Natural Occurrence of Alternaria Fungi and Associated Mycotoxins in Small-Grain Cereals from The Urals and West Siberia Regions of Russia
Abstract
:1. Introduction
2. Results
2.1. The Approbation of The qPCR Protocol
2.2. Content of Alternaria DNA in the Grain Samples
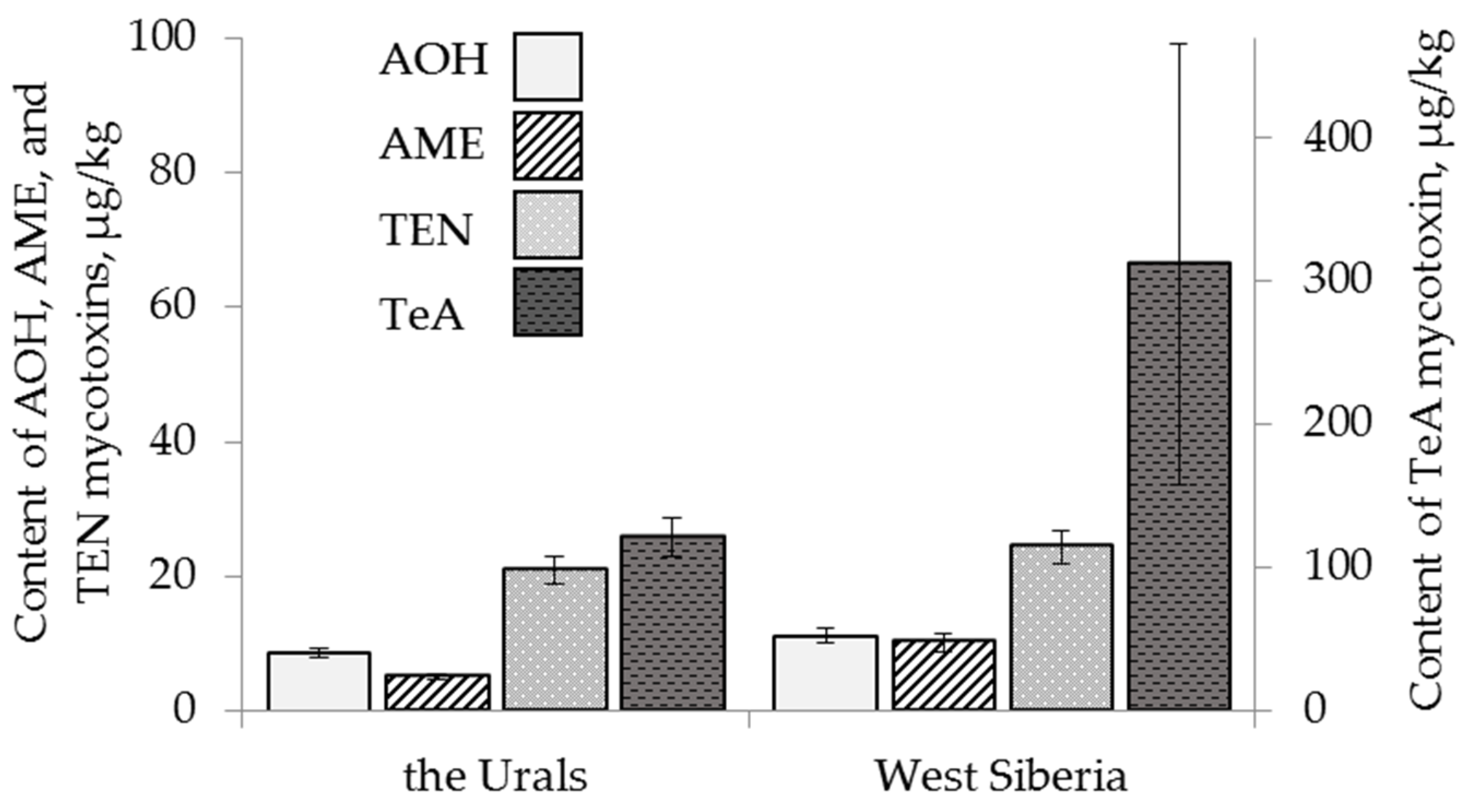
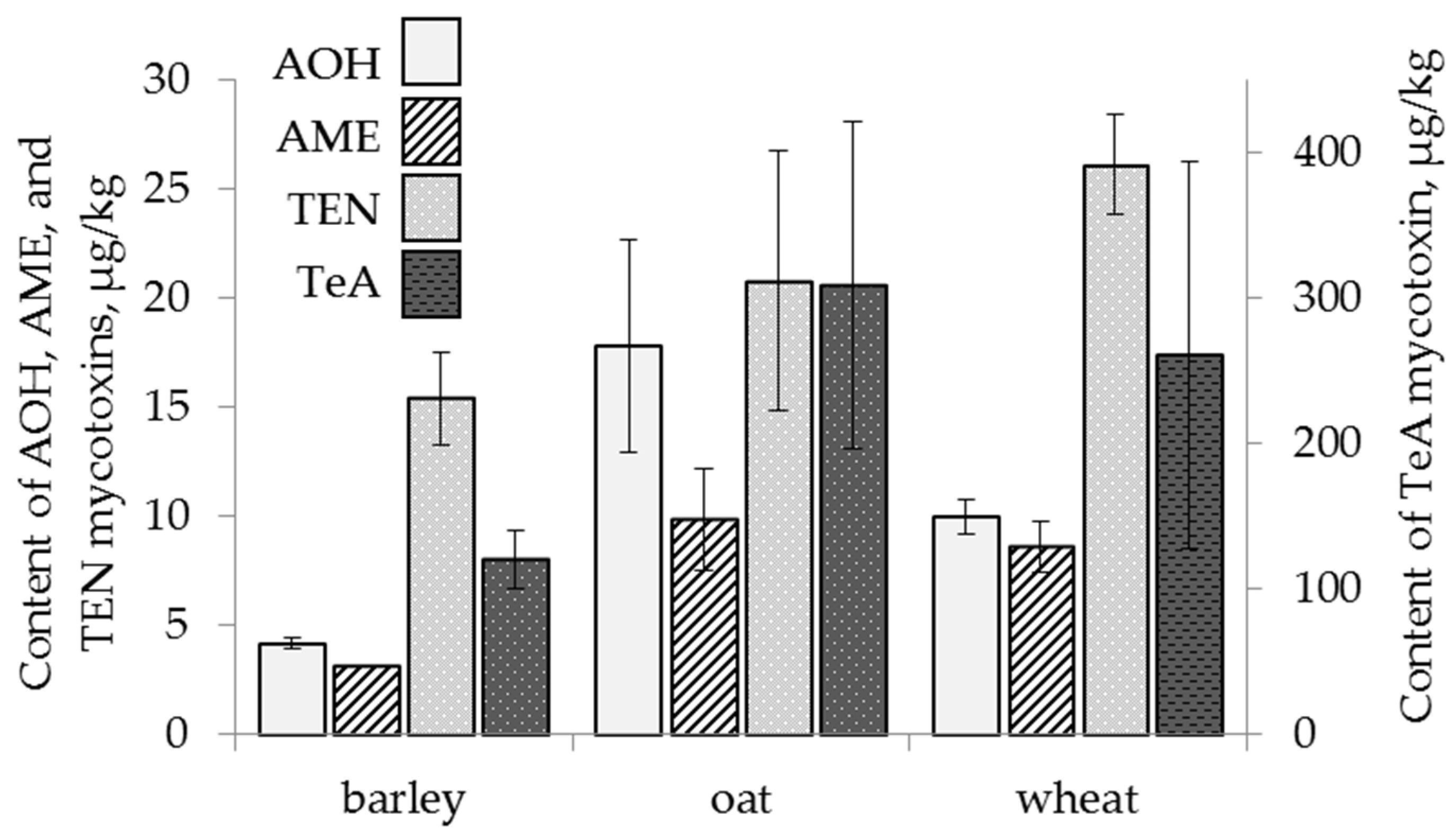
2.3. Content of Alternaria Mycotoxins in the Grain Samples
2.4. Factors Affecting Grain Contamination
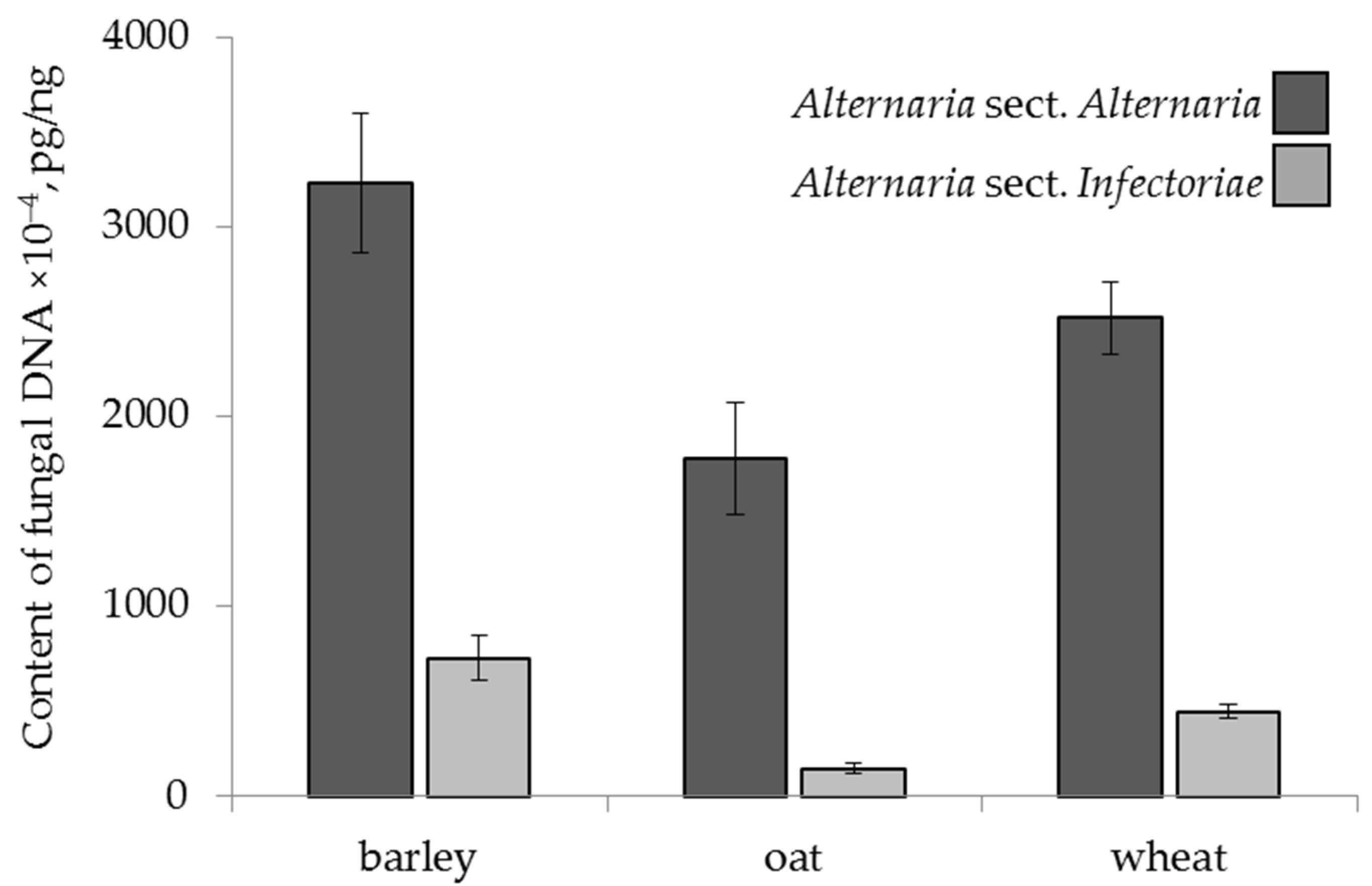
2.4.1. The Effect of Cereal Crop Species
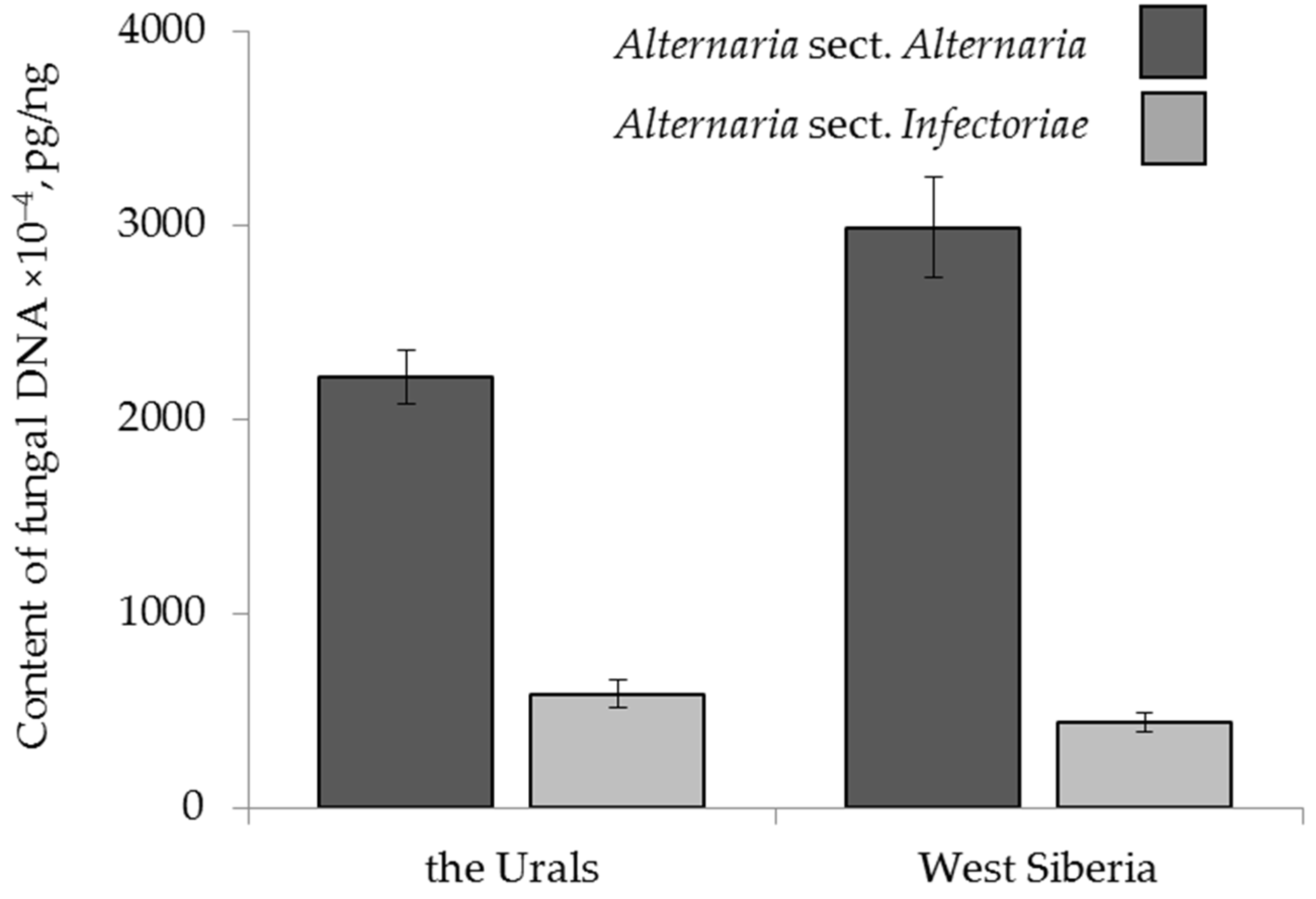
2.4.2. The Effect of Geographical Origin
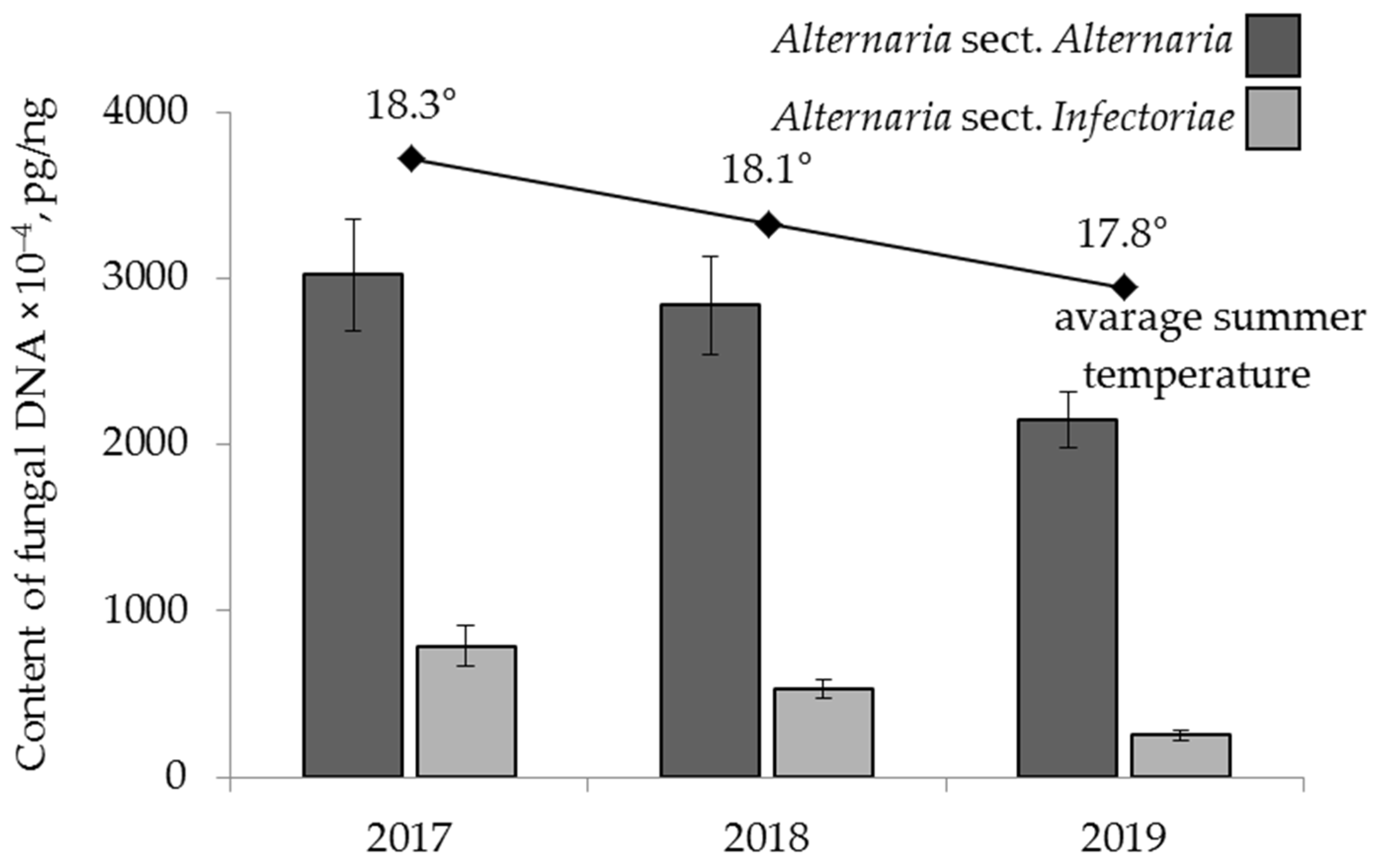
2.4.3. The Effect of Year
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Grain Samples and Weather Conditions of Vegetation Seasons in the Analysed Regions
5.2. Grinding Samples
5.3. DNA Extraction and Concentration Measurement
5.4. Detection of Fungal DNA Content in Grain Using qPCR
5.5. Analysis of Secondary Metabolites of Fungi by HPLC-MS/MS
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Thomma, B.P.H.J. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant. Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Polizzotto, R.; Andersen, B.; Martini, M.; Grisan, S.; Assante, G.; Musetti, R. A polyphasic approach for the characterization of endophytic Alternaria strains isolated from grapevines. J. Microbiol. Methods 2012, 88, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Nava, A.; Cooke, P.; Cook, D.; Creamer, R. Evidence of non-pathogenic relationship of Alternaria section Undifilum endophytes within three host locoweed plants species. Botany 2018, 96, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Patriarca, A. Alternaria in food products. Curr. Opin. Food Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1569748. [Google Scholar] [CrossRef] [Green Version]
