Distribution of Aspergillus Fungi and Recent Aflatoxin Reports, Health Risks, and Advances in Developments of Biological Mitigation Strategies in China
Abstract
1. Introduction
2. Reports of AF Distribution in Different Commodities in China
2.1. Cereals Crops
2.2. Peanuts, Pine Nuts, Nuts, Oils, and Other Oil Products
2.3. Chinese Herbal Medicines (CHMs), Spices, Tea, Fruits, and Vegetables
2.4. Animal Feed and Dairy Products
3. AF Detection in China
4. Health Impacts of AFs in China
5. AF Standards in China and Recent Updates
6. Nature of the Aspergillus Species
7. Distribution and Genetic Characteristics of Aspergillus Species in China
8. Atoxigenic A. flavus as AF Biocontrol Agents
AF Biocontrol Developments in China
9. Conclusions and Recommendations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frisvad, J.C.; Larsen, T.O.; De Vries, R.; Meijer, M.; Houbraken, J.; Cabañes, F.J.; Ehrlich, K.C.; Samson, R.A. Secondary metabolite profiling, growth profiles and other tools for species recognition and important Aspergillus mycotoxins. Stud. Mycol. 2007, 59, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Thrane, U.; Samson, R.A.; Pitt, J.I. Important mycotoxins and the fungi which produce them. In Advances in Experimental Medicine and Biology; 2006; Volume 571, pp. 3–31. ISBN 0065-2598. [Google Scholar]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Nov, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklen, F.; Mahakarnchanakul, W.; Samson, R.A.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stud. Mycol. 2019, 63, 1–63. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Allameh, A.; Kazeroon-Shiri, A.; Ranjbar-Bahadori, S.; Mirzahoseini, H.; Rezaee, M.B. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: Population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia 2006, 161, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Diener, U.L.; Sanders, J.C.T.H.; Lee, P.L.S.; Klich, M.A. Epidemiology of Aflatoxin Formation by Aspaergillus flavus. Ann. Rev. Phytopathol. 1987, 25, 249–270. [Google Scholar] [CrossRef]
- Blaize, M.; Mayaux, J.; Nabet, C.; Lampros, A.; Marcelin, A.; Thellier, M.; Piarroux, R.; Demoule, A. Fatal Invasive Aspergillosis and Coronavirus Disease in an Immunocompetent Patient. Emerg. Infect. Dis. 2020, 26, 1636–1637. [Google Scholar] [CrossRef]
- Lescure, F.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.; Behillil, S.; Gaymard, A. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Passone, M.A.; Resnik, S.; Etcheverry, M.G. Potential use of phenolic antioxidants on peanut to control growth and aflatoxin B1 accumulation by Aspergillus flavus and Aspergillus parasiticus. J. Sci. Food Agric. 2007, 87, 2121–2130. [Google Scholar] [CrossRef]
- Kumar, V.; Basu, M.S.; Rajendran, T.P. Mycotoxin research and mycoflora in some commercially important agricultural commodities. Crop Prot. 2008, 27, 891–905. [Google Scholar] [CrossRef]
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategies. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Control Strategies : Prevention and Detoxification in Foods. Foods 2020, 137, 1–48. [Google Scholar]
- Benkerroum, N. Aflatoxins : Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. [Google Scholar] [CrossRef]
- Pitt, J.I.; Manthong, C.; Siriacha, P.; Chotechaunmanirat, S.; Markwell, P.J. Studies on the biocontrol of aflatoxin in maize in Thailand. Biocontrol Sci. Technol. 2015, 25, 1070–1091. [Google Scholar] [CrossRef]
- Barros, G.; Torres, A.; Chulze, S. Aspergillus flavus population isolated from soil of Argentina’s peanut-growing region. Sclerotia production and toxigenic profile. J. Sci. Food Agric. 2005, 85, 2349–2353. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, F. Global burden of Aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Perspect. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Evaluation of Carcinogenic Risks to Humans; Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene and Styrene; Lyon, France, 2002. [Google Scholar]
- Mupunga, I.; Mngqawa, P.; Katerere, D.R. Peanuts, aflatoxins and undernutrition in children in Sub-Saharan Africa. Nutrients 2017, 9, 1287. [Google Scholar] [CrossRef] [PubMed]
- Okoth, S. Improving the Evidence Base on Aflatoxin Contamination and Exposure in Africa; The Technical Centre for Agricultural and Rural Cooperation, CTA: Wageningen, The Netherlands, 2016. [Google Scholar]
- Gong, Y.Y.; Egal, S.; Hounsa, A.; Turner, P.C.; Hall, A.J.; Cardwell, K.F.; Wild, C.P. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: The critical role of weaning. Int. J. Epidemiol. 2003, 32, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.C.; Hall, A.J.; Wild, C.P. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. A atoxins as a Cause of Hepatocellular Carcinoma. Reviews 2013, 22, 305–310. [Google Scholar]
- Chang, P.-K.; Ehrlich, K.C.; Fujii, I. Cyclopiazonic Acid Biosynthesis of Aspergillus flavus and Aspergillus oryzae. Toxins 2009, 1, 74–99. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, C.W. The isolation and structure of cyclopiazonic acid a toxic metabolite of Penicillium cyclopium Westling. Tetrahedron 1968, 24, 2101–2119. [Google Scholar] [CrossRef]
- Bamba, R.; Sumbali, G. Co-occurrence of aflatoxin B1 and cyclopiazonic acid in sour lime (Citrus aurantifolia Swingle) during post-harvest pathogenesis by Aspergillus flavus. Mycopathologia 2005, 159, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Astoreca, A.; Vaamonde, G.; Dalcero, A.; Marin, S.; Ramos, A. Abiotic factors and their interactions influence on the co-production of aflatoxin B1 and cyclopiazonic acid by Aspergillus flavus isolated from corn. Food Microbiol. 2014, 38, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.; Miller, J.; Groopman, J. Mycotoxin Control in Low- and Middle- Income Countries; 2015; ISBN 9789283225102. [Google Scholar]
- Udomkun, P.; Wiredu, A.N.; Nagle, M.; Bandyopadhyay, R.; Müller, J.; Vanlauwe, B. Mycotoxins in Sub-Saharan Africa: Present situation, socio-economic impact, awareness, and outlook. Food Control 2017, 72, 110–122. [Google Scholar] [CrossRef]
- Richard, J.L. Some major mycotoxins and their mycotoxicoses-An overview. Int. J. Food Microbiol. 2007, 119, 3–10. [Google Scholar] [CrossRef]
- Wu, F. Global impacts of aflatoxin in maize: Trade and human health. World Mycotoxin J. 2015, 8, 137–142. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization) Worldwide Regulations for mycotoxins in food and feed 2003. FAO Food Nutr. Pap. 2003, 81, 1–165.
