Zearalenone Exposure Triggered Cecal Physical Barrier Injury through the TGF-β1/Smads Signaling Pathway in Weaned Piglets
Abstract
:1. Introduction
2. Results
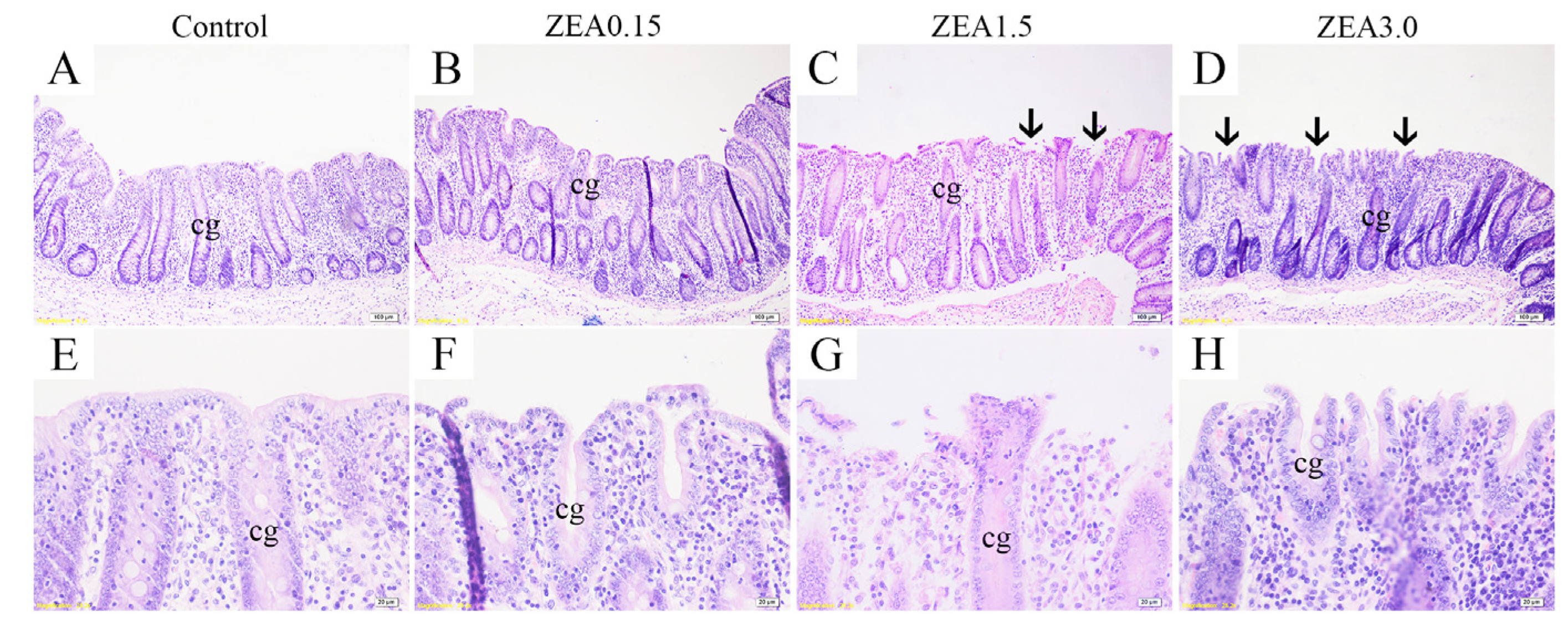
2.1. Effect of ZEA Exposure on Cecal Histomorphology in Pigs
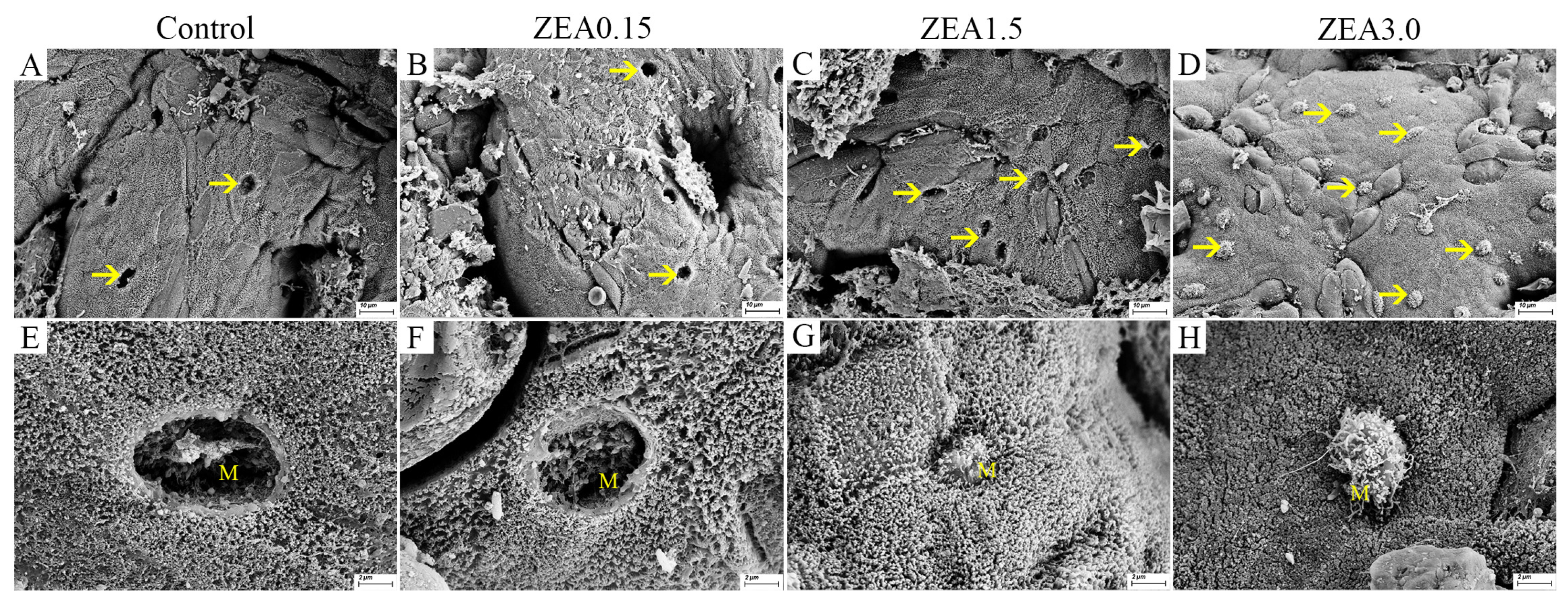
2.2. Effect of ZEA Exposure on Cecal Ultrastructure in Pigs
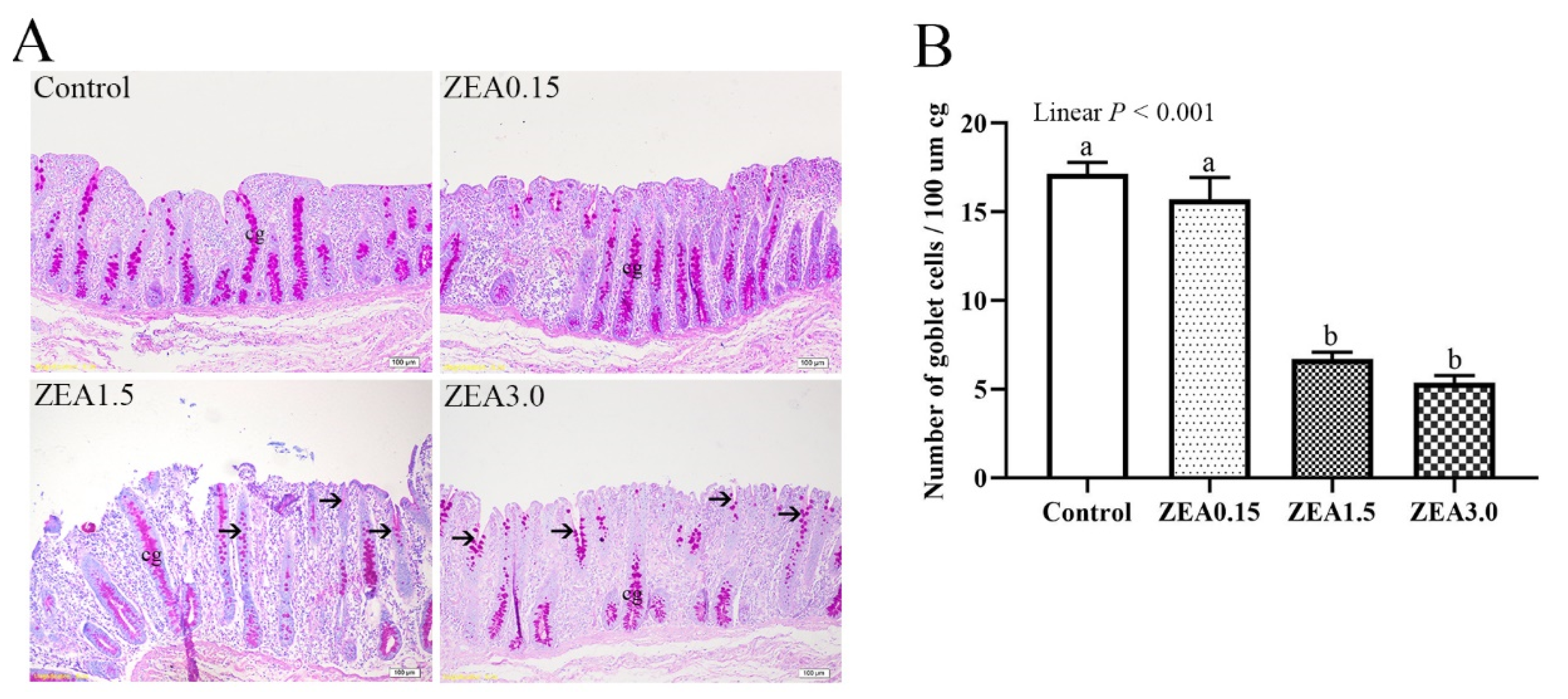
2.3. Effect of ZEA Exposure on Distribution and Number of Goblet Cells in the Cecum of Pigs
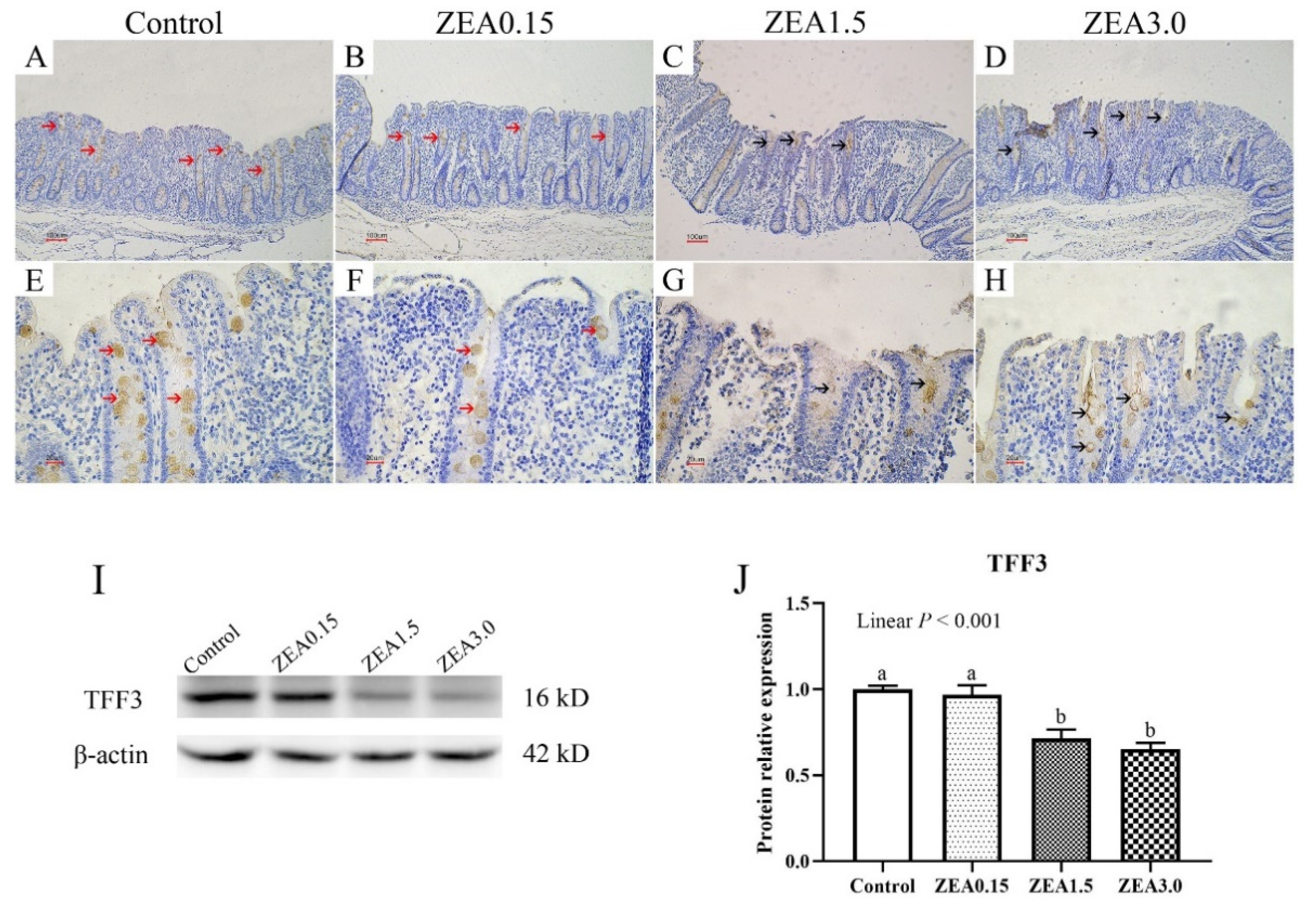
2.4. Effect of ZEA Exposure on Distribution and Expression of TFF3 in the Cecum of Pigs
2.5. Effect of ZEA on Expressions of Tight Junction Proteins in the Cecum of Pigs
2.6. Effect of ZEA on the TGF-β1/Smads Signaling Pathway in Cecum of Pigs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethical Approval
5.2. Experimental Design and Animal Management
5.3. Mycotoxins Determination in Diet
5.4. Sample Collection
5.5. Cecal Histology Examination
5.6. Ultrastructural Examination
5.7. Identification and Examination of Goblet Cells
5.8. Immunohistochemistry Analysis
5.9. Gene Expression
5.10. Western Blot Analysis
5.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, R.; Crisci, A.; Venâncio, A.; Cortiñas Abrahantes, J.; Dorne, J.-L.; Battilani, P.; Toscano, P. Occurrence and co-occurrence of mycotoxins in cereal-based feed and food. Microorganisms 2020, 8, 74. [Google Scholar] [CrossRef] [Green Version]
- Zinedine, A.; Soriano, J.M.; Molto, J.C.; Manes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Kriszt, R.; Krifaton, C.; Szoboszlay, S.; Cserháti, M.; Kriszt, B.; Kukolya, J.; Czéh, Á.; Fehér-Tóth, S.; Török, L.; Szőke, Z. A new zearalenone biodegradation strategy using non-pathogenic Rhodococcus