Characterization of the Composition and Biological Activity of the Venom from Vespa bicolor Fabricius, a Wasp from South China
Abstract
1. Introduction
2. Results
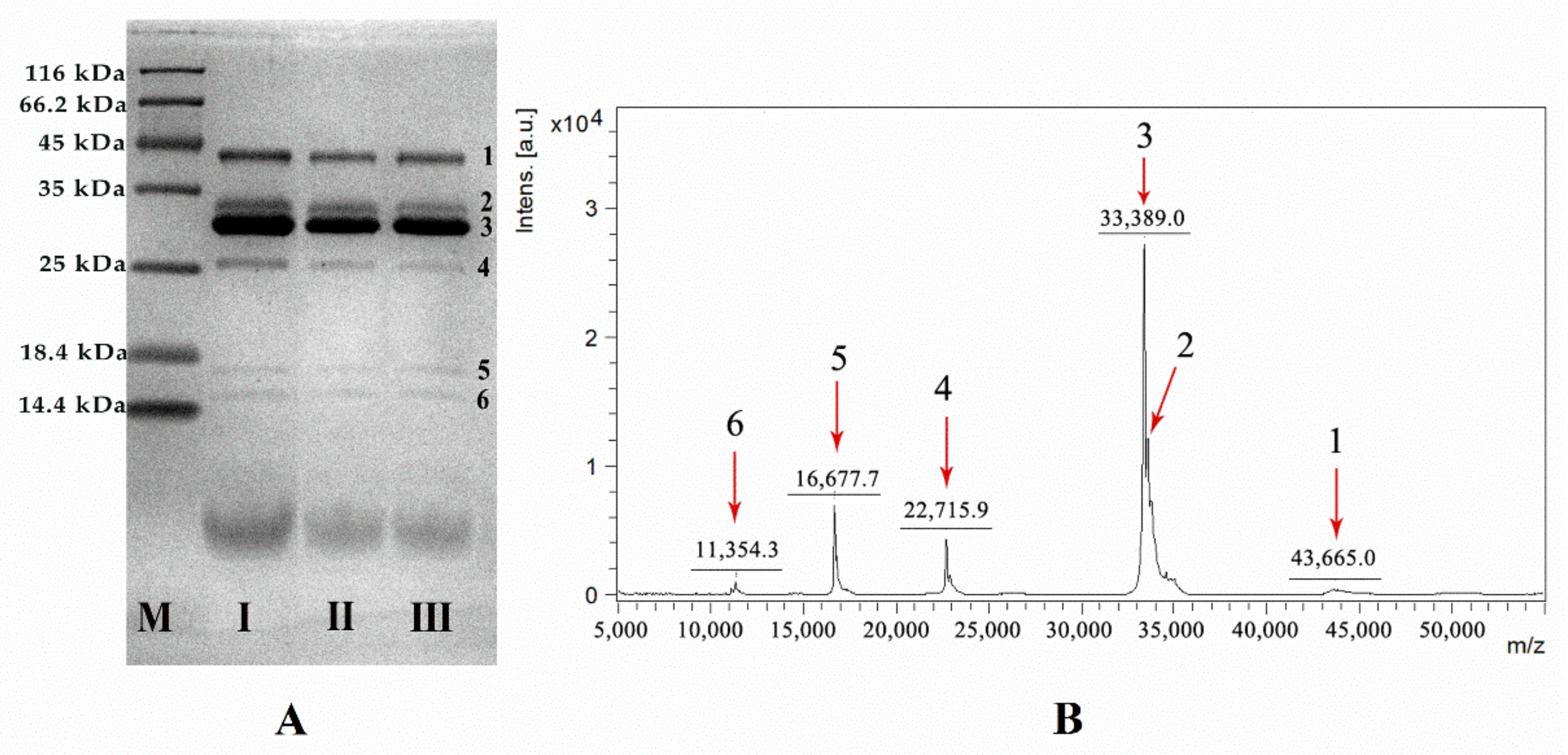
2.1. Protein Characterization of VBV
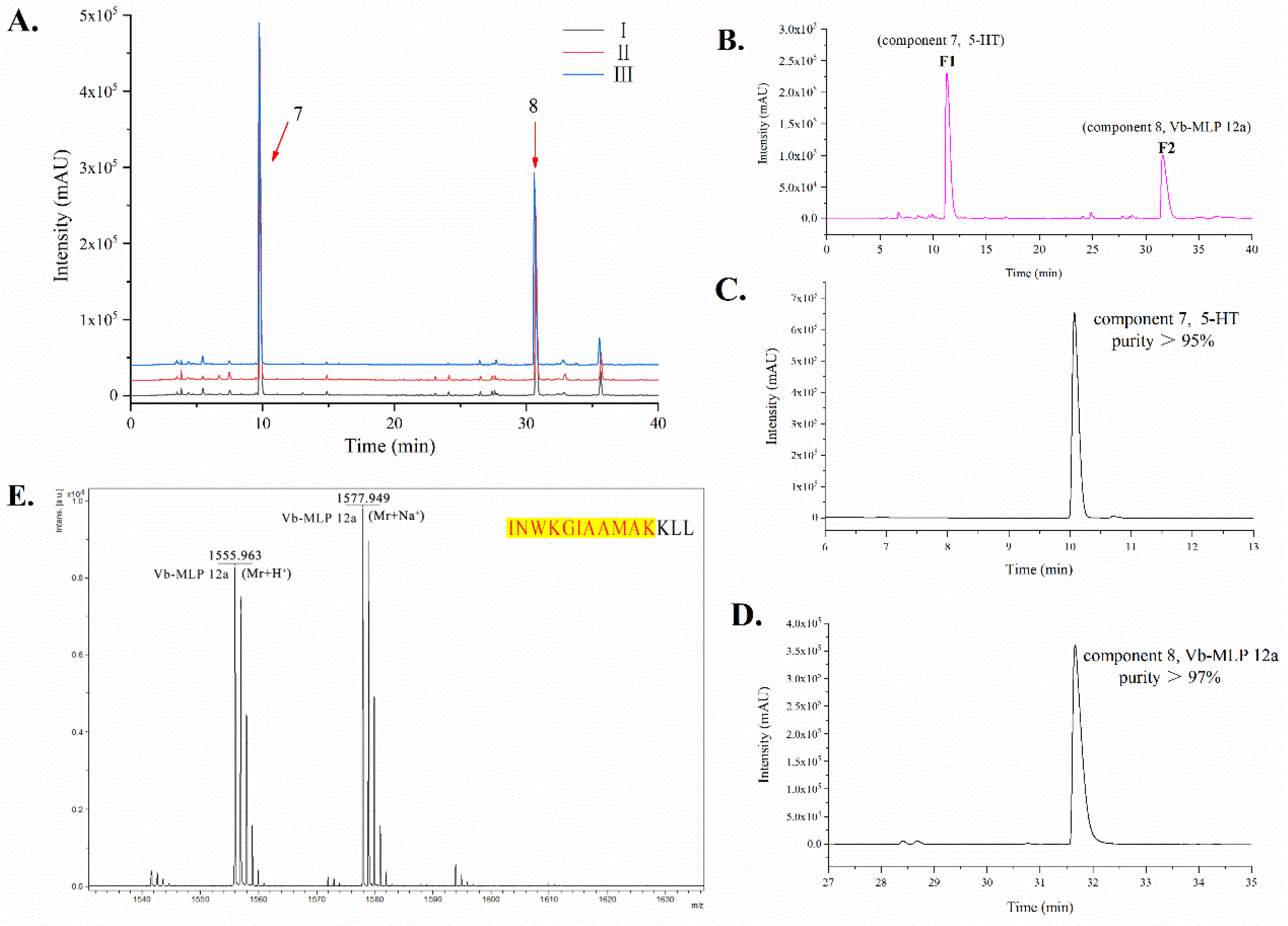
2.2. Chemical Characterization of VBV
2.3. Antimicrobial Activity
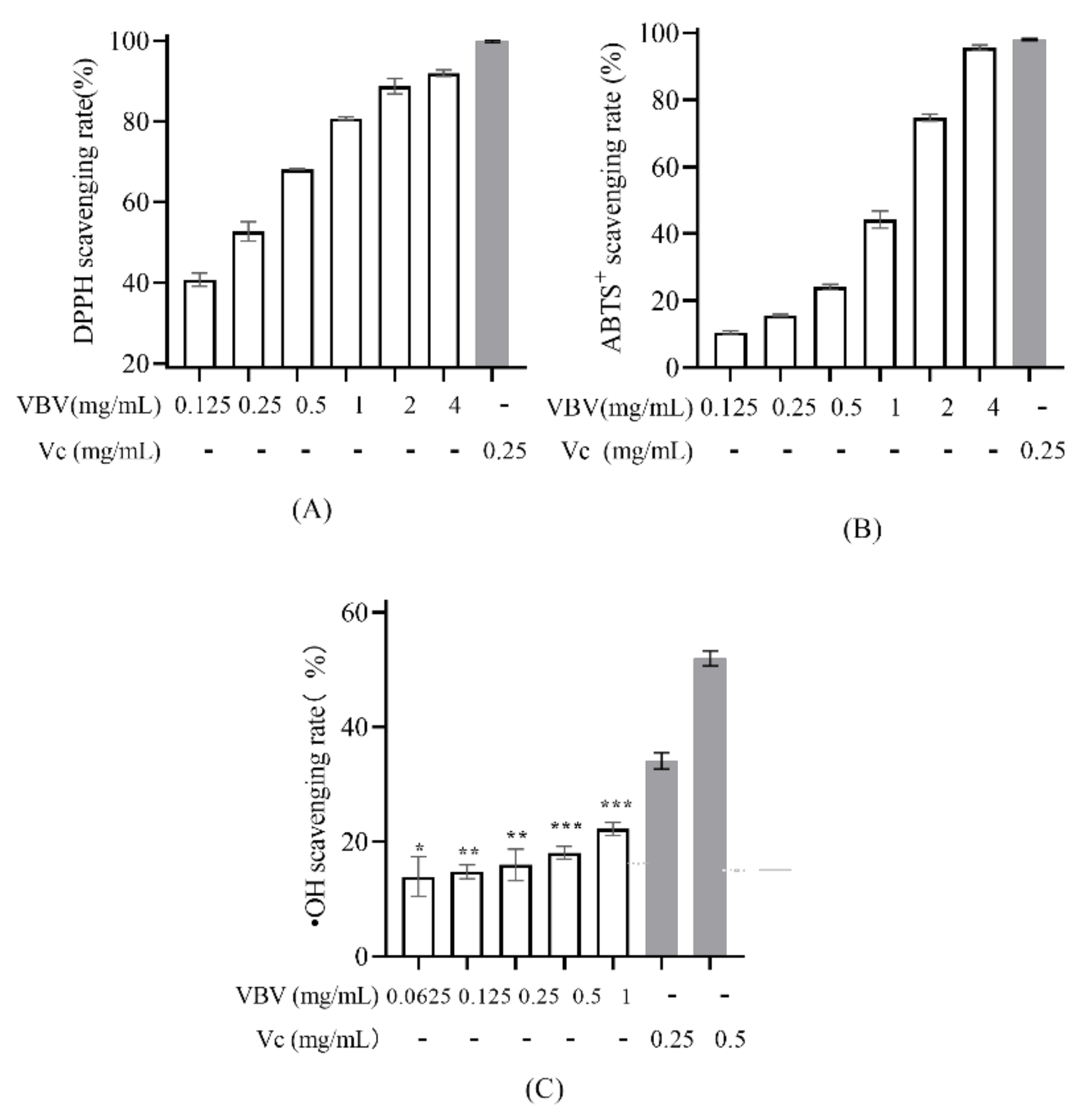
2.4. Antioxidant Activity
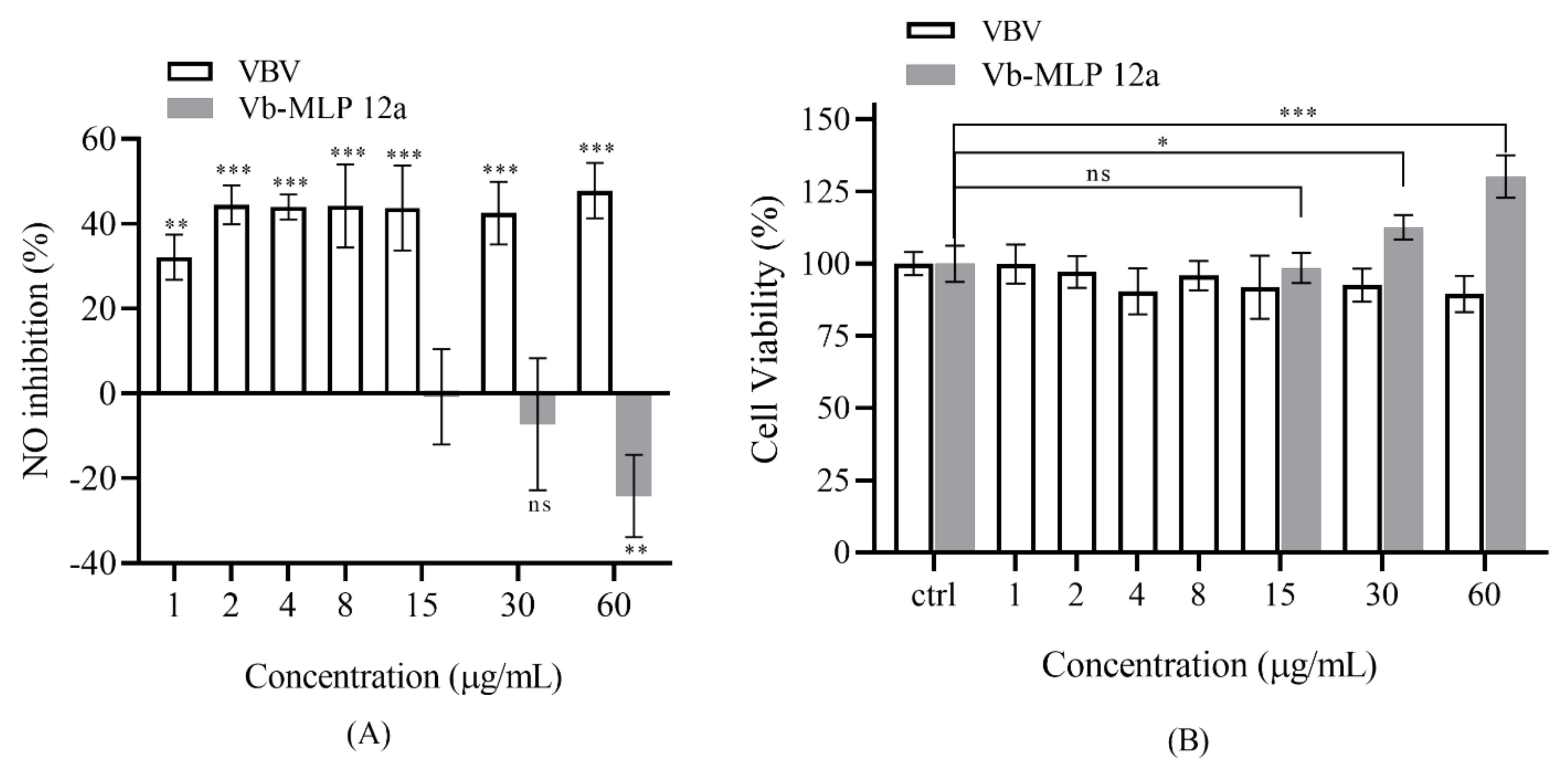
2.5. Anti-Inflammatory Assay
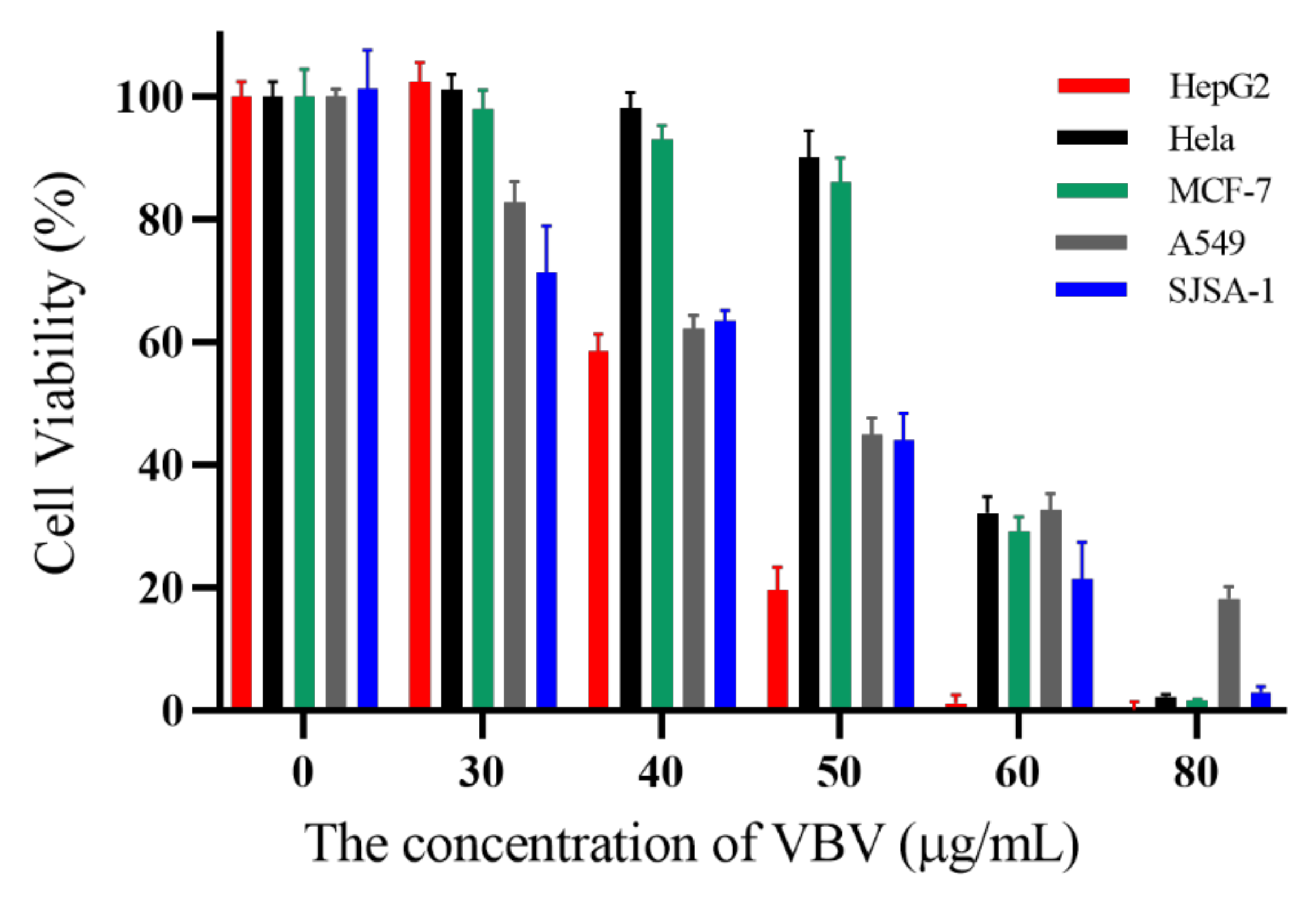
2.6. Cytotoxic Activity Assay on Cancer Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Venom
5.2. Protein Characterization of VBV
5.2.1. Protein Analysis of VBV by SDS-PAGE
5.2.2. Identification of Proteins of VBV by LC–MS/MS
5.3. Chemical Characterization of VBV
5.3.1. Chemical Analysis of VBV by HPLC
5.3.2. Identification of Chromatographic Peaks
5.4. Antibacterial Activity Assay
5.4.1. Inhibition Zone Diameter Measurements
5.4.2. Determination of MIC and MBC
5.5. Antioxidant Activity Assay
5.5.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
5.5.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) Diammonium Salt (ABTS) Assay
5.5.3. •OH Radical Scavenging Ability
5.6. Anti-Inflammatory Activity Assay
5.7. Cytotoxic Activity Assay on Cancer Cells
5.8. Statistical Analysis
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baek, J.H.; Oh, J.H.; Kim, Y.H.; Lee, S.H. Comparative transcriptome analysis of the venom sac and gland of social wasp Vespa tropica and solitary wasp Rhynchium brunneum. J. Asia-Pacific Èntomol. 2013, 16, 497–502. [Google Scholar] [CrossRef]