- Wu, F.; Guclu, H. Aflatoxin Regulations in a Network of Global Maize Trade. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Wu, L.X.; Ding, X.X.; Li, P.W.; Du, X.H. Aflatoxin contamination of peanuts at harvest in China from 2010 to 2013 and its relationship with climatic conditions. Food Control 2016, 60, 117–123. [Google Scholar] [CrossRef]
- Abbas, H.K.; Weaver, M.A.; Zablotowicz, R.M.; Horn, B.W.; Shier, W.T. Relationships between aflatoxin production and sclerotia formation among isolates of Aspergillus section Flavi from the Mississippi Delta. Eur. J. Plant Pathol. 2005, 112, 283–287. [Google Scholar] [CrossRef]
- Cotty, P.J.; Cardwell, K.F. Divergence of West African and North American Communities of Aspergillus SectionFlavi. Appl. Environ. Microbiol. 1999, 65, 2264–2266. [Google Scholar] [CrossRef]
- Horn, B.W.; Dorner, J.W. Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Appl. Environ. Microbiol. 1999, 65, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef]
- Donner, M.; Atehnkeng, J.; Sikora, R.A.; Bandyopadhyay, R.; Cotty, P.J. Distribution of Aspergillus section Flavi in soils of maize fields in three agroecological zones of Nigeria. Soil Biol. Biochem. 2009, 41, 37–44. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Donner, M.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Distribution and toxigenicity of Aspergillus species isolated from maize kernels from three agro-ecological zones in Nigeria. Int. J. Food Microbiol. 2008, 122, 74–84. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Z.; Yu, J. Identification of Aspergillus section Flavi in maize in northeastern China. Mycopathologia 2007, 164, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Mamo, F.T.; Shang, B.; Selvaraj, J.N.; Wang, Y.; Liu, Y. Isolation and characterization of Aspergillus flavus strains in China. J. Microbiol. 2018, 56, 1–9. [Google Scholar] [CrossRef]
- Vaamonde, G.; Patriarca, A.; Ferna, V.; Comerio, R.; Degrossi, C. Variability of aflatoxin and cyclopiazonic acid production by Aspergillus section Flavi from different substrates in Argentina. Int. J. Food Microbiol. 2003, 88, 79–84. [Google Scholar] [CrossRef]
- Tran-Dinh, N.; Pitt, J.I.; Markwell, P.J. Selection of nontoxigenic strains of Aspergillus flavus for biocontrol of aflatoxins in maize in Thailand. Biocontrol Sci. Technol. 2014, 3157, 1–20. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wei, D.-D.; Selvaraj, J.N.; Shang, B.; Zhang, C.-S.; Xing, F.-G.; Zhao, Y.-J.; Wang, Y.; Liu, Y. A strain of Aspergillus flavus from China shows potential as a biocontrol agent for aflatoxin contamination. Biocontrol Sci. Technol. 2015, 25, 583–592. [Google Scholar] [CrossRef]
- Yin, Y.; Lou, T.; Yan, L.; Michailides, T.; Ma, Z. Molecular characterization of toxigenic and atoxigenic Aspergillus flavus isolates, collected from peanut fields in China. J. Appl. Microbiol. 2009, 107, 1857–1865. [Google Scholar] [CrossRef]
- Wei, D.; Zhou, L.; Selvaraj, J.N.; Zhang, C.; Xing, F.; Zhao, Y.; Wang, Y.; Liu, Y. Molecular characterization of atoxigenic Aspergillus flavus isolates collected in China. J. Microbiol. 2014, 52, 559–565. [Google Scholar] [CrossRef]
- Zhang, C.; Selvaraj, J.N.; Yang, Q.; Liu, Y. A Survey of Aflatoxin-Producing Aspergillus sp. from Peanut Field Soils in Four Agroecological Zones of China. Toxins 2017, 9, 40. [Google Scholar] [CrossRef]
- Jiang, J.; Yan, L.; Ma, Z. Molecular characterization of an atoxigenic Aspergillus flavus strain AF051. Appl. Microbiol. Biotechnol. 2009, 83, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, T.; Su, J.; Liang, F.; Wei, Z.; Liang, Y.; Luo, H.; Kuang, S.-Y.; Qian, G.-S.; Sun, G.; et al. Hepatocellular Carcinoma and Aflatoxin Exposure in Zhuqing Village, Fusui County, People’s Republic of China. Cancer Epidemiol. Biomark. Prev. 2001, 10, 143–146. [Google Scholar]
- Li, F.; Yoshizawa, T.; Kawamura, O.; Luo, X.; Li, Y. Aflatoxins and Fumonisins in Corn from the High-Incidence Area for Human Hepatocellular Carcinoma in Guangxi, China. J. Agric. Food Chem. 2001, 2001, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Wang, X.; Zhou, T.; Yang, D.; Wang, Q.; Zhou, Y. Occurrence of four mycotoxins in cereal and oil products in Yangtze Delta region of China and their food safety risks. Food Control 2014, 35, 117–122. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, J.; Yu, J. Aflatoxins in stored maize and rice grains in Liaoning Province, China. J. Stored Prod. Res. 2006, 42, 468–479. [Google Scholar] [CrossRef]
- Lai, X.; Liu, R.; Ruan, C.; Zhang, H.; Liu, C. Occurrence of aflatoxins and ochratoxin A in rice samples from six provinces in China. Food Control 2015, 50, 401–404. [Google Scholar] [CrossRef]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. Part A 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.-M. Contamination of aflatoxins in different kinds of foods in China. Biomed. Environ. Sci. 2007, 20, 483–487. [Google Scholar]
- Taylor, P.; Jin, Q.; Liu, S.; Huang, X.; Zhu, G. Determination of Aflatoxin in Infant Cereals from Hangzhou, China. Anal. Lett. 2013, 46, 2319–2331. [Google Scholar] [CrossRef]
- Liu, B.; Hsu, Y.; Lu, C.; Yu, F. Detecting aflatoxin B1 in foods and feeds by using sensitive rapid enzyme-linked immunosorbent assay and gold nanoparticle immunochromatographic strip. Food Control 2013, 30, 184–189. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Liang, B.; Zhang, Y.; Zhong, X.; Luo, X. Probabilistic risk assessment of dietary exposure to aflatoxin B1 in. Sci. Rep. 2020, 10, 7973. [Google Scholar] [CrossRef] [PubMed]
- Suetens, C.; Haubruge, E. Mycotoxins in Stored Barley (Hordeum vulgare) in Tibet Autonomous Region (People’s Republic of China). Mt. Res. Dev. 2003, 23, 284–287. [Google Scholar]
- Ali, N. Aflatoxins in rice : Worldwide occurrence and public health perspectives. Toxicol. Rep. 2019, 6, 1188–1197. [Google Scholar] [CrossRef]
- Ding, X.; Li, P.; Bai, Y.; Zhou, H. Aflatoxin B1 in post-harvest peanuts and dietary risk in China. Food Control 2012, 23, 143–148. [Google Scholar] [CrossRef]
- Taylor, P.; Yang, L.; Liu, Y.; Miao, H.; Dong, B.; Yang, N.; Chang, F. Determination of aflatoxins in edible oil from markets in Hebei Province of China by liquid chromatography–tandem mass spectrometry. Food Addit. Contam. Part B Surveill. 2011, 4, 244–247. [Google Scholar] [CrossRef]
- Li, F.-Q.; Li, Y.-W.; Wang, Y.-R.; Luo, X.-Y. Natural Occurrence of Aflatoxins in Chinese Peanut Butter and Sesame Paste. J. Agric. Food Chem. 2009, 57, 3519–3524. [Google Scholar] [CrossRef]
- Yu-jiao, W.; Ji-yun, N.I.E.; Zhen, Y.A.N.; Zhi-xia, L.I.; Yang, C.; Farooq, S. Multi-mycotoxin exposure and risk assessments for Chinese consumption of nuts and dried fruits. J. Integr. Agric. 2018, 17, 1676–1690. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). A World Agricultural Production; 2017. Available online: usda.gov/psdonline/circulars/production.pdf (accessed on 23 June 2020).
- Yao, G. Peanut Production and Utilization in the People’s Republic of China; Peanut in Local and Global Food Systems Series Report No. 4: GA, USA, 2004. [Google Scholar]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Clever, J. China Standard for Maximum Levels of Mycotoxins in Foods (GB 2761-2017); Beijing, China, 2018. [Google Scholar]
- Foodnavigator Food. Available online: www.http://foodnavigator.com (accessed on 23 June 2020).
- European Rapid Alert System for Food and Feed (RASFF). Available online: https://ec.europa.eu/food/safety/rasff%0A (accessed on 10 September 2021).
