Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed
Abstract
1. Introduction
2. Results and Discussion
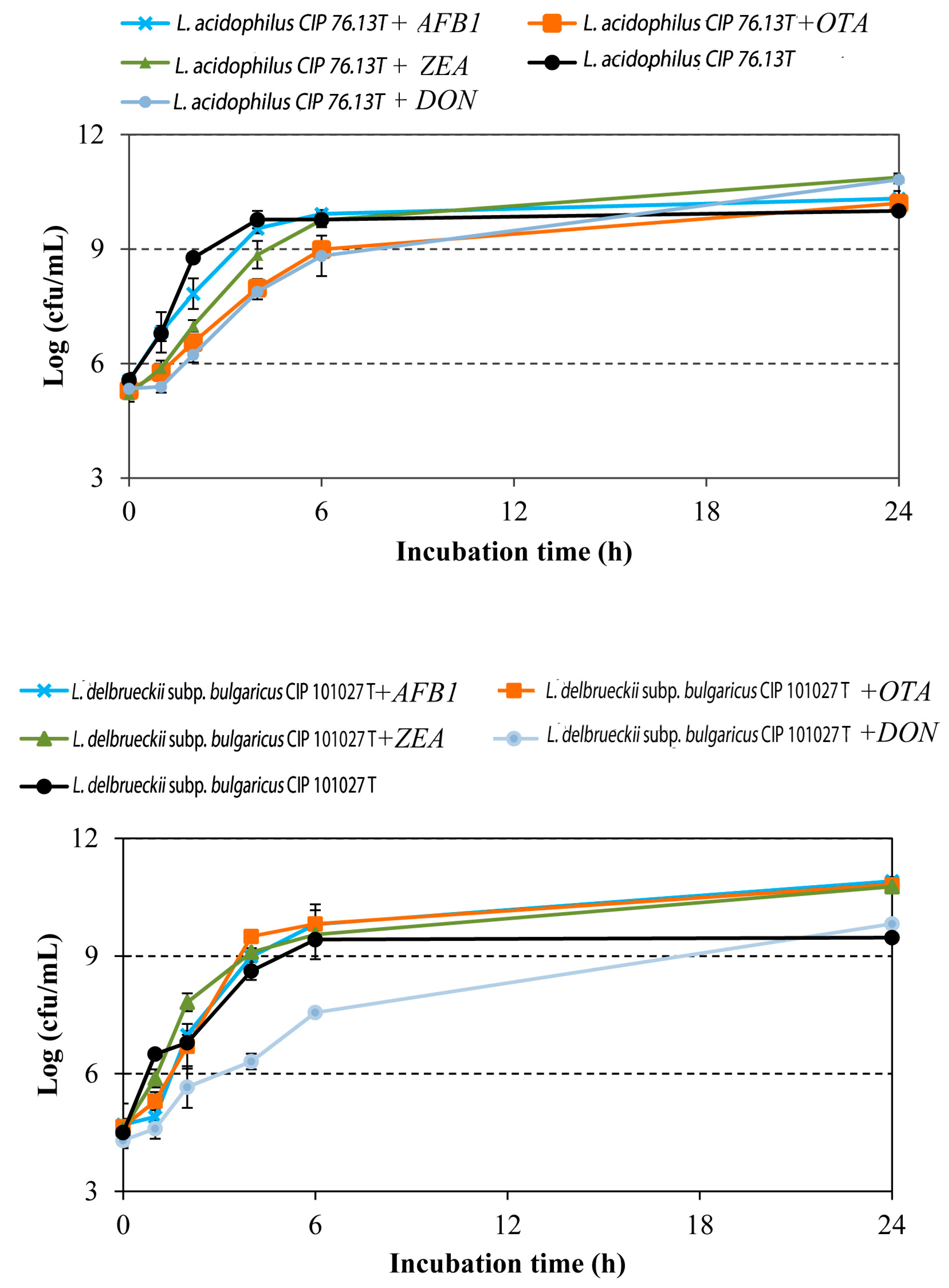
2.1. Effect of Mycotoxins on LAB Growth
2.2. Mycotoxin Removal by LABs
2.3. ZEA Removal in Animal Liquid Feed
3. Conclusions
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Bacterial Strains and Culture Conditions
4.3. Effect of Mycotoxins on Microbial Growth
4.4. Mycotoxin Removal by Viable and Heat Inactivated LAB Cells
4.5. Mycotoxin Desorption Experiments
4.6. Mycotoxin Removal by LAB from Animal LF
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Binder, E.; Tan, L.; Chin, L.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed. Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses—An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Streit, E.; Schwab, C.; Sulyok, M.; Naehrer, K.; Krska, R.; Schatzmayr, G. Multi-Mycotoxin Screening Reveals the Occurrence of 139 Different Secondary Metabolites in Feed and Feed Ingredients. Toxins 2013, 5, 504–523. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Regulation No. 32/2002 of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Union 2002. Available online: http://data.europa.eu/eli/dir/2002/32/oj (accessed on 28 November 2019).
