinct Mammalian Phospholipase A2s in Regulating Arachidonic Acid Release. J. Biol. Chem. 1998, 273, 14411–14423. [Google Scholar] [CrossRef]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; de Vries, A.H. The MARTINI Force Field: Coarse Grained Model for Biomolecular Simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef]
- Periole, X.; Cavalli, M.; Marrink, S.-J.; Ceruso, M.A. Combining an Elastic Network With a Coarse-Grained Molecular Force Field: Structure, Dynamics, and Intermolecular Recognition. J. Chem. Theory Comput. 2009, 5, 2531–2543. [Google Scholar] [CrossRef]
- de Jong, D.H.; Baoukina, S.; Ingólfsson, H.I.; Marrink, S.J. Martini Straight: Boosting Performance Using a Shorter Cutoff and GPUs. Comput. Phys. Commun. 2016, 199, 1–7. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from Ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Jämbeck, J.P.M.; Lyubartsev, A.P. Another Piece of the Membrane Puzzle: Extending Slipids Further. J. Chem. Theory Comput. 2013, 9, 774–784. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzmina, N.; Volynsky, P.; Boldyrev, I.; Alekseeva, A. sPLA2 Wobbles on the Lipid Bilayer between Three Positions, Each Involved in the Hydrolysis Process. Toxins 2022, 14, 669. https://doi.org/10.3390/toxins14100669
Kuzmina N, Volynsky P, Boldyrev I, Alekseeva A. sPLA2 Wobbles on the Lipid Bilayer between Three Positions, Each Involved in the Hydrolysis Process. Toxins. 2022; 14(10):669. https://doi.org/10.3390/toxins14100669
Chicago/Turabian StyleKuzmina, Natalia, Pavel Volynsky, Ivan Boldyrev, and Anna Alekseeva. 2022. "sPLA2 Wobbles on the Lipid Bilayer between Three Positions, Each Involved in the Hydrolysis Process" Toxins 14, no. 10: 669. https://doi.org/10.3390/toxins14100669
APA StyleKuzmina, N., Volynsky, P., Boldyrev, I., & Alekseeva, A. (2022). sPLA2 Wobbles on the Lipid Bilayer between Three Positions, Each Involved in the Hydrolysis Process. Toxins, 14(10), 669. https://doi.org/10.3390/toxins14100669