- Gao, R.; Hu, Y.; Dan, Y.; Hao, L.; Liu, X.; Song, J. Chinese herbal medicine resources: Where we stand. Chin. Herb. Med. 2020, 12, 3–13. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, X.; Zhang, C.; Logrieco, A.F.; Yang, M. A Review of Current Methods for Analysis of Mycotoxins in Herbal Medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef]
- Chen, L.; Guo, W.; Zheng, Y.; Zhou, J.; Liu, T.; Chen, W.; Liang, D. Occurrence and Characterization of Fungi and Mycotoxins in Contaminated Medicinal Herbs. Toxins 2020, 12, 30. [Google Scholar] [CrossRef]
- Dan, Y.; Qian, Z.; Peng, Y.; Chen, C.; Liu, Y.; Tai, W.; Qi, J. Revision and Improvement of Criterion on Traditional Chinese Medicines in Chinese Pharmacopoeia 2015. Chinese Herb. Med. 2016, 8, 196–208. [Google Scholar] [CrossRef]
- Ashiq, S.; Hussain, M.; Ahmad, B. Natural occurrence of mycotoxins in medicinal plants: A review. Fungal Genet. Biol. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Ren, Y.; Zhu, J.; Cai, Z.; Chen, Y.; Luan, L.; Wu, Y. Multianalysis of 35 mycotoxins in traditional Chinese medicines by ultra-high-performance liquid chromatography-tandem mass spectrometry coupled with accelerated solvent extraction. J. Agric. Food Chem. 2012, 60, 8233–8247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, D.; Tan, L.; Yu, B.; Cao, W. Analysis of aflatoxins in traditional Chinese medicines : Classification of analytical method on the basis of matrix variations. Sci. Rep. 2016, 6, 30822. [Google Scholar] [CrossRef]
- Han, Z.; Zheng, Y.; Luan, L.; Cai, Z.; Ren, Y.; Wu, Y. An ultra-high-performance liquid chromatography-tandem mass spectrometry method for simultaneous determination of aflatoxins B1, B2, G1, G2, M1 and M2 in traditional Chinese medicines. Anal. Chim. Acta 2010, 664, 165–171. [Google Scholar] [CrossRef]
- Wen, J.; Kong, W.; Hu, Y.; Wang, J.; Yang, M. Multi-mycotoxins analysis in ginger and related products by UHPLC-FLR detection and LC-MS/MS con fi rmation. Food Control 2014, 43, 82–87. [Google Scholar] [CrossRef]
- Su, C.; Hu, Y.; Gao, D.A.N.; Luo, Y.I.; Chen, A.J.; Jiao, X.; Gao, W. Occurrence of Toxigenic Fungi and Mycotoxins on Root Herbs from Chinese Markets. J. Food Prot. 2018, 81, 754–761. [Google Scholar] [CrossRef]
- Han, Z.; Dong, M.; Han, W.; Shen, Y.; Nie, D.; Shi, W.; Zhao, Z. Occurrence and exposure assessment of multiple mycotoxins in dried fruits based on liquid chromatography-tandem mass spectrometry. World Mycotoxin J. 2016, 9, 465–474. [Google Scholar] [CrossRef]
- Kensler, T.W.; Roebuck, B.D.; Wogan, G.N.; Groopman, J.D. Aflatoxin: A 50-year Odyssey of mechanistic and translational toxicology. Toxicol. Sci. 2011, 120, 28–48. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in Food and Feed: Present Status and Future Concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Antonissen, G.; Ochieng, P.E.; Croubels, S.; Okoth, S.; Kangethe, E.K.; Faas, J.; Lindahl, J.F. A Review of the Impact of Mycotoxins on Dairy Cattle Health : Challenges for Food Safety and Dairy Production in Sub-Saharan Africa. Toxins 2020, 12, 222. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Pahlow, G.; Muck, R.E.; Driehuis, F. Microbiology of Ensiling. In Silage Science and Technology; Buxton, D.R., Muck, R.E., Harrison, J.H., Eds.; American Society of Agronomy, 2003; pp. 31–93. [Google Scholar]
- Diaz, D.E.; Hagler, W.M., Jr.; Blackwelder, J.T.; Eve, J.A.; Brinton, A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin Binders II : Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Prandini, A.; Tansini, G.; Sigolo, S.; Filippi, L.; Laporta, M.; Piva, G. On the occurrence of aflatoxin M 1 in milk and dairy products. Food Chem. Toxicol. 2009, 47, 984–991. [Google Scholar] [CrossRef]
- Giovati, L.; Magliani, W.; Ciociola, T.; Santinoli, C.; Conti, S.; Polonelli, L. AFM1 in milk: Physical, biological, and prophylactic methods to mitigate contamination. Toxins 2015, 7, 4330–4349. [Google Scholar] [CrossRef]
- Han, R.W.; Zheng, N.; Wang, J.Q.; Zhen, Y.P. Survey of a flatoxin in dairy cow feed and raw milk in China. Food Chem. 2013, 34, 35–39. [Google Scholar] [CrossRef]
- Yu, F.A.N.; Xiao-ying, L.I.; Li-hong, Z.; Ya-xiong, J.I.A.; Cheng, J.I.; Qiu-gang, M.A.; Yu, C.; Liang, W. Investigation on Contamination Situation of Aflatoxin in Detected Feeds and Feedstuffs in Beijing Area. Sci. Agric. Sin. 2012, 45, 5102–5109. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, Y.; Yue, T. Aflatoxin M 1 in Milk Products in China and Dietary Risk Assessment. J. Food Prot. 2013, 76, 849–853. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.Y.; Zheng, N.; Zhang, Y.D.; Du, R.H.; Zheng, B.Q.; Wang, J.Q.; Zheng, N.; Zhang, Y.D.; Du, R.H.; Zheng, B.Q.; et al. A survey of seasonal variations of aflatoxin M1 in raw milk in Tangshan region of China during 2012–2014. Food Control 2016, 69, 30–35. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Li, P.; Zhang, Q.; Ding, X.; Zhang, W. Europium Nanospheres-Based Time-Resolved Fluorescence for Rapid and Ultrasensitive Determination of Total A fl atoxin in Feed. J. Agric. Food Chem. 2015, 63, 10313–10318. [Google Scholar] [CrossRef]
- Xu, N.; Xiao, Y.; Xie, Q.; Li, Y.; Ye, J.; Ren, D. Occurrence of aflatoxin B1 in total mixed rations and aflatoxin M1 in raw and commercial dairy milk in northern China during winter season. Food Control 2021, 124, 107916. [Google Scholar] [CrossRef]
- Xiong, J.; Xiong, L.; Zhou, H.; Liu, Y.; Wu, L. Occurrence of aflatoxin B1 in dairy cow feedstuff and aflatoxin M1 in UHT and pasteurized milk in central China. Food Control 2018. [Google Scholar] [CrossRef]
- Zheng, N.; Sun, P.; Wang, J.Q.; Zhen, Y.P.; Han, R.W.; Xu, X.M. Occurrence of a fl atoxin M1 in UHT milk and pasteurized milk in China market. Food Control 2013, 29, 198–201. [Google Scholar] [CrossRef]
- Cigić, I.K.; Prosen, H. An overview of conventional and emerging analytical methods for the determination of mycotoxins. Int. J. Mol. Sci. 2009, 10, 62–115. [Google Scholar] [CrossRef]
- Chawla, G.; Ranjan, C. Principle, Instrumentation, and Applications of UPLC: A Novel Technique of Liquid Chromatography. Open Chem. J. 2016, 3, 1–16. [Google Scholar] [CrossRef]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; Arco, G.D. Lateral-flow immunoassays for mycotoxins and phycotoxins: A review. Anal. Bioanal. Chem. 2013, 405, 467–480. [Google Scholar] [CrossRef]
- Tumukunde, E.; Ma, G.; Li, D.; Yuan, J.; Qin, L.; Wang, S. Current research and prevention of aflatoxins in China. World Mycotoxin J. 2020, 13, 121–138. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Wu, X.; Pan, M.; Hu, N.; Wang, J.; Wang, S. Quartz crystal microbalance sensor based on covalent organic framework composite and molecularly imprinted polymer of poly(o-aminothiophenol) with gold nanoparticles for the determination of aflatoxin B1. Sens. Actuators B Chem. 2019, 291, 293–297. [Google Scholar] [CrossRef]
- Yagati, A.K.; Chavan, S.G.; Baek, C.; Lee, M.H.; Min, J. Label-free impedance sensing of aflatoxin B1 with polyaniline nanofibers/Au nanoparticle electrode array. Sensors 2018, 18, 1320. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tang, D.; Zhang, J.; Tang, D. Novel quartz crystal microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay. Anal. Chim. Acta 2018, 1031, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control (CDC). Outbreak of Aflatoxin Poisoning Eastern and Central Provinces, Kenya, January–July 2004; 2004; Volume 53.