- Kulik, T.; Treder, K.; Załuski, D. Quantification of Alternaria, Cladosporium, Fusarium and Penicillium verrucosum in conventional and organic grains by qPCR. J. Phytopathol. 2015, 163, 522–528. [Google Scholar] [CrossRef]
- Barkat, E.; Hardy, G.; Ren, Y.; Calver, M.; Bayliss, K. Fungal contaminants of stored wheat vary between Australian states. Australas. Plant. Pathol. 2016, 45, 621–628. [Google Scholar] [CrossRef] [Green Version]
- Gannibal, P.B. Factors affecting Alternaria appearance in grains in European Russia. Sel’skokhozyaistvennaya Biol. 2018, 53, 605–615. [Google Scholar] [CrossRef]
- Gashgari, R.; Ameen, F.; Al-Homaidi, E.; Gherbawy, Y.; Al Nadhari, S.; Vijayan, V. Mycotoxigenic fungi contaminating wheat; toxicity of different Alternaria compacta strains. Saudi J. Biol. Sci. 2019, 26, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Somma, S.; Amatulli, M.T.; Masiello, M.; Moretti, A.; Logrieco, A.F. Alternaria species associated to wheat black point identified through a multilocus sequence approach. Int. J. Food Microbiol. 2019, 293, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Puvača, N.; Bursic, V.; Gorica, V.; Budakov, D.; Petrovic, A.; Merkuri, J.; Avantaggiato, G.; Cara, M. Ascomycete fungi (Alternaria spp.) characterization as major feed grains pathogens. J. Agron. Technol. Eng. Manag. 2020, 3, 499–505. [Google Scholar]
- Orina, A.S.; Gavrilova, O.P.; Gagkaeva, T.Y.; Gannibal, P.B. Micromycetes Alternaria spp. and Bipolaris sorokiniana and mycotoxins in the grain from the Ural region. Mikol. Fitopatol. 2020, 54, 365–377. (In Russian) [Google Scholar] [CrossRef]