- European Commission. Commission Recommendation No. 576 of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, L229, 7–9. [Google Scholar]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Streit, E.; Naehrer, K.; Rodrigues, I.; Schatzmayr, G. Mycotoxin occurrence in feed and feed raw materials worldwide: Long-term analysis with special focus on Europe and Asia. J. Sci. Food Agric. 2013, 93, 2892–2899. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and Methodologies for Developing Microbial Detoxification Systems to Mitigate Mycotoxins. Toxins 2017, 9, 130. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of Mycotoxin-Contaminated Feedstuffs and Compound Feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Fanelli, F.; Zucca, P.; Liuzzi, V.C.; Quintieri, L.; Cimmarusti, M.T.; Monaci, L.; Haidukowski, M.; Logrieco, A.F.; Sanjust, E.; et al. Aflatoxin B1 and M1 Degradation by Lac2 from Pleurotus pulmonarius and Redox Mediators. Toxins 2016, 8, 245. [Google Scholar] [CrossRef]
- Temba, B.; Sultanbawa, Y.; Kriticos, D.J.; Fox, G.P.; Harvey, J.J.W.; Fletcher, M.T. Tools for Defusing a Major Global Food and Feed Safety Risk: Nonbiological Postharvest Procedures to Decontaminate Mycotoxins in Foods and Feeds. J. Agric. Food Chem. 2016, 64, 8959–8972. [Google Scholar] [CrossRef] [PubMed]
- Kabak, B.; Dobson, A.D.W.; Var, I. Strategies to Prevent Mycotoxin Contamination of Food and Animal Feed: A Review. Crit. Rev. Food Sci. Nutr. 2006, 46, 593–619. [Google Scholar] [CrossRef]
- Jard, G.; Liboz, T.; Mathieu, F.; Guyonvarch, A.; Lebrihi, A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit. Contam. Part A 2011, 28, 1590–1609. [Google Scholar] [CrossRef]
- Missotten, J.; Goris, J.; Michiels, J.; Van Coillie, E.; Herman, L.; De Smet, S.; Dierick, N.; Heyndrickx, M. Screening of isolated lactic acid bacteria as potential beneficial strains for fermented liquid pig feed production. Anim. Feed. Sci. Technol. 2009, 150, 122–138. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented liquid feed—Microbial and nutritional aspects and impact on enteric diseases in pigs. Anim. Feed. Sci. Technol. 2012, 173, 17–40. [Google Scholar] [CrossRef]
- Plumed-Ferrer, C.; Kivelä, I.; Hyvönen, P.; Von Wright, A. Survival, growth and persistence under farm conditions of a Lac-tobacillus plantarum strain inoculated into liquid pig feed. J. Appl. Microbiol. 2005, 99, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Missotten, J.A.; Michiels, J.; DeGroote, J.; De Smet, S. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.S.; Jin, S.S.; Jung, S.W.; Fang, L.H.; Kim, Y.Y. Evaluation of dry feeding and liquid feeding to lactating sows under high temperature environment. J. Anim. Sci. Technol. 2016, 58, 36. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Mao, C.; Wen, H.; Chen, Z.; Lai, T.; Li, L.; Lu, W.; Wu, H. Influence of ad Libitum Feeding of Piglets with Bacillus Subtilis Fermented Liquid Feed on Gut Flora, Luminal Contents and Health. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Kantas, D.; Papatsiros, V.G.; Tassis, P.D.; Giavasis, I.; Bouki, P.; Tzika, E.D. A feed additive containing Bacillus toyonensis (Toyocerin®) protects against enteric pathogens in postweaning piglets. J. Appl. Microbiol. 2015, 118, 727–738. [Google Scholar] [CrossRef]
- Yeo, S.; Lee, S.; Park, H.; Shin, H.; Holzapfel, W.; Huh, C.S. Development of putative probiotics as feed additives: Validation in a porcine-specific gastrointestinal tract model. Appl. Microbiol. Biotechnol. 2016, 100, 10043–10054. [Google Scholar] [CrossRef]
- Rundberget, T.; Skaar, I.; Flåøyen, A. The presence of Penicillium and Penicillium mycotoxins in food wastes. Int. J. Food Microbiol. 2004, 90, 181–188. [Google Scholar] [CrossRef]
- Sultana, N.; Hanif, N.Q. Mycotoxin contamination in cattle feed and feed ingredients. Pakistan Vet. J. 2019, 29, 211–213. [Google Scholar]
- Adamse, P.; van Egmond, H.J.; Driessen, J.J.M.; de Rijk, T.C.; de Jong, J.; de Nijs, W.C.M. Trend Analysis of Mycotoxins in Animal Feed. Rikilt-Institute for Food Safety No. 2011.017. , 2012. Available online: https://edepot.wur.nl/197668 (accessed on 1 February 2012).