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Appl. Environ. Microbiol. 2007, 73, 2762–2764. [Google Scholar] [CrossRef] [PubMed]
- Azziz-baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; Mccoy, L.F.; Misore, A.; Decock, K.; et al. Case–Control Study of an Acute Aflatoxicosis Outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1784. [Google Scholar] [CrossRef]
- Aydın, M.; Aydın, S.; Bacanlı, M.; Basaran, N. Aflatoxin levels in chronic hepatitis B patients with cirrhosis or hepatocellular carcinoma in Balıkesir, Turkey. J. Viral Hepat. 2015, 22, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yang, H.; Wu, H.; Liu, J.; Wang, L.; Lu, S.; Lee, M.; Jen, C.; You, S.; Santella, R.M.; et al. Aflatoxin B 1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int. J. Cancer 2017, 141, 711–720. [Google Scholar] [CrossRef]
- Lopez-Valdes, S.; Mario, M.C. The Relationship of Aflatoxin B1 and Hepatocellular Carcinoma: A Mini Review. J. Liver Res. Disord. Ther. 2017, 3, 5–6. [Google Scholar] [CrossRef][Green Version]
- Denissenko, M.F.; Koudriakova, T.B.; Smith, L.; Connor, T.R.O.; Riggs, A.D.; Pfeifer, G.P. The p53 codon 249 mutational hotspot in hepatocellular carcinoma is not related to selective formation or persistence of a ¯ atoxin B 1 adducts. Oncogen 1998, 17, 3007–3014. [Google Scholar] [CrossRef][Green Version]
- Verma, R.J. Aflatoxin Cause DNA Damage. Int. J. Hum. Genet. 2004, 4, 231–236. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L. Epidemiology of Aflatoxin Exposure and Human Liver Cancer. In Aflatoxin and Food Safety; Taylor & Francis Group, LLC, 2005; pp. 195–211. [Google Scholar]
- Obuseh, F.A.; Jolly, P.E.; Yi Jiang, F.M.B.S.; Waterbor, J.; Ellis, W.O.; Piyathilake, C.J.; Desmond, R.A.; Afriyie-Gyawu, E.; Phillips, T.D. Aflatoxin B1 albumin adducts in plasma and aflatoxin M1 in urine are associated with plasma concentrations of vitamins A and E. Int. J. Vitam. Nutr. Res. 2010, 80, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Gong, Y.Y.; Kimanya, M.E.; Shirima, C.P. Comparison of urinary aflatoxin M1 and aflatoxin albumin adducts as biomarkers for assessing aflatoxin exposure in Tanzanian children. Biomarkers 2018, 23, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.K.; Yuan, J.-M.; Yu, M.C.; Wogan, G.N.; Qian, G.-S.; Tu, J.-T.; Groopman, J.D.; Gao, Y.-T.; E.Henderson, B. Urinary aflatoxin biomarkers and risk of hepatocellular carcinoma. Lancet 1992, 339, 944–946. [Google Scholar] [CrossRef]
- Qian, G.; Ross, R.K.; Yu, M.C.; Yuan, J.; Gao, Y.; Henderson, B.E.; Wogan, G.N.; Groopman, J.D. A Follow-Up Study of Urinary Markers of Aflatoxin Liver Cancer Risk in Shanghai, People’s Republic Exposure and of China. Cancer Epidemiol. 1994, 3, 3–10. [Google Scholar]
- Guangxi, S.; Yeh, F.; Yu, M.C.; Mo, C.; Luo, S.; Tong, M.J.; Henderson, B.E. Hepatitis B Virus, Aflatoxins, and Hepatocellular Carcinoma in. Cancer Res. 1989, 49, 2506–2509. [Google Scholar]
- Tao, P.; Zhi-ming, L.I.U.; Tang-wei, L.I.U.; Le-qun, L.I.; Min-hao, P.; Xue, Q.I.N.; Lu-nam, Y.A.N.; Ren-xiang, L.; Zong-liang, W.E.I.; Lian-wen, W. Associated Factors in Modulating Aflatoxin B1–Albumin Adduct Level in Three Chinese Populations. Dig. Dis. Sci. 2005, 50, 525–532. [Google Scholar] [CrossRef]
- Wan, L.; Hatch, M.; Chen, C.; You, S.; Lu, S.; Mei-huei, W.; Wus, W.; Wang, L.; Wang, Q. Aflatoxin Exposure and Risk of Hepatocellular Carcinoma In Taiwan. Int. J. Cancer 1996, 67, 620–625. [Google Scholar]
- Chen, C.; Wang, L.; Lu, S.; Wu, M.; You, S.; Zhang, Y.; Wang, L. Elevated Aflatoxin Exposure and Increased Risk of Hepatocellular Carcinoma. Hepatology 1996, 24, 38–42. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Q.; Yang, H.; Ahsan, H.; Tsai, W.; Wang, L.; Chen, S.; Chen, C.; Santella, R.M. Aflatoxin B 1 Exposure, Hepatitis B Virus Infection, and Hepatocellular Carcinoma in Taiwan. Cancer Epidemiol. Biomark. Prev. 2009, 18, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Merican, I.; Guan, R.; Amarapuka, D.; Alexander, M.J.; Chutaputti, A.; Chien, R.N.; Hasnian, S.S.; Leung, N.; Lesmana, L.; Phiet, P.H.; et al. Chronic hepatitis B virus infection in Asian countries. J. Gastroenterol. Hepatol. 2000, 15, 1356–1361. [Google Scholar] [CrossRef]
- Meng, E.C.H.; Hu, R.; Shi, X.; Zhang, S. Maize in China: Production Systems, Constraints, and Research Priorities; CIMMYT: Mexico D.F., Mexico, 2006; ISBN 9706481451. [Google Scholar]
- Sun, Z.; Chen, T.; Thorgeirsson, S.S.; Zhan, Q.; Chen, J.; Park, J.; Lu, P.; Hsia, C.C.; Wang, N.; Xu, L.; et al. Dramatic reduction of liver cancer incidence in young adults: 28 year follow-up of etiological interventions in an endemic area of China. Carcinogenesis 2013, 34, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- EC European Commission, amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins. Off. J. Eur. Union 2010, 165, 8–12.
- Zhang, H.; He, J.; Li, B. Aflatoxin Contamination and Research in China. In Aflatoxins-Detection, Measurement and Control; Torres-Pacheco, I., Ed.; InTech: Shanghai, China, 2009; pp. 22–36. [Google Scholar]
- European Commission (EC). Commission recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding. Off. J. Eur. Union 2006, 229, 7–8. [Google Scholar]
- Woo, C.S.J.; El-Nezami, H. Mycotoxins in Asia: Is China in danger? Qual. Assur. Saf. Crop. Foods 2015, 7, 3–25. [Google Scholar] [CrossRef]
- CHEMLINKED National Food Safety Standard Limits of Mycotoxins in Foods. Available online: https://food.chemlinked.com (accessed on 23 June 2020).