- Kwaśna, H.; Kosiak, B. Lewia avenicola sp. nov. and its Alternaria anamorph from oat grain, with a key to the species of Lewia. Mycol. Res. 2003, 107, 371–376. [Google Scholar] [CrossRef]
- Kwaśna, H.; Ward, E.; Kosiak, B. Lewia hordeicola sp. nov. from barley grain. Mycologia 2006, 98, 662–668. [Google Scholar] [CrossRef]
- Poursafar, A.; Ghosta, Y.; Orina, A.S.; Gannibal, P.B.; Javan-Nikkhah, M.; Lawrence, D.P. Taxonomic study on Alternaria sections Infectoriae and Pseudoalternaria associated with black (sooty) head mold of wheat and barley in Iran. Mycol. Prog. 2018, 17, 343–356. [Google Scholar] [CrossRef]
- Kumar, A.; Karre, S.; Dhokane, D.; Kage, U.; Hukkeri, S.; Kushalappa, A.C. Real-time quantitative PCR based method for the quantification of fungal biomass to discriminate quantitative resistance in barley and wheat genotypes to Fusarium head blight. J. Cereal Sci. 2015, 64, 16–22. [Google Scholar] [CrossRef]
- Kulik, T.; Bilska, K.; Żelechowski, M. Promising perspectives for detection, identification, and quantification of plant pathogenic fungi and oomycetes through targeting mitochondrial DNA. Int. J. Mol. Sci. 2020, 21, 2645. [Google Scholar] [CrossRef] [Green Version]
- Alexander, J.; Benford, D.; Boobis, A.; Ceccatelli, S.; Cottrill, B.; Cravedi, J.-P.; Di Domenico, A.; Doerge, D.; Dogliotti, E.; Edler, L.; et al. Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 2407–2504. [Google Scholar] [CrossRef]
- Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary exposure assessment to Alternaria toxins in the European population. EFSA J. 2016, 14, 4654. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F.; Zhang, L. Natural occurrence of Alternaria toxins in the 2015 wheat from Anhui province, China. Toxins 2016, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef] [Green Version]