- Cano-Garrido, O.; Seras-Franzoso, J.; Garcia-Fruitós, E. Lactic acid bacteria: Reviewing the potential of a promising delivery live vector for biomedical purposes. Microb. Cell Factories 2015, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Siragusa, S.; Caputo, L.; Ragni, A.; Burzigotti, R.; Gobbetti, M. Survival and persistence of Lactobacillus plantarum 4.1 and Lactobacillus reuteri 3S7 in the gastrointestinal tract of pigs. Veter Microbiol. 2007, 123, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs from Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters During Food-Animal Production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef]
- Rabelo, C.H.S.; Valente, A.L.S.; Barbero, R.P.; Basso, F.C.; Reis, R.A. Performance of finishing beef cattle fed diets containing maize silages inoculated with lactic-acid bacteria and Bacillus subtilis. Anim. Prod. Sci. 2019, 59, 266. [Google Scholar] [CrossRef]
- Dalié, D.; Deschamps, A.; Richard-Forget, F. Lactic acid bacteria—Potential for control of mould growth and mycotoxins: A review. Food Control. 2010, 21, 370–380. [Google Scholar] [CrossRef]
- Ahlberg, S.H.; Joutsjoki, V.; Korhonen, H.J. Potential of lactic acid bacteria in aflatoxin risk mitigation. Int. J. Food Microbiol. 2015, 207, 87–102. [Google Scholar] [CrossRef] [PubMed]
- El-Nezami, H.; Gratz, S. Control of mycotoxin contamination in foods using lactic acid bacteria. In Protective Cultures, Antimicrobial Metabolites and Bacteriophages for Food and Beverage Biopreservation; Woodhead Publishing Series in Food Science, Technology and Nutrition; Elsevier: Amsterdam, The Netherlands, 2011; pp. 449–459. [Google Scholar]
- Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini-Rev. Med. Chem. 2014, 14, 84–98. [Google Scholar] [CrossRef]
- Chen, S.-W.; Wang, H.-T.; Shih, W.-Y.; Ciou, Y.-A.; Chang, Y.-Y.; Ananda, L.; Wang, S.-Y.; Hsu, J.-T. Application of Zearalenone (ZEN)-Detoxifying Bacillus in Animal Feed Decontamination through Fermentation. Toxins 2019, 11, 330. [Google Scholar] [CrossRef]
- Vadopalas, L.; Ruzauskas, M.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Zokaityte, E.; Bartkevics, V.; Pugajeva, I.; Reinolds, I.; Badaras, S.; et al. Combination of Antimicrobial Starters for Feed Fermentation: Influence on Piglet Feces Microbiota and Health and Growth Performance, Including Mycotoxin Biotransformation in vivo. Front. Veter. Sci. 2020, 7, 528990. [Google Scholar] [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin removal ability of Lactobacillus acidophilus cip 76.13 and L. brevis cip 102806t isolated from humans. J. Clin. Gastroent. 2020, 54, S29. [Google Scholar] [CrossRef]
- Ali-Vehmas, T.; Rizzo, A.; Westermarck, T.; Atroshi, F. Measurement of Antibacterial Activities of T-2 Toxin, Deoxynivalenol, Ochratoxin A, Aflatoxin B1 and Fumonisin B1 Using Microtitration Tray-based Turbidimetric Techniques. J. Veter-Med. Ser. A 1998, 45, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, H.R.; Hesseltine, C.W. Survey of the sensitivity of microorganisms to aflatoxin. Appl. Microbiol. 1966, 14, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Zhang, R.-Q.; Zhai, Q.-Y.; Liu, J.-C.; Li, N.; Liu, W.-X.; Li, L.; Shen, W. Metagenomic analysis of gut microbiota alteration in a mouse model exposed to mycotoxin deoxynivalenol. Toxicol. Appl. Pharmacol. 2019, 372, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic Acid Bacteria as Antifungal and Anti-Mycotoxigenic Agents: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef]
- Piotrowska, M. The Adsorption of Ochratoxin A by Lactobacillus Species. Toxins 2014, 6, 2826–2839. [Google Scholar] [CrossRef] [PubMed]
- Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Nwinyi, O.C.; Mavumengwana, V. Review on microbial degradation of aflatoxins. Crit. Rev. Food Sci. Nutr. 2017, 57, 3208–3217. [Google Scholar] [CrossRef]
- Turbic, A.; Ahokas, J.T.; Haskard, C.A. Selective in vitro binding of dietary mutagens, individually or in combination, by lactic acid bacteria. Food Addit. Contam. 2002, 19, 144–152. [Google Scholar] [CrossRef]
- Bueno, D.J.; Casale, C.H.; Pizzolitto, R.P.; Salvano, M.A.; Oliver, G. Physical Adsorption of Aflatoxin B1 by Lactic Acid Bacteria and Saccharomyces cerevisiae: A Theoretical Model. J. Food Prot. 2007, 70, 2148–2154. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-W.; Hsu, J.-T.; Chou, Y.-A.; Wang, H.-T. The application of digestive tract lactic acid bacteria with high esterase activity for zearalenone detoxification. J. Sci. Food Agric. 2018, 98, 3870–3879. [Google Scholar] [CrossRef] [PubMed]
- Chlebicz, A.; Śliżewska, K. In Vitro Detoxification of Aflatoxin B1, Deoxynivalenol, Fumonisins, T-2 Toxin and Zearalenone by Probiotic Bacteria from Genus Lactobacillus and Saccharomyces cerevisiae Yeast. Probiotics Antimicrob. Proteins 2020, 12, 289–301. [Google Scholar] [CrossRef] [PubMed]
- García, G.R.; Payros, D.; Pinton, P.; Dogi, C.A.; Laffitte, J.; Neves, M.; Pereyra, M.L.G.; Cavaglieri, L.R.; Oswald, I.P. Intestinal toxicity of deoxynivalenol is limited by Lactobacillus rhamnosus RC007 in pig jejunum explants. Arch. Toxicol. 2017, 92, 983–993. [Google Scholar] [CrossRef]
- Ben Taheur, F.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.-C.; Yi, P.-J.; Lee, T.-Y.; Liu, J.-R. Probiotic characteristics and zearalenone-removal ability of a Bacillus licheniformis strain. PLoS ONE 2018, 13, e0194866. [Google Scholar] [CrossRef]
- Wang, J.; Yang, F.; Yang, P.; Liu, J.; Lv, Z. Microbial reduction of zearalenone by a new isolated Lysinibacillus sp. ZJ-2016-1. World Mycotoxin J. 2018, 11, 571–578. [Google Scholar] [CrossRef]
- Brooks, P.H.; Beal, J.D.; Niven, S. Liquid feeding of pigs: Potential for reducing environmental impact and for improving productivity and food safety. Recent Adv. Anim. Nutr. Aust. 2001, 13, 49–63. [Google Scholar]
- Hurst, D.; Clarke, L.; Lean, I.J. Effect of liquid feeding at different water-to-feed ratios on the growth performance of growing-finishing pigs. Animal 2008, 2, 1297–1302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mokoena, M.P.; Chelule, P.K.; Gqaleni, N. Reduction of Fumonisin B1 and Zearalenone by Lactic Acid Bacteria in Fermented Maize Meal. J. Food Prot. 2005, 68, 2095–2099. [Google Scholar] [CrossRef] [PubMed]
- Chelule, P.; Mbongwa, H.; Carries, S.; Gqaleni, N. Lactic acid fermentation improves the quality of amahewu, a traditional South African maize-based porridge. Food Chem. 2010, 122, 656–661. [Google Scholar] [CrossRef]
- Niderkorn, V.; Morgavi, D.P.; Pujos, E.; Tissandier, A.; Boudra, H. Screening of fermentative bacteria for their ability to bind and biotransform deoxynivalenol, zearalenone and fumonisins in anin vitrosimulated corn silage model. Food Addit. Contam. 2007, 24, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; D’Ascanio, V.; Santovito, E.; Logrieco, A.F.; Avantaggiato, G. Comparative efficacy of agricultural by-products in sequestering mycotoxins. J. Sci. Food Agric. 2019, 99, 1623–1634. [Google Scholar] [CrossRef]
- Adunphatcharaphon, S.; Petchkongkaew, A.; Greco, D.; D’Ascanio, V.; Visessanguan, W.; Avantaggiato, G. The Effectiveness of Durian Peel as a Multi-Mycotoxin Adsorbent. Toxins 2020, 12, 108. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO) 17372:2008. Animal Feeding Stuffs-Determination of Zearalenone by Immunoaffinity Column Chromatography and High-Performance Liquid Chromatography. Available online: https://www.iso.org/standard/43605 (accessed on 1 February 2008).