- Okun, D.O.; Khamis, F.M.; Muluvi, G.M.; Ngeranwa, J.J.; Ombura, F.O.; Yongo, M.O. Distribution of indigenous strains of atoxigenic and toxigenic Aspergillus flavus and Aspergillus parasiticus in maize and peanuts agro-ecological zones of Kenya. Agric. Food Secur. 2015, 4, 14. [Google Scholar] [CrossRef]
- Yu, J.; Chang, P.; Cary, J.W.; Wright, M.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E. Comparative mapping of aflatoxin pathway gene clusters in Aspergillus parasiticus and Aspergillus flavus Comparative Mapping of Aflatoxin Pathway Gene Clusters in Aspergillus parasiticus and Aspergillus flavus. Appl. Environ. Microbiol. 1995, 61, 2365–2371. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Current understanding on aflatoxin biosynthesis and future perspective in reducing aflatoxin contamination. Toxins 2012, 4, 1024–1057. [Google Scholar] [CrossRef]
- Cleveland, T.E.; Yu, J.; Fedorova, N.; Bhatnagar, D.; Payne, G.A.; Nierman, W.C.; Bennett, J.W. Potential of Aspergillus flavus genomics for applications in biotechnology. Trends Biotechnol. 2009, 27, 151–157. [Google Scholar] [CrossRef]
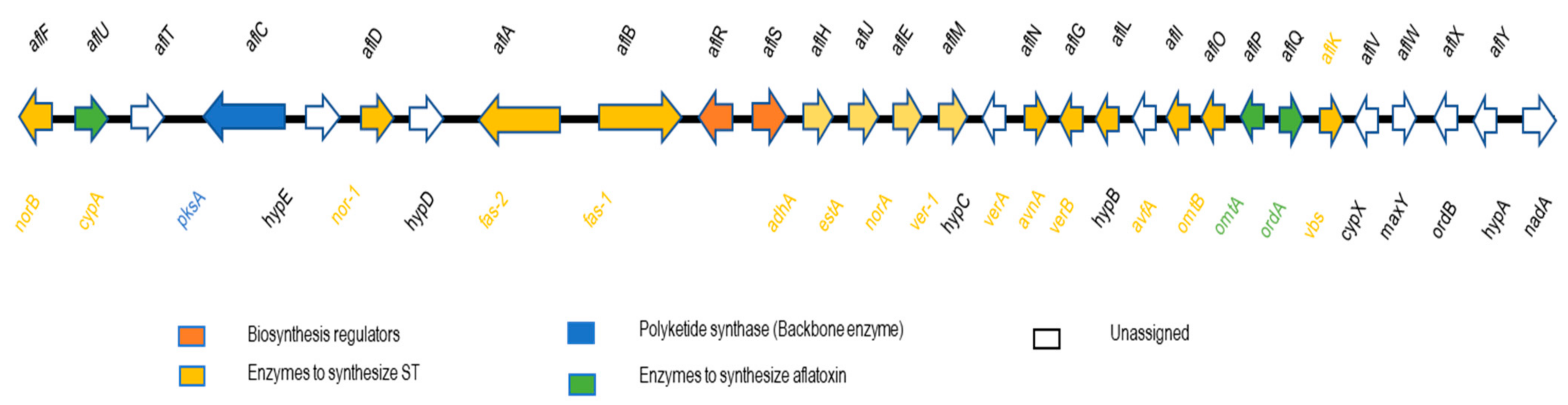
- Li, Q.; He, Z. Advances in research of the structural gene characteristics of the a fl atoxin biosynthetic gene cluster. J. Plant Sci. Phytopathol. 2018, 2, 68–82. [Google Scholar]
- Adhikari, B.N.; Bandyopadhyay, R.; Cotty, P.J. Degeneration of aflatoxin gene clusters in Aspergillus flavus from Africa and North America. AMB Express 2016. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.K.; Matsushima, K.; Takahashi, T.; Yu, J.; Abe, K.; Bhatnagar, D.; Yuan, G.F.; Koyama, Y.; Cleveland, T.E. Understanding nonaflatoxigenicity of Aspergillus sojae: A windfall of aflatoxin biosynthesis research. Appl. Microbiol. Biotechnol. 2007, 76, 977–984. [Google Scholar] [CrossRef]
- Rasheed, U.; Wu, H.; Wei, J.; Ou, X.; Qin, P.; Yao, X.; Chen, H.; Chen, A.J.; Liu, B. A polyphasic study of Aspergillus section Flavi isolated from corn in Guangxi, China- a hot spot of aflatoxin contamination. Int. J. Food Microbiol. 2019, 310, 108307. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Kang, Y.; Lei, Y.; Wan, L.; Huai, D.; Jiang, H.; Ren, X.; Liao, B. Genetic diversity of atoxigenic Aspergillus flavus isolates from peanut kernels in China. Oil Crop Sci. 2018, 3, 42–49. [Google Scholar] [CrossRef]
- Lai, X.; Zhang, H.; Liu, R.; Liu, C. Potential for aflatoxin B 1 and B 2 production by Aspergillus flavus strains isolated from rice samples. Saudi J. Biol. Sci. 2015, 22, 176–180. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, Z.; Jin, S.; Wang, L. Genetic diversity and toxin-producing characters of Aspergillus flavus from China. Biodivers. Sci. 2019, 27, 842–853. [Google Scholar] [CrossRef]
- Chang, P.K.; Horn, B.W.; Dorner, J.W. Clustered genes involved in cyclopiazonic acid production are next to the aflatoxin biosynthesis gene cluster in Aspergillus flavus. Fungal Genet. Biol. 2009, 46, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Horn, B.W.; Dorner, J.W. Effect of nontoxigenic Aspergillus flavus and A. parasiticus on aflatoxin contamination of wounded peanut seeds inoculated with agricultural soil containing natural fungal populations. Biocontrol Sci. Technol. 2009, 19, 249–262. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Bruns, H.A.; Abel, C.A. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci. Technol. 2006, 16, 437–449. [Google Scholar] [CrossRef]
- Alaniz Zanon, M.S.; Barros, G.G.; Chulze, S.N. Non-aflatoxigenic Aspergillus flavus as potential biocontrol agents to reduce aflatoxin contamination in peanuts harvested in Northern Argentina. Int. J. Food Microbiol. 2016, 231, 63–68. [Google Scholar] [CrossRef]
- Atehnkeng, J.; Ojiambo, P.S.; Ikotun, T.; Sikora, R.A.; Cotty, P.J.; Bandyopadhyay, R. Evaluation of atoxigenic isolates of Aspergillus flavus as potential biocontrol agents for aflatoxin in maize. Food Addit. Contam.-Part A Chem. Anal. Control. Expo. Risk Assess. 2008, 25, 1264–1271. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Mycotoxins in Australia: Biocontrol of aflatoxin in peanuts. Mycopathologia 2006, 162, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Dorner, J.W.; Cole, R.J. Effect of application of nontoxigenic strains of Aspergillus flavus and A. parasiticus on subsequent aflatoxin contamination of peanuts in storage. J. Stored Prod. Res. 2002, 38, 329–339. [Google Scholar] [CrossRef]
- Abbas, H.K.; Zablotowicz, R.M.; Horn, B.W.; Phillips, N.A.; Johnson, B.J.; Jin, X.; Abel, C.A. Comparison of major biocontrol strains of non-aflatoxigenic Aspergillus flavus for the reduction of aflatoxins and cyclopiazonic acid in maize. Food Addit. Contam. 2011, 28, 198–208. [Google Scholar] [CrossRef]
- King, E.D.; Bassi, A.B.; Ross, J.C.; Druebbisch, B. An industry perspective on the use of “atoxigenic” strains of Aspergillus flavus as biological control agents and the significance of cyclopiazonic acid. Toxin Rev. 2011, 30, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G. Sex and recombination in aflatoxigenic Aspergilli: Global implications. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Olarte, R.A.; Horn, B.W.; Dorner, J.W.; Monacell, J.T.; Singh, R.; Stone, E.A.; Carbone, I. Effect of sexual recombination on population diversity in aflatoxin production by Aspergillus flavus and evidence for cryptic heterokaryosis. Mol. Ecol. 2012, 21, 1453–1476. [Google Scholar] [CrossRef] [PubMed]