- Topi, D.; Tavcar-Kalcher, G.; Pavšič-Vrtač, K.; Babič, J.; Jakovac-Strajn, B. Alternaria mycotoxins in grains from Albania: Alternariol, alternariol monomethyl ether, tenuazonic acid and tentoxin. World Mycotoxin J. 2018, 12, 1–12. [Google Scholar] [CrossRef]
- Castañares, E.; Pavicich, M.; Dinolfo, M.; Moreyra, F.; Stenglein, S.; Patriarca, A. Natural occurrence of Alternaria mycotoxins in malting barley grains in the main producing region of Argentina. J. Sci. Food Agri. 2020, 100, 1004–1011. [Google Scholar] [CrossRef]
- Babič, J.; Tavčar-Kalcher, G.; Celar, F.A.; Kos, K.; Knific, T.; Jakovac-Strajn, B. Occurrence of Alternaria and other toxins in cereal grains intended for animal feeding collected in Slovenia: A three-year study. Toxins 2021, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites from Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Rychlik, M.; Lepper, H.; Weidner, C.; Asam, S. Risk Evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 2016, 68, 181–185. [Google Scholar] [CrossRef]
- Food Chemistry Institute of the Association of the German Confectionery Industry. Alternaria Toxins: Occurrence, Toxicity, Analytical Methods, Maximum Levels. 2020. Available online: https://www.lci-koeln.de/deutsch/veroeffentlichungen/lci-focus/alternaria-toxins-occurrence-toxicity-analytical-methods-maximum-levels (accessed on 17 August 2021).
- Tralamazza, S.M.; Piacentini, K.C.; Iwase, C.H.T.; de Oliveira Rocha, L. Toxigenic Alternaria species: Impact in cereals worldwide. Curr. Opin. Food Sci. 2018, 23, 57. [Google Scholar] [CrossRef]