- Dias, N.B.; De Souza, B.M.; Gomes, P.C.; Brigatte, P.; Palma, M.S. Peptidome profiling of venom from the social wasp Polybia paulista. Toxicon 2015, 107, 290–303. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, D.K.; Thounaojam, M.C.; Mitra, A.; Basu, A. Vespa tropica venom suppresses lipopolysaccharide-mediated secretion of pro-inflammatory cyto-chemokines by abrogating nuclear factor-kappa B activation in microglia. Inflamm. Res. 2014, 63, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Jalaei, J.; Fazeli, M.; Rajaian, H.; Ghalehsoukhteh, S.L.; Dehghani, A.; Winter, D. In vitro antihistamine-releasing activity of a peptide derived from wasp venom of Vespa orientalis. Asian Pac. J. Trop. Biomed. 2016, 6, 259–264. [Google Scholar] [CrossRef]
- Le, T.N.; Da Silva, D.; Colas, C.; Darrouzet, É.; Baril, P.; Leseurre, L.; Maunit, B. Asian hornet Vespa velutina nigrithorax venom: Evaluation and identification of the bioactive compound responsible for human keratinocyte protection against oxidative stress. Toxicon 2020, 176, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Lee, W.-H.; Zhang, Y. Antimicrobial peptides from the venom gland of the social wasp Vespa tropica. Toxicon 2013, 74, 151–157. [Google Scholar] [CrossRef]
- Dos Santos-Pinto, J.R.A.; Perez-Riverol, A.; Lasa, A.M.; Palma, M.S. Diversity of peptidic and proteinaceous toxins from social Hymenoptera venoms. Toxicon 2018, 148, 172–196. [Google Scholar] [CrossRef]
- Ho, C.-L.; Chen, W.-C.; Lin, Y.-L. Structures and biological activities of new wasp venom peptides isolated from the black-bellied hornet (Vespa basalis) venom. Toxicon 1998, 36, 609–617. [Google Scholar] [CrossRef]
- An, S.; Chen, L.; Wei, J.-F.; Yang, X.; Ma, N.; Xu, X.; Xu, X.; He, S.; Lu, J.; Lai, R. Purification and Characterization of Two New Allergens from the Venom of Vespa magnifica. PLoS ONE 2012, 7, e31920. [Google Scholar] [CrossRef]