- Atehnkeng, J.; Donner, M.; Peter, S.; Ikotun, B.; Augusto, J.; Cotty, J.; Bandyopadhyay, R. Environmental distribution and genetic diversity of vegetative compatibility groups determine biocontrol strategies to mitigate aflatoxin contamination of maize by Aspergillus flavus. Microb. Biotechnol. 2016, 9, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, K.C.; Cotty, P.J. An isolate of Aspergillus flavus used to reduce aflatoxin contamination in cottonseed has a defective polyketide synthase gene. Appl. Microbiol. Biotechnol. 2004, 65, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Carraro, A.; De Giacomo, A.; Giannossi, M.L.; Medici, L.; Muscarella, M.; Palazzo, L.; Quaranta, V.; Summa, V.; Tateo, F. Clay minerals as adsorbents of aflatoxin M1from contaminated milk and effects on milk quality. Appl. Clay Sci. 2014, 88–89, 92–99. [Google Scholar] [CrossRef]
- Fowler, J.; Li, W.; Bailey, C. Effects of a calcium bentonite clay in diets containing aflatoxin when measuring liver residues of aflatoxin B1in starter broiler chicks. Toxins 2015, 7, 3455–3464. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, H.; Shoeibi, S.; Yazdanpanah, H.; Amirahmadi, M.; Mousavi, A.; Bovo, F.; Sant, A.S. Removal of a fl atoxin B 1 by roasting with lemon juice and / or citric acid in contaminated pistachio nuts. Food Control 2017, 71, 279–284. [Google Scholar] [CrossRef]
- Sani, A.M.; Azizi, E.G.; Salehi, E.A.; Rahimi, K. Reduction of aflatoxin in rice by different cooking methods. Toxicol. Ind. Health 2014, 30, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.; Cavaglieri, L.; Vital, H.; Cristofolini, A.; Merkis, C.; Astoreca, A.; Orlando, J.; Carú, M.; Dalcero, A.; Rosa, C.A.R. Effect of gamma radiation on Aspergillus flavus and Aspergillus ochraceus ultrastructure and mycotoxin production. Radiat. Phys. Chem. 2011, 80, 658–663. [Google Scholar] [CrossRef]
- Inan, F.; Pala, M.; Doymaz, I. Use of ozone in detoxification of aflatoxin B1 in red pepper. J. Stored Prod. Res. 2007, 43, 425–429. [Google Scholar] [CrossRef]
- Diao, E.; Hou, H.; Dong, H. Ozonolysis mechanism and influencing factors of aflatoxin B1: A review. Trends Food Sci. Technol. 2013, 33, 21–26. [Google Scholar] [CrossRef]
- Yan, L.; Song, W.; Chen, Y.; Kang, Y.; Lei, Y.; Huai, D.; Wang, Z.; Wang, X.; Liao, B. Effect of non-aflatoxigenic strains of Aspergillus flavus on aflatoxin contamination of pre-harvest peanuts in fields in China. Oil Crop Sci. 2021, 6, 81–86. [Google Scholar] [CrossRef]
| Province | Crops | Origin | Period | Test | Detection Limit (µg/kg) | Mycotoxin | Total Samples | Incidences (%) | Range of Positive Samples/Maximum Value (µg/kg) | Mean ± SEM of Positive Samples/Mean (μg/kg) | Level of Contamination above Chinese Regulatory Limit (%) (µg/kg) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liaoning | Maize | Farmer stores | 2003 | HPLC | AFs | 73 | 97 | - | 0.99 | All < 20 | [52] | |
| Whole grain rice | 16 | 100 | - | 3.87 | ||||||||
| Brown rice | 37 | 97 | - | 0.88 | ||||||||
| Heilongjiang | Rice | Farmer stores, granaries, and markets | 2009–2011 | DLLME HPLC | AFs | 62 | 69 | 0.033–0.17 | 0.062 ± 0.042 | All < 20 | [53] | |
| Liaoning | 30 | 96.7 | ND | ND | ||||||||
| Jilin | 59 | 40 | 0.030–0.98 | 0.12 ± 0.25 | ||||||||
| Guangdong | 138 | 53 | 0.19–4.1 | 0.44 ± 0.90 | ||||||||
| Guangxi | 67 | 81 | 0.032–21 | 1.3 ± 3.7 | ||||||||
| Hainan | 14 | 93 | 0.032–0.71 | 10.23 ± 0.32 | ||||||||
| Heilongjiang | LOQ = 0.009 | AFB1 | 62 | 69.3 | 0.033–0.14 | 0.058 ± 0.034 | ||||||
| Liaoning | 30 | 97 | <LOQ | <LOQ | ||||||||
| Jilin | 59 | 39 | 0.030–0.90 | 0.11 ± 0.23 | ||||||||
| Guangdong | 138 | 73 | 0.030–3.7 | 0.41 ± 0.81 | ||||||||
| Guangxi | 67 | 53 | 0.032–20 | 1.2 ± 3.4 | ||||||||
| Hainan | 14 | 93 | 0.032–0.66 | 0.21 ± 0.30 | ||||||||
| Heilongjiang | LOQ = 0.006 | AFB2 | 62 | 14.5 | 0.022 | 0.022 | ||||||
| Liaoning | 30 | 6.6 | <LOQ | <LOQ | ||||||||
| Jilin | 59 | 6.5 | 0.086 | 0.086 | ||||||||
| Guangdong | 138 | 13 | 0.020–0.47 | 0.11 ± 0.15 | ||||||||
| Guangxi | 67 | 37.3 | 0.029–1.6 | 0.19 ± 0.36 | ||||||||
| Hainan | 14 | 14.3 | 0.051 | 0.051 | ||||||||
| Shandong Province (Huantai County) | Maize | Individual households | 2010 | ELISA | 0.1 | AFB1 | 31 | 100 | 0.4–2.2 | [54] | ||
| Rice | 9 | 100 | 0.1–1.2 | |||||||||
| Wheat flour | 9 | 100 | 0.3–0.9 | |||||||||
| Jiangsu Province (Huaian City) | Maize | 43 | 100 | 1.2–136.8 | ||||||||
| Rice | 10 | 100 | 0.2–0.7 | |||||||||
| Wheat flour | 7 | 100 | 0.1–0.3 | |||||||||
| Guangxi Zhuang Autonomous (Fusui County) | Maize | 34 | 100 | 1.0–50.0 | ||||||||
| Rice | 10 | 100 | 0.3–1.4 | |||||||||
| Wheat flour | - | - | - | |||||||||
| Eight regions (Chongqing, Fujian, Guangdong, Guangxi, Hubei, Jiangsu, Shanghai, Zhejiang) | Maize | Local food markets | 2007 | HPLC | 0.012 ;B1 0.008; B2; 0.036;G1 | AFs | 74 | 52 | 0.02–1098.36 | [55] | ||
| AFB1 | 74 | 46 | 0.14–970.32 | 23.91 above 20 | ||||||||
| AFB2 | 74 | 41 | 0.02–128.04 | |||||||||
| AFG1 | 74 | 9 | 0.36–4.76 | |||||||||
| Rice | AFs | 84 | 23 | 0.15–3.88 | ||||||||
| AFB1 | 84 | 16 | 0.15–3.22 | |||||||||
| AFB2 | 84 | 3 | 0.06–0.24 | |||||||||
| AFG1 | 84 | 7 | 0.36–1.59 | |||||||||