- Andersen, B.; Krøger, E.; Roberts, R.G. Chemical and morphological segregation of Alternaria arborescens, A. infectoria and A. tenuissima species-groups. Mycol. Res. 2002, 106, 170–182. [Google Scholar] [CrossRef]
- Zwickel, T.; Kahl, S.M.; Rychlik, M.; Müller, M.E.H. Chemotaxonomy of mycotoxigenic small-spored Alternaria fungi—Do multitoxin mixtures act as an indicator for species differentiation? Front. Microbiol. 2018, 9, 1368. [Google Scholar] [CrossRef] [Green Version]
- Masiello, M.; Somma, S.; Susca, A.; Ghionna, V.; Logrieco, A.F.; Franzoni, M.; Ravaglia, S.; Meca, G.; Moretti, A. Molecular identification and mycotoxin production by Alternaria species occurring on durum wheat, showing black point symptoms. Toxins 2020, 12, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Wei, D.; Li, H.; Wang, L.; Jiang, N.; Li, Y.; Wang, M. Natural occurrence of Alternaria mycotoxins in wheat and potential of reducing associated risks using magnolol. J. Sci. Food Agric. 2021, 101, 3071–3077. [Google Scholar] [CrossRef] [PubMed]
- Kononenko, G.P.; Burkin, A.A.; Zotova, E.V. Mycotoxilogical monitoring. Part 2. Wheat, barley, oat and maize grain. Vet. Segodnya 2020, 2, 139–145. [Google Scholar] [CrossRef]
- Kiseleva, M.G.; Sedova, I.B.; Chalyy, Z.A.; Zakharova, L.P.; Aristarkhova, T.V.; Tutelyan, V.A. Multi-mycotoxin screening of food grain produced in Russia in 2018. Sel’skokhozyaistvennaya Biol. 2021, 56, 559–577. [Google Scholar] [CrossRef]
- Federal State Statistics Service. Bulletins on the State of Agriculture (Electronic Versions). Available online: https://www.gks.ru/compendium/document/13277 (accessed on 17 August 2021). (In Russian).