pyridinivorans K408 strain. PLoS ONE 2012, 7, e43608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Zhang, S.; Wang, J.; Shan, A.; Xu, L. Changes in intestinal barrier functions and gut microbiota in rats exposed to zearalenone. Ecotoxicol. Environ. Saf. 2020, 204, 111072. [Google Scholar] [CrossRef]
- Yang, L.-J.; Zhou, M.; Huang, L.B.; Yang, W.-R.; Yang, Z.-B.; Jiang, S.-Z.; Ge, J.-S. Zearalenone-promoted follicle growth through modulation of Wnt-1/β-catenin signaling pathway and expression of estrogen receptor genes in ovaries of postweaning piglets. J. Agric. Food Chem. 2018, 66, 7899–7906. [Google Scholar] [CrossRef]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef] [Green Version]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Videmann, B.; Mazallon, M.; Tep, J.; Lecoeur, S. Metabolism and transfer of the mycotoxin zearalenone in human intestinal Caco-2 cells. Food Chem. Toxicol. 2008, 46, 3279–3286. [Google Scholar] [CrossRef] [PubMed]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Tan, X.; Wang, H.; Wang, Q.; Huang, P.; Li, Y.; Li, J.; Huang, J.; Yang, H.; Yin, Y. Changes in cecal morphology, cell proliferation, antioxidant enzyme, volatile fatty acids, lipopolysaccharide, and cytokines in piglets during the postweaning period. J. Anim. Sci. 2020, 98, skaa046. [Google Scholar] [CrossRef]
- Williams, B.A.; Verstegen, M.W.; Tamminga, S. Fermentation in the large intestine of single-stomached animals and its relationship to animal health. Nutr. Res. Rev. 2001, 14, 207–228. [Google Scholar] [CrossRef]
- Heo, J.M.; Opapeju, F.; Pluske, J.; Kim, J.; Hampson, D.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Hanuszewska, M.; Petrusewicz-Kosinska, M.; Gajecka, M.; Zielonka, L.; Gajecki, M. The effects of deoxynivalenol and zearalenone on the pig large intestine. A light and electron microscopy study. Toxins 2018, 10, 148. [Google Scholar] [CrossRef] [Green Version]