| Yangtze Delta region (Hangzhou, Ningbo, Shanghai, Suzhou and Wuxi cities) | Rice, wheat, maize, oats, soya bean | Supermarkets and wholesale markets | 2010 | IAC-fluorometer | 1 | AFs | 76 | 14.5 | 1.1–35.0 | 6.9 | 4.0 beyond Chinese (20) and 6.6 beyond EU(4) | [51] |
| 1 | AFB1 | 76 | 14.5 | 1.0–32.2 | 6.6 | 4 beyond Chinese limit (20) and 9.2 beyond EU limit (2) | ||||||
| Hangzhou | Cereal based infant food | Supermarkets | 2012 | UPLC-MS/MS | 0.001 | AFB1 | 30 | 6.6 | 0.016–0.024 | [56] | ||
| 0.001 | AFB2 | 0 | ND | |||||||||
| 0.002 | AFG1 | 0 | ND | |||||||||
| 0.006 | AFG2 | 0 | ND | |||||||||
| 0.008 | AFM1 | 0 | ND | |||||||||
| 0.004 | AFM2 | 0 | ND | |||||||||
| Chongzuo County and Guilin suburbs, Guangxi autonomous region | Maize | Individual households | 1998 | HPLC | 1 | AFB1 | 40 | 45 | 9–2496 | 460 ± 732 | 76% AFB1positive samples above Chinese limit (20) | [50] |
| 2.5 | AFB2 | 35 | 11–320 | 82 ± 102 | ||||||||
| 10 | AFG1 | 22.5 | 12–21 | 15 ± 3 | ||||||||
| 10 | AFG2 | 0 | ND | ND | ||||||||
| Taiwan | Coffee, red yeast rice and maize | Local stores | 2013 | ELISA | 1–2 | AFB1 | 36 | 55.5 | 1.7–234.0 | 30% are beyond the Taiwan limit (15) | [57] | |
| Eleven districts of Guangzhou | Rice and rice products | Household supply retail shops | 2015–2017 | HPLC | 0.1 | AFB1 | 490 | 1.42 | 0.28–1.00 | 0.13 ± 0.001 | [58] | |
| Wheat and wheat products | 436 | 1.4 | 0.28–1.46 | 0.13 ± 0.001 | ||||||||
| 339 | 0.9 | 1.50–6.30 | 0.17 ± 0.001 | |||||||||
| Maize and maize products | ||||||||||||
| Guangxi ; Zhuqing Village, Fusui, | Maize | Households | 1999 | ELISA | - | AFB1 | 30 | 76.7 | 0.4–128.1 | 23.7 ± 6.6 | 30% beyond (20) | [49] |
| Rice | 30 | 23.3 | 0.3–2.0 | 1.1 ± 0.3 | ||||||||
| Shigatze Prefecture of Tibet Autonomous Region | Barley | Farms | 1998 | CD-ELISA | AFs | 25 | 4 0.0 | - | 0.04 | [59] |
| Province | Crops | Origin of Sample | Period | Analytical Method | Detection Limit (µg/kg) | Mycotoxin | Total Samples | Incidences (%) | Range of Positive Samples/Maximum Value (µg/kg) | Mean ± SEM of Positive Samples/Mean (μg/kg) | Level Contamination above Chinese Regulatory Limit (%) | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Twelve provinces, including Liaoning, Shandong, Henan, Hebei, Jiangsu, Anhui, Jiangxi, Hubei, Hunan, Guangdong, Guangxi, and Fujian | Peanut with pod | From farm | 2011/2012 | HPLC | 3:1 for LOD | AFB1 | 1040 | 25 | 0.01–720 | 2.13 | 1% beyond Chinese regulation (20) and 3.7% above EU regulation (2) | [61] | |
| Liaoning | Peanut | From farm and storage | 2015 | HPLC | 0.2 for AFB1; 0.05 for AFB2; 0.2 for AFG1; 0.05 for AFG2 | AFB1 | 408 | 3.19 | 0.15–116.64 | 0.43 ± 6.23 | - | [32] | |
| AFB2 | 3.68 | 0.05–27.36 | 0.11 ± 1.50 | ||||||||||
| AFG1 | 0.25 | 3.61 | 0.01 ± 0.18 | ||||||||||
| AFG2 | 0.74 | 0.27–1.15 | 0.00 ± 0.06 | ||||||||||
| Total AF | 4.90 | 0.05–144.00 | 0.55 ± 7.80 | ||||||||||
| Henan | AFB1 | 1190 | 19.00 | 0.06–483.00 | 7.57 ± 41.12 | ||||||||
| AFB2 | 11.68 | 0.01–61.50 | 0.82 ± 4.67 | ||||||||||
| AFG1 | 1.18 | 0.33–460.00 | 0.81 ± 15.23 | ||||||||||
| AFG2 | 4.03 | 0.05–104.00 | 0.23 ± 3.33 | ||||||||||
| Total AF | 19.00 | 0.06–1023.2 | 9.43 ± 54.98 | ||||||||||
| Sichuan | AFB1 | 455 | 15.60 | 15.56 ± 86.73 | 15.56 ± 86.73 | ||||||||
| AFB2 | 13.19 | 2.34 ± 13.40 | 2.34 ± 13.40 | ||||||||||
| AFG1 | 0.22 | 0.07 ± 1.57 | 0.07 ± 1.57 | ||||||||||
| AFG2 | 5.27 | 0.22 ± 1.82 | 0.22 ± 1.82 | ||||||||||
| Total AF | 15.60 | 18.19 ± 100.38 | 18.19 ± 100.38 | ||||||||||
| Guangdong | AFB1 | 441 | 11.56 | 0.22–341.41 | 4.73 ± 29.84 | ||||||||
| AFB2 | 11.79 | 0.05–30.38 | 0.51 ± 2.96 | ||||||||||
| AFG1 | 0.91 | 0.50–11.50 | 0.04 ± 0.57 | ||||||||||
| AFG2 | 3.17 | 0.21–5.74 | 0.06 ± 0.41 | ||||||||||
| Total AF | 14.29 | 0.06–373.69 | 5.34 ± 32.90 | ||||||||||
| Eight regions (Chongqing, Fujian, Guangdong, Guangxi, Hubei, Jiangsu, Shanghai, Zhejiang) | Peanut | Local food markets | 2007 | HPLC | - | Total AF | 65 | 15 | 0.03–28.39 | Average 27.44 | [55] | ||
| Walnut | 48 | 31 | 0.02–1.20 | ||||||||||
| Pine Nut | 12 | 2 | 0.19–0.25 | ||||||||||
| Peanut | AFB1 | 65 | 9 | 0.15–22.39 | |||||||||
| Walnut | 48 | 21 | 0.14–0.32 | ||||||||||
| Pine Nut | 12 | 2 | 0.19–0.23 | ||||||||||
| Peanut | AFB2 | 65 | 5 | 0.03–6.00 | |||||||||
| Walnut | 48 | 12 | 0.02–0.70 | ||||||||||
| Pine Nut | 12 | 1 | 0.02 | ||||||||||
| Peanut | AFG1 | 65 | 4 | 0.42–11.73 | |||||||||
| Walnut | 48 | 8 | 0.36–0.83 | ||||||||||
| Pine Nut | 12 | 0 | - | ||||||||||
| Shandong Province (Huantai County), Jiangsu Province (Huaian City), and Guangxi Zhuang Autonomous (Fusui County) | Plant oil | Individual households | 2010/2011 | ELISA | 0.1 | AFB1 | 39 | 100 | 0.5–114.4 | Median level is 52.3 beyond the Chinese standard 10 | [54] | ||
| Peanut | 17 | 100 | 0.1–0.7 | ||||||||||
| Hebei Province | Shijiazhuang | Edible oil (peanut, blended, soybean, maize, sunflower, fish oil) | Local markets | 2011 | LC–MS/MS | AFB1 | 40 | 32.5 | 0.14–2.72 | [62] | |||
| AFB2 | 12.5 | 0.15–0.36 | |||||||||||
| AFG1 | 7.5 | 0.01–0.02 | |||||||||||
| Baoding | AFB1 | 18 | 22.2 | 0.16–1.88 | |||||||||
| AFB2 | 5.56 | 0–0.18 | |||||||||||
| Tangshan | AFG1 | 0 | - | ||||||||||
| AFB1 | 18 | 27.8 | 0.15–0.45 | ||||||||||
| AFB2 | 0 | - | |||||||||||
| AFG1 | 0 | - | |||||||||||
| Beijing, Shanghai, Changchun, Chengdu, Shijiazhuang, and Zhengzhou | Peanut butter | Retail markets | 2007 | LC | 1 | AFT | 50 | 82 | 0.77–70.64 | 8.51 | 39% for total AFs set by EU (4) 37% AFB1 set by EU (2) and 2% AFB1 exceed the Chinese regulations (20); | [63] | |