- Toropova, E.Y.; Kirichenko, A.A.; Kazakova, O.A.; Porsev, I.N. Alternaria disease of grain of spring wheat and barley in Western Siberia and Eastern Trans-Urals. Zashchita I Karantin Rasteniy 2015, 1, 20–22. (In Russian) [Google Scholar]
- Turzhanova, A.; Khapilina, O.N.; Tumenbayeva, A.; Shevtsov, V.; Raiser, O.; Kalendar, R. Genetic diversity of Alternaria species associated with black point in wheat grains. Peer J. 2020, 5, e9097. [Google Scholar] [CrossRef]
- Gannibal, P.B. Alternaria disease of grain—modern view of the problem. Zashchita I Karantin Rasteniy 2014, 6, 11–15. (In Russian) [Google Scholar]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef] [Green Version]
- Woudenberg, J.H.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstantinova, P.; Bonants, P.J.M.; van Gent-Pelzer, M.P.E.; van der Zouwen, P.; van den Bulk, R. Development of specific primers for detection and identification of Alternaria spp. in carrot material by PCR and comparison with blotter and plating assays. Mycol. Res. 2002, 106, 23–33. [Google Scholar] [CrossRef]
- Gannibal, P.B.; Yli-Mattila, T. Morphological and UP-PCR analyses and design of a PCR assay for differentiation of Alternaria infectoria species-group. Mikol. I Fitopatol. 2007, 41, 313–322. [Google Scholar]
- Andersen, B.; Thrane, U. Differentiation of Alternaria infectoria and Alternaria alternata based on morphology, metabolite profiles, and cultural characteristics. Can. J. Microbiol. 1996, 42, 685–689. [Google Scholar] [CrossRef]
- Kosiak, B.; Torp, M.; Skjerve, E.; Andersen, B. Alternaria and Fusarium in Norwegian grains of reduced quality—a matched pair sample study. Int. J. Food Microbiol. 2004, 93, 51–62. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.B.; Peever, T.L.; Pryor, B.M. The sections of Alternaria: Formalizing species-group concepts. Mycologia 2013, 105, 530–546. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.T.; Kim, J.; Jeon, S.J.; Lee, C.W.; Magan, N.; Lee, H.B. Mycotoxin production of Alternaria strains isolated from Korean barley grains determined by LC-MS/MS. Int. J. Food Microbiol. 2018, 268, 44–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, P.M.; Zhao, W.; Feng, S.; Lau, B.P. Alternaria toxins aternariol and alternariol monomethyl ether in grain foods in Canada. Mycotoxin Res. 2012, 28, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crudo, F.; Varga, E.; Aichinger, G.; Galaverna, G.; Marko, D.; Dall’Asta, C.; Dellafiora, L. Co-occurrence and combinatory effects of Alternaria mycotoxins and other xenobiotics of food origin: Current scenario and future perspectives. Toxins 2019, 11, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azcarate, M.P.; Patriarca, A.; Terminiello, L.; Fernández, P.V. Alternaria toxins in wheat during the 2004 to 2005 Argentinean harvest. J. Food Prot. 2008, 71, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.E.; Korn, U. Alternaria mycotoxins in wheat—A 10 years survey in the northeast of Germany. Food Control 2013, 34, 191–197. [Google Scholar] [CrossRef]
- Li, F.; Yoshizawa, T. Alternaria mycotoxins in weathered wheat from China. J. Agric. Food Chem. 2000, 48, 2920–2924. [Google Scholar] [CrossRef] [PubMed]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Shao, B.; Yang, D.; Li, F.; Zhu, J. Natural occurrence of Alternaria toxins in wheat based products and their dietary exposure in China. PLoS ONE 2015, 10, e0132019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Logrieco, A.; Bottalico, A.; Solfrizzo, M.; Mule, G. Incidence of Alternaria species in grains from Mediterranean countries and their ability to produce mycotoxins. Mycologia 1990, 82, 501–505. [Google Scholar] [CrossRef]
- Reinholds, I.; Jansons, M.; Fedorenko, D.; Pugajeva, I.; Zute, S.; Bartkiene, E.; Bartkevics, V. Mycotoxins in cereals and pulses harvested in Latvia by nanoLC-Orbitrap MS. Food Addit. Contam. Part B 2021, 14, 115–123. [Google Scholar] [CrossRef]