- Dignass, A.U. Mechanisms and modulation of intestinal epithelial repair. Inflamm. Bowel Dis. 2001, 7, 68–77. [Google Scholar] [CrossRef]
- Barnard, J.A.; Beauchamp, R.D.; Coffey, R.J.; Moses, H.L. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc. Natl. Acad. Sci. USA 1989, 86, 1578–1582. [Google Scholar] [CrossRef] [Green Version]
- Roche, J.K.; Martins, C.A.; Cosme, R.; Fayer, R.; Guerrant, R.L. Transforming growth factor β1 ameliorates intestinal epithelial barrier disruption by Cryptosporidium parvum in vitro in the absence of mucosal T lymphocytes. Infect. Immun. 2000, 68, 5635–5644. [Google Scholar] [CrossRef] [Green Version]
- Howe, K.L.; Reardon, C.; Wang, A.; Nazli, A.; McKay, D.M. Transforming growth factor-β regulation of epithelial tight junction proteins enhances barrier function and blocks enterohemorrhagic Escherichia coli O157: H7-induced increased permeability. Am. J. Pathol. 2005, 167, 1587–1597. [Google Scholar] [CrossRef]
- Hering, N.A.; Andres, S.; Fromm, A.; van Tol, E.A.; Amasheh, M.; Mankertz, J.; Fromm, M.; Schulzke, J.D. Transforming growth factor-β, a whey protein component, strengthens the intestinal barrier by upregulating claudin-4 in HT-29/B6 cells. J. Nutr. 2011, 141, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Mashima, H.; Sakai, T.; Matsuhashi, T.; Jin, M.; Ohnishi, H. Functional roles of TGF-β 1 in intestinal epithelial cells through Smad-dependent and non-Smad pathways. Dig. Dis. Sci. 2013, 58, 1207–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehra, A.; Wrana, J.L. TGF-β and the Smad signal transduction pathway. Biochem. Cell Biol. 2002, 80, 605–622. [Google Scholar] [CrossRef]