- Jacomini, D.L.J.; Pereira, F.D.C.; Pinto, J.R.A.D.S.; Dos Santos, L.D.; Neto, A.J.D.S.; Giratto, D.T.; Palma, M.S.; Zollner, R.D.L.; Braga, M.R.B. Hyaluronidase from the venom of the social wasp Polybia paulista (Hymenoptera, Vespidae): Cloning, structural modeling, purification, and immunological analysis. Toxicon 2013, 64, 70–80. [Google Scholar] [CrossRef]
- Rungsa, P.; Peigneur, S.; Daduang, S.; Tytgat, J. Purification and biochemical characterization of VesT1s, a novel phospholipase A1 isoform isolated from the venom of the greater banded wasp Vespa tropica. Toxicon 2018, 148, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Sukprasert, S.; Rungsa, P.; Uawonggul, N.; Incamnoi, P.; Thammasirirak, S.; Daduang, J.; Daduang, S. Purification and structural characterisation of phospholipase A1 (Vespapase, Ves a 1) from Thai banded tiger wasp (Vespa affinis) venom. Toxicon 2013, 61, 151–164. [Google Scholar] [CrossRef]
- Hoffman, D.R.; Wood, C.L. Allergens in Hymenoptera venom XI. Isolation of protein allergens from Vespula maculifrons (yellow jacket) venom. J. Allergy Clin. Immunol. 1984, 74, 93–103. [Google Scholar] [CrossRef]
- Bazon, M.L.; Silveira, L.H.; Simioni, P.U.; Brochetto-Braga, M.R. Current Advances in Immunological Studies on the Vespidae Venom Antigen 5: Therapeutic and Prophylaxis to Hypersensitivity Responses. Toxins 2018, 10, 305. [Google Scholar] [CrossRef]
- Rungsa, P.; Incamnoi, P.; Sukprasert, S.; Uawonggul, N.; Klaynongsruang, S.; Daduang, J.; Patramanon, R.; Roytrakul, S.; Daduang, S. Comparative proteomic analysis of two wasps venom, Vespa tropica and Vespa affinis. Toxicon 2016, 119, 159–167. [Google Scholar] [CrossRef]
- Perez-Riverol, A.; Pereira, F.D.C.; Lasa, A.M.; Fernandes, L.G.R.; Dos Santos-Pinto, J.R.A.; Justo-Jacomini, D.L.; De Azevedo, G.O.; Bazon, M.L.; Palma, M.S.; Zollner, R.D.L.; et al. Molecular cloning, expression and IgE-immunoreactivity of phospholipase A1, a major allergen from Polybia paulista (Hymenoptera: Vespidae) venom. Toxicon 2016, 124, 44–52. [Google Scholar] [CrossRef]