- Zwickel, T.; Kahl, S.M.; Klaffke, H.; Rychlik, M.; Müller, M.E. Spotlight on the underdogs—An analysis of underrepresented Alternaria mycotoxins formed depending on varying substrate, time and temperature conditions. Toxins 2016, 8, 344. [Google Scholar] [CrossRef] [Green Version]
- Janić Hajnal, E.; Orčić, D.; Torbica, A.; Kos, J.; Mastilović, J.; Škrinjar, M. Alternaria toxins in wheat from the autonomous province of Vojvodina, Serbia: A preliminary survey. Food Addit. Contam. Part A 2015, 32, 361–370. [Google Scholar] [CrossRef]
- Schiro, G.; Verch, G.; Grimm, V.; Müller, M.E.H. Alternaria and Fusarium fungi: Differences in distribution and spore deposition in a topographically heterogeneous wheat field. J. Fungi 2018, 4, 63. [Google Scholar] [CrossRef] [Green Version]
- Malachová, A.; Sulyok, M.; Beltrán, E.; Berthiller, F.; Krska, R. Optimization and validation of a quantitative liquid chromatography-tandem mass spectrometric method covering 295 bacterial and fungal metabolites including all regulated mycotoxins in four model food matrices. J. Chromatogr. A 2014, 1362, 145–156. [Google Scholar] [CrossRef] [Green Version]
| Strain ID | Fungi | Results of qPCR with Primer Pairs (Ct 1) | |
|---|---|---|---|
| AAF2/AAR3 | AinfF3/AinfR4 | ||
| MFP556081 | A. tenuissima (sect. Alternaria) | 10.9 | 33.7 |
| MFP028011 | Alternaria sp. (sect. Alternaria) | 11.5 | 34.4 |
| MFP094121 | Alternaria sp. (sect. Pseudoalternaria) | 34.6 | 35.5 |
| MFP457051 | Alternaria sp. (sect. Pseudoalternaria) | 32.9 | 37.7 |
| MFP094331 | Alternaria sp. (sect. Infectoriae) | 34.1 | 20.8 |
| MFP778011 | Alternaria sp. (sect. Infectoriae) | 35.5 | 21.5 |
| MFG59013 | Bipolaris sorokiniana | n.d. 2 | n.d. |
| MFG232100 | Cladosporium sp. | n.d. | 34.5 |
| MFG60204 | Fusarium avenaceum | n.d. | 35.4 |
| MFG102100 | F. culmorum | n.d. | n.d. |
| MFG11039 | F. sporotrichioides | n.d. | n.d. |
| MFG14000 | Trichothecium roseum | n.d. | 38.8 |
| Cereal | Region | Crop Year (Number of Samples) | The Range of Alternaria DNA Amounts ×10−4, pg/ng of Total DNA | |
|---|---|---|---|---|
| A. sect. Alternaria | A. sect. Infectoriae | |||
| wheat | the Urals | 2017 (13) | 881–3079 | 117–2388 |
| 2018 (23) | 736–4580 | 36–1462 | ||
| 2019 (10) | 1165–3826 | 55–544 | ||
| West Siberia | 2017 (26) | 1027–6442 | 158–877 | |
| 2018 (21) | 1213–21,731 | 91–1187 | ||
| 2019 (23) | 426–3851 | 35–1204 | ||
| barley | the Urals | 2018 (15) | 497–5568 | 26–3616 |
| 2019 (8) | 361–7146 | 43–1051 | ||
| West Siberia | 2017 (2) | 12,721; 9695 | 4237; 2746 | |
| 2018 (15) | 53–10,526 | 252–1472 | ||
| 2019 (9) | 820–4776 | 103–535 | ||
| oat | the Urals | 2018 (2) | 1093; 1452 | 88; 326 |
| 2019 (4) | 313–3323 | 11–227 | ||
| West Siberia | 2018 (5) | 905–3472 | 79–327 | |
| 2019 (2) | 624; 526 | 139; 91 | ||
| Region | Year of Crop (No. of Samples) | Number of Contaminated Samples/ Range of Mycotoxin Amounts, µg/kg | |||
|---|---|---|---|---|---|
| AOH | AME | TEN | TeA | ||
| wheat | |||||
| the Urals | 2017 (13) | 3/2–4 | nd/0 | 13/4–48 | 13/15–226 |
| 2018 (23) | 6/3–26 | 3/5–5 | 21/3–79 | 16/35–454 | |
| 2019 (10) | 1/15 | nd 1/0 | 7/5–19 | 10/19–97 | |
| West Siberia | 2017 (26) | 13/2–44 | 9/3–56 | 26/9–131 | 26/17–545 |
| 2018 (21) | 7/3–14 | 2/3; 4 | 20/4–83 | 16/37–14,963 | |
| 2019 (23) | 6/7–17 | 3/4–6 | 17/3–36 | 23/16–241 | |
| barley | |||||
| the Urals | 2018 (15) | 3/2–8 | 1/3 | 14/5–80 | 13/15–593 |
| 2019 (8) | 1/3 | nd/0 | 7/5–10 | 7/14–570 | |
| West Siberia | 2017 (2) | nd/0 | nd/0 | 2/9; 12 | nd/0 |
| 2018 (15) | nd/0 | nd/0 | 13/5–38 | 7/30–349 | |
| 2019 (9) | 2/4; 5 | nd/0 | 7/3–6 | 9/9–113 | |
| oat | |||||
| the Urals | 2018 (2) | 1/19 | 1/3 | 2/16; 21 | 2/65; 228 |
| 2019 (4) | 2/11; 13 | 2/4; 11 | 4/3–15 | 3/59–276 | |
| West Siberia | 2018 (5) | 1/4 | nd/0 | 5/13–88 | 5/164–405 |
| 2019 (2) | 2/7; 53 | 1/22 | 2/15; 15 | 2/280; 1579 | |
| Factors | Analysed Parameters | |||||
|---|---|---|---|---|---|---|
| A. sect. Alternaria DNA | A. sect. Infectoriae DNA | AOH | AME | TEN | TeA | |