- Dai, X.; Wang, B. Role of gut barrier function in the pathogenesis of nonalcoholic fatty liver disease. Gastroent. Res. Pract. 2015, 2015, 287348. [Google Scholar] [CrossRef] [Green Version]
- Regoli, M.; Bertelli, E.; Borghesi, C.; Nicoletti, C. Three-dimensional (3D-) reconstruction of M cells in rabbit peyer’s patches: Definition of the intraepithelial compartment of the follicle-associated epithelium. Anat. Rec. 1995, 243, 19–26. [Google Scholar] [CrossRef]
- Williams, I.R.; Owen, R.L. M cells: Specialized antigen sampling cells in the follicle-associated epithelium. In Mucosal Immunology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 211–229. [Google Scholar]
- Ma, T.Y.; Anderson, J.M.; Turner, J.R. Tight junctions and the intestinal barrier. In Physiology of the Gastrointestinal Tract; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1043–1088. [Google Scholar]
- Ren, Z.; Guo, C.; Yu, S.; Zhu, L.; Wang, Y.; Hu, H.; Deng, J. Progress in mycotoxins affecting intestinal mucosal barrier function. Int. J. Mol. Sci. 2019, 20, 2777. [Google Scholar] [CrossRef] [Green Version]
- Groh, K.J.; Geueke, B.; Muncke, J. Food contact materials and gut health: Implications for toxicity assessment and relevance of high molecular weight migrants. Food Chem. Toxicol. 2017, 109, 1–18. [Google Scholar] [CrossRef]
- Natividad, J.M.; Verdu, E.F. Modulation of intestinal barrier by intestinal microbiota: Pathological and therapeutic implications. Pharmacol. Res. 2013, 69, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ho, S.B. Intestinal goblet cells and mucins in health and disease: Recent insights and progress. Curr. Gastroenterol. Rep. 2010, 12, 319–330. [Google Scholar] [CrossRef] [Green Version]