- Yang, H.; Xu, X.; Ma, D.; Zhang, K.; Lai, R. A phospholipase A1 platelet activator from the wasp venom of Vespa magnifica (Smith). Toxicon 2008, 51, 289–296. [Google Scholar] [CrossRef]
- Bazon, M.L.; Perez-Riverol, A.; Dos Santos-Pinto, J.R.A.; Fernandes, L.G.R.; Lasa, A.M.; Justo-Jacomini, D.L.; Palma, M.S.; De Lima Zollner, R.; Brochetto-Braga, M.R. Heterologous Expression, Purification and Immunoreactivity of the Antigen 5 from Polybia paulista Wasp Venom. Toxins 2017, 9, 259. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Baek, J.H.; Yoon, K.A. Differential Properties of Venom Peptides and Proteins in Solitary vs. Social Hunting Wasps. Toxins 2016, 8, 32. [Google Scholar] [CrossRef]
- Herrera, C.; Leza, M.; Martínez-Lopez, E. Diversity of compounds in Vespa spp. venom and the epidemiology of its sting: A global appraisal. Arch. Toxicol. 2020, 94, 3609–3627. [Google Scholar] [CrossRef] [PubMed]
- Hilchie, A.L.; Sharon, A.; Haney, E.F.; Hoskin, D.W.; Bally, M.; Franco, O.; Corcoran, J.A.; Hancock, R.E. Mastoparan is a membranolytic anti-cancer peptide that works synergistically with gemcitabine in a mouse model of mammary carcinoma. Biochim. Biophys. Acta (BBA)—Biomembr. 2016, 1858, 3195–3204. [Google Scholar] [CrossRef]
- Lopes, K.S.; Campos, G.A.A.; Camargo, L.C.; De Souza, A.C.B.; Ibituruna, B.V.; Magalhães, A.C.M.; Da Rocha, L.F.; Garcia, A.B.; Rodrigues, M.C.; Ribeiro, D.M.; et al. Characterization of two peptides isolated from the venom of social wasp Chartergellus communis (Hyme-noptera: Vespidae): Influence of multiple alanine residues and C-terminal amidation on biological effects. Peptides 2017, 95, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.; Neto, L.M.; Neves, R.C.; Gonçalves, J.C.; Trentini, M.M.; Mucury-Filho, R.; Smidt, K.S.; Fensterseifer, I.C.; Silva, O.N.; Lima, L.D.; et