| cereal species | F 1 = 3.50 p = 0.03 | F = 7.48 p = 0.001 | F = 7.25 p = 0.0009 | ns 2 | F = 3.32 p = 0.04 | ns |
| geographic origin | F = 6.03 p = 0.02 | ns | ns | ns | ns | ns |
| crop year | ns | F = 12.03 p = 0.00001 | ns | F = 3.06 p = 0.049 | F = 9.48 p = 0.002 | ns |
| Cereals (No. of Samples) | The Proportion of Samples Containing Mycotoxin, % | |||
|---|---|---|---|---|
| AOH | AME | TEN | TeA | |
| Barley (49) | 12 | 2 | 88 | 73 |
| Oat (13) | 46 | 31 | 100 | 92 |
| Wheat (116) | 31 | 15 | 90 | 90 |
| Region | Year | Month | Average Month Temperature, °C | Average Summer Temperature, °C | Rainfall, mm | Days with Precipitation |
|---|---|---|---|---|---|---|
| the Urals | 2017 | June | +16.6 | +17.7 | 72 | 19 |
| July | +18.5 | 109 | 19 | |||
| August | +18.0 | 57 | 13 | |||
| 2018 | June | +15.0 | +17.5 | 36 | 19 | |
| July | +21.0 | 90 | 13 | |||
| August | +16.5 | 65 | 19 | |||
| 2019 | June | +15.9 | +17.5 | 64 | 18 | |
| July | +20.3 | 75 | 14 | |||
| August | +16.4 | 85 | 18 | |||
| West Siberia | 2017 | June | +20.2 | +18.9 | 50 | 14 |
| July | +19.0 | 94 | 23 | |||
| August | +17.5 | 69 | 16 | |||
| 2018 | June | +19.9 | +18.6 | 64 | 16 | |
| July | +18.9 | 52 | 14 | |||
| August | +17.1 | 26 | 13 | |||
| 2019 | June | +16.6 | +18.1 | 48 | 16 | |
| July | +19.3 | 83 | 12 | |||
| August | +18.5 | 61 | 17 |
| Target | The Primers | Primer Sequence (5′ → 3′) | Protocol | References |
|---|---|---|---|---|
| A. sect. Alternaria | AAF2 | TGCAATCAGCGTCAGTAACAAA | 50° for 2 min; 95° for 10 min; [95° for 15 s; 67° for 60 s; 72° for 5 s] × 40 | [43] |
| AAR3 | ATGGATGCTAGACCTTTGCTGAT | |||
| A. sect. Infectoriae | AinfF3 | CTCGATGTCCGCCTCAGTAG | 50° for 2 min; 95° for 10 min; [95° for 15 s; 65° for 60 s; 72° for 3 s] × 40 | [44] |
| Analyte | Retention Time, min | MS/MS Parameters | ||||
|---|---|---|---|---|---|---|
| m/z Q1 | m/z Q3 1 | DP (V) 2 | CE (V) 3 | CXP (V) 4 | ||
| Alternariol (AOH) | 9.67 | 257.0 | 213.0/215.0 | −100 | −34/−36 | −11/−11 |
| Alternariolmethylether (AME) | 11.40 | 271.0 | 256.0/227.0 | −95 | −32/−50 | −13/−9 |
| Tentoxin (TEN) | 8.84 | 413.3 | 141.0/271.1 | −105 | −30/−24 | −11/−24 |
| Tenuazonic acid (TeA) | 8.04 | 196.1 | 139.0/112.1 | −120 | −28/−28 | −7/−7 |
| Analyte | Precision (±), % | LOD, µg/kg | LOQ, µg/kg |
|---|---|---|---|
| Alternariol (AOH) | 10–21 | ||
| wheat | 0.79 | 2.39 | |
| barley | 0.82 | 2.46 | |
| oat | 0.82 | 2.60 | |
| Alternariolmethylether (AME) | 7–23 | ||
| wheat | 0.69 | 2.15 | |
| barley | 0.70 | 2.15 | |
| oat | 0.82 | 2.67 | |
| Tentoxin (TEN) | 8–11 | ||
| wheat | 0.79 | 2.00 | |
| barley | 0.82 | 2.15 | |
| oat | 0.82 | 2.22 | |
| Tenuazonic acid (TeA) | 13–21 | ||
| wheat | 6.3 | 14.80 | |
| barley | 3.40 | 9.00 | |
| oat | 18.44 | 51.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orina, A.S.; Gavrilova, O.P.; Gogina, N.N.; Gannibal, P.B.; Gagkaeva, T.Y. Natural Occurrence of Alternaria Fungi and Associated Mycotoxins in Small-Grain Cereals from The Urals and West Siberia Regions of Russia. Toxins 2021, 13, 681. https://doi.org/10.3390/toxins13100681
Orina AS, Gavrilova OP, Gogina NN, Gannibal PB, Gagkaeva TY. Natural Occurrence of Alternaria Fungi and Associated Mycotoxins in Small-Grain Cereals from The Urals and West Siberia Regions of Russia. Toxins. 2021; 13(10):681. https://doi.org/10.3390/toxins13100681
Chicago/Turabian StyleOrina, Aleksandra S., Olga P. Gavrilova, Nadezhda N. Gogina, Philipp B. Gannibal, and Tatiana Yu. Gagkaeva. 2021. "Natural Occurrence of Alternaria Fungi and Associated Mycotoxins in Small-Grain Cereals from The Urals and West Siberia Regions of Russia" Toxins 13, no. 10: 681. https://doi.org/10.3390/toxins13100681
APA StyleOrina, A. S., Gavrilova, O. P., Gogina, N. N., Gannibal, P. B., & Gagkaeva, T. Y. (2021). Natural Occurrence of Alternaria Fungi and Associated Mycotoxins in Small-Grain Cereals from The Urals and West Siberia Regions of Russia. Toxins, 13(10), 681. https://doi.org/10.3390/toxins13100681