- Maloy, K.J.; Powrie, F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature 2011, 474, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Taupin, D.; Podolsky, D.K. Trefoil factors: Initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 2003, 4, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Kjellev, S. The trefoil factor family—Small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009, 66, 1350–1369. [Google Scholar] [CrossRef]
- Xiao, K.; Song, Z.-H.; Jiao, L.-F.; Ke, Y.-L.; Hu, C.-H. Developmental changes of TGF-β1 and smads signaling pathway in intestinal adaption of weaned pigs. PLoS ONE 2014, 9, e104589. [Google Scholar] [CrossRef]
- Van’t Land, B.; Meijer, H.; Frerichs, J.; Koetsier, M.; Jager, D.; Smeets, R.; M’Rabet, L.; Hoijer, M. Transforming growth factor-β2 protects the small intestine during methotrexate treatment in rats possibly by reducing stem cell cycling. Br. J. Cancer 2002, 87, 113–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, K.; Cao, S.; Jiao, L.; Song, Z.; Lu, J.; Hu, C. TGF-β1 protects intestinal integrity and influences Smads and MAPK signal pathways in IPEC-J2 after TNF-α challenge. Innate Immun. 2017, 23, 276–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Z.H.; Xiao, K.; Ke, Y.L.; Jiao, L.F.; Hu, C.H. Zinc oxide influences mitogen-activated protein kinase and TGF-β1 signaling pathways, and enhances intestinal barrier integrity in weaned pigs. Innate Immun. 2015, 21, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H. Fine tuning and cross-talking of TGF-β signal by inhibitory Smads. BMB Rep. 2005, 38, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lonn, P.; Moren, A.; Raja, E.; Dahl, M.; Moustakas, A. Regulating the stability of TGFβ receptors and Smads. Cell Res. 2009, 19, 21–35. [Google Scholar] [CrossRef]