al. Evaluation of the antimicrobial activity of the mastoparan Polybia-MPII isolated from venom of the social wasp Pseudopolybia vespiceps testacea (Vespidae, Hymenoptera). Int. J. Antimicrob. Agents 2017, 49, 167–175. [Google Scholar] [CrossRef]
- Yang, M.J.; Lin, W.-Y.; Lu, K.-H.; Tu, W.-C. Evaluating antioxidative activities of amino acid substitutions on mastoparan-B. Peptides 2011, 32, 2037–2043. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-H.; Tzen, J.T.; Shyu, C.-L.; Yang, M.J.; Tu, W.-C. Structural and biological characterization of mastoparans in the venom of Vespa species in Taiwan. Peptides 2011, 32, 2027–2036. [Google Scholar] [CrossRef]
- Frangieh, J.; Salma, Y.; Haddad, K.; Mattei, C.; Legros, C.; Fajloun, Z.; El Obeid, D. First Characterization of The Venom from Apis mellifera syriaca, A Honeybee from The Middle East Region. Toxins 2019, 11, 191. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Lu, Z.; Zhai, L.; Jiang, J.; Liu, J.; Yu, H. A novel serine protease inhibitor from the venom of Vespa bicolor Fabricius. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 153, 116–120. [Google Scholar] [CrossRef]
- Yin, X.; Tang, F.; Huang, Y.; Huang, X. Medicinal Liquor Useful for Treating Rheumatism Comprises Hornet, Vespa bicolor and White Wine. Patent Application No. CN107397762-A2017, 28 November 2017. [Google Scholar]
- Chen, W.; Yang, X.; Yang, X.; Zhai, L.; Lu, Z.; Liu, J.; Yu, H. Antimicrobial peptides from the venoms of Vespa bicolor Fabricius. Peptides 2008, 29, 1887–1892. [Google Scholar] [CrossRef]
- Yan, H.-L.; Chen, W.-L.; Chen, L.-L.; Lai, R.; Liu, J.-Z. A Novel Bioactive Peptide with Myotropic Activity from Wasp Venoms. Chin. J. Nat. Med. 2011, 9, 317–320. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Lu, Q.; Yang, H.; Zhang, Y.; Lai, R. Two families of antimicrobial peptides from wasp (Vespa magnifica) venom. Toxicon 2006, 47, 249–253. [Google Scholar] [CrossRef]