| Mycotoxin Reduction (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| LAB Strain | ZEA | DON | AFB1 | OTA | ||||
| MRS | PBS | MRS | PBS | MRS | PBS | MRS | PBS | |
| VC L. acidophilus CIP: 76.13T | 28.3 ± 1.8 ay | 57.4 ± 3.4 az | 8.8 ± 1.2 ay | 30.5 ± 2.6 az | 33.5 ± 3.1 ay | 6.3 ± 2.7 az | 7.1 ± 3.8 ay | 8.3 ± 2.3 ay |
| VC L. delbrueckii subsp. bulgaricus CIP: 101027T | 28.5 ± 1.8 ay | 56.4 ± 4.2 az | 5.4 ± 0.8 aby | 30.0 ± 0.5 az | 30.8 ± 3.6 ay | 15.9 ± 1.4 bz | 15.3 ± 2.5 by | 4.0 ± 1.2 az |
| HIC L. acidophilus CIP: 76.13T | 11.9 ± 1.8 by | 12.8 ± 4.3 by | 3.1 ± 0.8 by | 14.1 ± 1.1 bz | 27.4 ± 1.9 aby | 11.9 ± 2.4 bz | 2.2 ± 2.5 cy | 13.2 ± 1.0 bz |
| HIC L. delbrueckii subsp. bulgaricus CIP: 101027T | 10.7 ± 1.8 by | 11.7 ± 3.8 by | 6.8 ± 2.4 aby | 19.4 ± 4.8 bz | 22.8 ± 0.5 by | 14.3 ± 2.4 bz | 1.1 ± 0.9 cy | 22.5 ± 3.4 cz |
| LAB Strain | ZEA Desorbed (%) | |
|---|---|---|
| VC | HIC | |
| L. acidophilus CIP: 76.13T | 1.9 ± 0.5 a | 55.5 ± 2.2 b |
| L. delbrueckii subsp. bulgaricus CIP: 101027T | 1.9 ± 0.2 a | 82.9 ± 5.4 c |
| Spiking Level (µg/mL) | Recovery % (RSDr, %) |
|---|---|
| 0.1 | 81 (7) |
| 1.0 | 92 (6) |
| LAB Strain | ZEA Reduction (%) | Log cfu/mL | |||
|---|---|---|---|---|---|
| 24 h | 48 h | 0 h | 24 h | 48 h | |
| L. acidophilus CIP: 76.13T | 14 ± 2 a | 22 ± 2 b | 5.6 ± 0.1 a | 7.5 ± 0.1 b | 5.6 ± 0.8 a |
| L. delbrueckii subsp. bulgaricus CIP: 101027T | 10 ± 2 a | 23 ± 2 b | 5.8 ± 0.1 a | 9.5 ± 0.6 c | 8.9 ± 1.1 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed. Toxins 2021, 13, 185. https://doi.org/10.3390/toxins13030185
Ragoubi C, Quintieri L, Greco D, Mehrez A, Maatouk I, D’Ascanio V, Landoulsi A, Avantaggiato G. Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed. Toxins. 2021; 13(3):185. https://doi.org/10.3390/toxins13030185
Chicago/Turabian StyleRagoubi, Chaima, Laura Quintieri, Donato Greco, Amel Mehrez, Imed Maatouk, Vito D’Ascanio, Ahmed Landoulsi, and Giuseppina Avantaggiato. 2021. "Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed" Toxins 13, no. 3: 185. https://doi.org/10.3390/toxins13030185
APA StyleRagoubi, C., Quintieri, L., Greco, D., Mehrez, A., Maatouk, I., D’Ascanio, V., Landoulsi, A., & Avantaggiato, G. (2021). Mycotoxin Removal by Lactobacillus spp. and Their Application in Animal Liquid Feed. Toxins, 13(3), 185. https://doi.org/10.3390/toxins13030185