- Heldin, C.-H.; Moustakas, A. Role of Smads in TGFβ signaling. Cell Tissue Res. 2012, 347, 21–36. [Google Scholar] [CrossRef]
- Halttunen, T.; Marttinen, A.; Rantala, I.; Kainulainen, H.; Maki, M. Fibroblasts and transforming growth factor beta induce organization and differentiation of T84 human epithelial cells. Gastroenterology 1996, 111, 1252–1262. [Google Scholar] [CrossRef]
- Jiang, S.; Yang, Z.; Yang, W.; Gao, J.; Liu, F.; Broomhead, J.; Chi, F. Effects of purified zearalenone on growth performance, organ size, serum metabolites, and oxidative stress in postweaning gilts. J. Anim. Sci. 2011, 89, 3008–3015. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Yang, C.; Huang, L.; Niu, Q.; Jiang, S.; Chi, F. Zearalenone altered the serum hormones, morphologic and apoptotic measurements of genital organs in post-weaning gilts. Asian Australas. J. Anim. Sci. 2015, 28, 171. [Google Scholar] [CrossRef] [Green Version]
- Dai, M.; Jiang, S.; Yuan, X.; Yang, W.; Yang, Z.; Huang, L. Effects of zearalenone-diet on expression of ghrelin and PCNA genes in ovaries of post-weaning piglets. Anim. Reprod. Sci. 2016, 168, 126–137. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, L.; Shao, M.; Wang, Y.; Yang, W.; Huang, L.; Zhou, X.; Jiang, S.; Yang, Z. Effects of zearalenone exposure on the TGF-β1/Smad3 signaling pathway and the expression of proliferation or apoptosis related genes of post-weaning gilts. Toxins 2018, 10, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Xu, C.; Yang, Z.; Yang, W.; Huang, L.; Wang, S.; Liu, F.; Liu, M.; Wang, Y.; Jiang, S. Effects of dietary zearalenone exposure on the growth performance, small intestine disaccharidase, and antioxidant activities of weaned gilts. Animals 2020, 10, 2157. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Yuan, X.; Yang, W.; Jiao, N.; Li, Y.; Liu, F.; Liu, M.; Yang, Z.; Huang, L.; Jiang, S. The Effects of Zearalenone on the Localization and Expression of Reproductive Hormones in the Ovaries of Weaned Gilts. Toxins 2021, 13, 626. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Jiang, S.; Huang, L.; Ge, J.; Wang, Y.; Yang, W. Zearalenone induced oxidative stress in the jejunum in postweaning gilts through modulation of the Keap1–Nrf2 signaling pathway and relevant genes. J. Anim. Sci. 2019, 97, 1722–1733. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Jing, C.; Liang, M.; Jiang, S.; Huang, L.; Jiao, N.; Li, Y.; Yang, W. Zearalenone Exposure Triggered Cecal Physical Barrier Injury through the TGF-β1/Smads Signaling Pathway in Weaned Piglets. Toxins 2021, 13, 902. https://doi.org/10.3390/toxins13120902
Zhang P, Jing C, Liang M, Jiang S, Huang L, Jiao N, Li Y, Yang W. Zearalenone Exposure Triggered Cecal Physical Barrier Injury through the TGF-β1/Smads Signaling Pathway in Weaned Piglets. Toxins. 2021; 13(12):902. https://doi.org/10.3390/toxins13120902
Chicago/Turabian StyleZhang, Pengfei, Changwei Jing, Ming Liang, Shuzhen Jiang, Libo Huang, Ning Jiao, Yang Li, and Weiren Yang. 2021. "Zearalenone Exposure Triggered Cecal Physical Barrier Injury through the TGF-β1/Smads Signaling Pathway in Weaned Piglets" Toxins 13, no. 12: 902. https://doi.org/10.3390/toxins13120902
APA StyleZhang, P., Jing, C., Liang, M., Jiang, S., Huang, L., Jiao, N., Li, Y., & Yang, W. (2021). Zearalenone Exposure Triggered Cecal Physical Barrier Injury through the TGF-β1/Smads Signaling Pathway in Weaned Piglets. Toxins, 13(12), 902. https://doi.org/10.3390/toxins13120902