- Pénez, N.; Culioli, G.; Pérez, T.; Briand, J.-F.; Thomas, O.P.; Blache, Y. Antifouling Properties of Simple Indole and Purine Alkaloids from the Mediterranean Gorgonian Paramuricea clavata. J. Nat. Prod. 2011, 74, 2304–2308. [Google Scholar] [CrossRef]
- Zhou, S.-T.; Luan, K.; Ni, L.-L.; Wang, Y.; Yuan, S.-M.; Che, Y.-H.; Yang, Z.-Z.; Zhang, C.-G.; Yang, Z.-B. A Strategy for Quality Control of Vespa magnifica (Smith) Venom Based on HPLC Fingerprint Analysis and Multi-Component Separation Combined with Quantitative Analysis. Molecules 2019, 24, 2920. [Google Scholar] [CrossRef]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with an-ti-inflammatory activity. Comb. Chem. High Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef]
- De Azevedo, R.A.; Figueiredo, C.R.; Ferreira, A.K.; Matsuo, A.L.; Massaoka, M.H.; Girola, N.; Auada, A.V.; Farias, C.F.; Pasqualoto, K.F.; Rodrigues, C.P.; et al. Mastoparan induces apoptosis in B1 6F10-Nex2 melanoma cells via the intrinsic mitochondrial pathway and displays antitumor activity in vivo. Peptides 2015, 68, 113–119. [Google Scholar] [CrossRef]
- Rocha, T.; De Souza, B.M.; Palma, M.S.; Cruz-Hoefling, M.A.D. Myotoxic effects of mastoparan from Polybia paulista (Hy-menoptera, Epiponini) wasp venom in mice skeletal muscle. Toxicon 2007, 50, 589–599. [Google Scholar] [CrossRef]
- Huerta-Reyes, M.; Anselme, C.; Cherqui, L.; Decocq, G. Exploration Through the Venoms from Hymenoptera as Potential Therapeutic Agents in Cancer Therapy. Int. J. Pharmacol. 2017, 13, 507–515. [Google Scholar] [CrossRef]
- Koo, C.-Y.; Sen, Y.-P.; Bay, B.-H.; Yip, G.W. Targeting heparan sulfate proteoglycans in breast cancer treatment. Recent Patents Anti-Cancer Drug Discov. 2008, 3, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Bafna, S.; Kaur, S.; Batra, S.K. Membrane-bound mucins: The mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010, 29, 2893–2904. [Google Scholar] [CrossRef] [PubMed]
- Feyissa, B.A.; Renaud, J.; Nasrollahi, V.; Kohalmi, S.E.; Hannoufa, A. Transcriptome-IPMS analysis reveals a tissue-dependent miR156/SPL13 regulatory mechanism in alfalfa drought tolerance. BMC Genom. 2020, 21, 721. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Dounighi, N.M. Purification and characterization of a novel type of neurotoxic peptides from the venom of the Iranian scorpion Hemiscorpius lepturus. Iran J. Basic Med. Sci. 2019, 23, 195–201. [Google Scholar]
- Sukprasert, S.; Uawonggul, N.; Jamjanya, T.; Thammasirirak, S.; Daduang, J.; Daduang, S. Characterization of the allergen Sol gem 2 from the fire ant venom, Solenopsis geminata. J. Venom. Anim. Toxins Incl. Trop. Dis. 2012, 18, 325–334. [Google Scholar] [CrossRef][Green Version]
- Zhou, G.; Wu, J.; Xia, C.; Liu, S.; Jiang, F.; Liu, Z.; Zhou, Y.; Ji, Y. Identifying the toxins in hornet (Vespa basalis) venom that induce rat pain responses. Toxicon 2020, 179, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Jalaei, J.; Fazeli, M.; Rajaian, H.; Shekarforoush, S.S. In vitro antibacterial effect of wasp (Vespa orientalis) venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2014, 20, 22. [Google Scholar] [CrossRef]
- Pan, C.-Y.; Rajanbabu, V.; Chen, J.-Y.; Her, G.M.; Nan, F.-H. Evaluation of the epinecidin-1 peptide as an active ingredient in cleaning solutions against pathogens. Peptides 2010, 31, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Miazga-Karska, M.; Michalak, K.; Ginalska, G. Anti-Acne Action of Peptides Isolated from Burdock Root—Preliminary Studies and Pilot Testing. Molecules 2020, 25, 2027. [Google Scholar] [CrossRef]
- Meng, Y.-C.; Mo, X.-G.; He, T.-T.; Wen, X.-X.; Nieh, J.-C.; Yang, X.-W.; Tan, K. New bioactive peptides from the venom gland of a social hornet Vespa velutina. Toxicon 2021, 199, 94–100. [Google Scholar] [CrossRef]
- Abdel-Aty, A.M.; Hsalama, W.; Hamed, M.; Fahmy, A.S.; Mohamed, S.A. Phenolic-antioxidant capacity of mango seed kernels: Therapeutic effect against viper venoms. Rev. Bras. Farm. 2018, 28, 594–601. [Google Scholar] [CrossRef]
| Microorganisms | Inhibition Zone 1 (mm) | MIC 2 (µg/mL) | MBC 3 (µg/mL) |
|---|---|---|---|
| S. aureus | 12.3 ± 0.2 | 25 | 50 |
| E. coli | 10.2 ± 0.5 | 100 | 200 |
| P. aeruginosa | ND 4 | ND | ND |
| Pr. acnes | 19.0 ± 0.3 | 12.5 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-H.; Zhang, Y.; Fang, D.-Q.; Chen, J.; Wang, J.-A.; Jiang, L.; Lv, Z.-F. Characterization of the Composition and Biological Activity of the Venom from Vespa bicolor Fabricius, a Wasp from South China. Toxins 2022, 14, 59. https://doi.org/10.3390/toxins14010059
Wu Y-H, Zhang Y, Fang D-Q, Chen J, Wang J-A, Jiang L, Lv Z-F. Characterization of the Composition and Biological Activity of the Venom from Vespa bicolor Fabricius, a Wasp from South China. Toxins. 2022; 14(1):59. https://doi.org/10.3390/toxins14010059
Chicago/Turabian StyleWu, Yong-Hua, Yu Zhang, Dan-Qiao Fang, Jing Chen, Jing-An Wang, Lin Jiang, and Zhu-Fen Lv. 2022. "Characterization of the Composition and Biological Activity of the Venom from Vespa bicolor Fabricius, a Wasp from South China" Toxins 14, no. 1: 59. https://doi.org/10.3390/toxins14010059
APA StyleWu, Y.-H., Zhang, Y., Fang, D.-Q., Chen, J., Wang, J.-A., Jiang, L., & Lv, Z.-F. (2022). Characterization of the Composition and Biological Activity of the Venom from Vespa bicolor Fabricius, a Wasp from South China. Toxins, 14(1), 59. https://doi.org/10.3390/toxins14010059