| 0.15 | AFB1 | 0.39–68.51 | 6.12 6 | ||||||||||
| AFB2 | 0–5.52 | 0.67 | |||||||||||
| AFG1 | 0–21.22 | 2 | |||||||||||
| AFG2 | 0–6.36 | 0.4 | |||||||||||
| Sesame paste | 1 | AFT | 50 | 37 | 0.54–56.89 | 6.75 | 24% beyond the limits total AFs of EU (4) 19% and 32% of sesame AFB1 exceed Chinese (5) and European Union (EU) (2) | ||||||
| 0.15 | AFB1 | 0.39–20.45 | 4.31 | ||||||||||
| AFB2 | 0–4.92 | 0.63 | |||||||||||
| AFG1 | 0–26.28 | 1.44 | |||||||||||
| AFG2 | 0–5.75 | 0.37 | |||||||||||
| Eleven districts of Guangzhou | Nuts | Household supply retail shops | 2015–2017 | HPLC | 0.1 | AFB1 | 96 | 3.1 | 0.62–1.37 | 0.14 ± 0.001 | [58] | ||
| Vegetable oil | 365 | 38.9 | 0.26–283.0 | 6.32 ± 25.99 | |||||||||
| Commercial vegetable oil | 269 | 25 | 0.35–7.30 | 0.67 ± 1.81 | |||||||||
| Home-made peanut oil | 96 | 75.5 | 0.26–283.0 | 38.74 ± 47.45 | The mean Is 7 times larger that the Chinese maximum limit (5) | ||||||||
| 21 provinces, autonomous regions and municipalities | Nuts | Local markets and supermarkets | 2018 | UPLC | LOD; 0.05–1.00; LOQ; 0.10–5.00 | AFB1 | 133 | 3.8 | 1.3–40.7 | 9.3 ± 0.28 | [64] | ||
| AFB2 | 15 | 0.2–1.2 | 1.9 ± 0.02 | ||||||||||
| AFG1 | ND | ND | ND | ||||||||||
| AFG2 | 2.3 | 1.1–1.6 | 1.3 ± 0.02 | ||||||||||
| Guangxi; Zhuqing Village, Fusui, | Peanut | Households | 2013 | ELISA | - | AFB1 | 30 | 66.7 | 0.1–52.5 | 7.8 ± 3.2 | [49] | ||
| Yangtze Delta region (Hangzhou, Ningbo, Shanghai, Suzhou and Wuxi cities) | Peanut, soya bean, and oil. | Supermarkets and wholesale markets | IAC-fluorometer | 1 | AFs | 76 | 14.5 | 1.1–35.0 | 6.9 | 4.0 | [51] | ||
| AFB1 | 76 | 1.0–32.2 | 6.6 | ||||||||||
| Province | Product | Origin of the Sample | Study Year | Analytical Method | Mycotoxin | Detection Limit (µg/kg) | Total Samples (n) | Incidences (%) | Range of Positive Samples/Maximum Value (µg/kg) | Mean ± SEM of Positive Samples/Mean (μg/kg) | Level Contamination above Chinese Regulatory Limit (%) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Eleven districts of Guangzhou | Tea | Household supply retail shops | 2019/2020 | HPLC | AFB1 | 0.1 | 128 | 17.9 | 0.25~4.0 | 0.36 ± 0.62 | [58] | |
| Anhui, Fujian, Gansu, Guangdong, Guizhou, Hubei, Shanxi, Xinjiang, Yunnan, Zhejiang | Traditional Chinese medicines (TCM) | Herbal market | 2019/2020 | HPLC | AFB1 | 0.012–1.3 | 48 | 70.8 | 0.12–3.05 | All < 5 | [73] | |
| AFB2 | 0.43–0.5 | AFB1 limit 2–10, AF’s ; 4–20 (Chinese AFB1 ≤ 5 ; AFs ≤ 10) | ||||||||||
| AFG1 | ND–0.85 | |||||||||||
| AFG2 | 0.87–2.11 | |||||||||||
| China | TCM | Regulated enterprises | 2011 | UHPLC/MS/MS | AF’s | LOD; 0.01–1.56 | 60 | 40 | 0.2–19.5 | [75,76] | ||
| AFB1 | 1.2–9.8 | |||||||||||
| AFB2 | 0.2–7.1 | |||||||||||
| AFG1 | 0.6–2.5 | |||||||||||
| AFG2 | 0.2–4.8 | |||||||||||
| Chongqing China | TCM | Local markets and drug stores | 2015 | UPLC-MS/MS | AF’s | LOD; 0.008–0.022 | 22 | 63 | 0.2–7.5 | 18.2 exceeded the maximum limit set by EU (4) | [77] | |
| AFB1 | 0.2–4.8 | |||||||||||
| AFB2 | 0.1–2.3 | |||||||||||
| AFG1 | 0.1–0.8 | |||||||||||
| AFG2 | 0.1–0.2 | |||||||||||
| Zhejiang | TCM | Regulated enterprises | 2009/2010 | (UHPLC–MS/MS | AFB1 | LOD; 0.01–1.56 | 30 | 68.8 | - | 1.40 | [78] | |
| AFB2 | 50.0 | 1.27 | ||||||||||
| AFG1 | 43.8 | 0.50 | ||||||||||
| AFG2 | 43.8 | 0.94 | ||||||||||
| AFM1 | 6.6 | 0.7 | ||||||||||
| Beijing | Ginger | Local markets | 2013/2014 | UHPLC-FLR | AFB1 | 0.005–0.2 | 30 | 5/30 | 0.3–1.38 | 0.073 | [79] | |
| AFB2 | 30 | ND | - | |||||||||
| AFG1 | 30 | ND | - | |||||||||
| AFG2 | 30 | ND | - | |||||||||
| Hebei province and Guangxi provinces | Chinese yam, American ginseng, Ginseng, Notoginseng, Astragalus, Polygala, Bupleurum, Liquorice | Markets | 2013 | UPLC-MS/MS | AFB1 | LOD ≤ 0.05 and LOQ ≤ 0.1 | 48 | 35.4 | ND-13.3 | 14.58 exceed 5 | [80] | |
| AFB2 | 2 | ND-8.2 | ||||||||||
| AFs | 37.5 | ND-21.5 | 8.33 exceed 10 | |||||||||
| Shanghai | Pistachios | Markets | 2014–2015 | LC-MS/MS | AFB1 | 0.03 | 25 | 4 | ND-0.8 | 0.8 | - | [81] |
| AFB2 | 0.2 | 0 | ND | ND | ||||||||
| AFG1 | 0.2 | 0 | ND | ND | ||||||||
| AFG2 | 0.3 | 0 | ND | ND | ||||||||
| Dried longans | AFB1 | 0.1 | 28 | 0 | ND | ND | ||||||
| AFB2 | 0.1 | 3.6 | ND-0.2 | 0.2 | ||||||||
| AFG1 | 0.2 | 0 | ND | ND | ||||||||
| AFG2 | 0.3 | 0 | ND | ND | ||||||||
| Raisins | AFB1 | 0.1 | 32 | 0 | ND | ND | ||||||
| AFB2 | 0.3 | 0 | ND | ND | ||||||||
| AFG1 | 0.3 | 0 | ND | ND | ||||||||
| AFG2 | 0.3 | 0 | ND | ND | ||||||||
| Dried dates | AFB1 | 0.1 | 40 | 0 | ND | ND | ||||||
| AFB2 | 0.1 | 0 | ND | ND | ||||||||
| AFG1 | 0.3 | 0 | ND | ND | ||||||||
| AFG2 | 0.3 | 0 | ND | ND | ||||||||
| 21 provinces, autonomous regions and municipalities | Dried jujube | Local markets and supermarkets | 2018 | UPLC-MS/MS | AFB1 | LOD; 0.05–1.00 and LOQ; 0.10–5.00 | 35 | 0 | ND | ND | [64] | |
| AFB2 | 0 | ND | ND | |||||||||
| AFG1 | 8.6 | 0.2–0.6 | 0.4 ± 0.03 | |||||||||
| AFG2 | 2.9 | 0.4 | 0.4 ± 0.06 | |||||||||
| Raisins | AFB1 | 30 | 0 | ND | ND | |||||||
| AFB2 | 0 | ND | ND | |||||||||
| AFG1 | 0 | ND | ND | |||||||||
| AFG2 | 20 | 0.5–1.4 | 0.9 ± 0.02 | |||||||||
| Dried figs | AFB1 | 20 | 15 | 1.8–384.1 | 129.5 ± 0.68 | |||||||
| AFB2 | 5 | 2.5 | 2.5 ± 0.21 | |||||||||
| AFG1 | 15 | 0.4–17.8 | 5.9 ± 0.33 | |||||||||
| AFG2 | 15 | 0.6–1.2 | 0.9 ± 0.05 | |||||||||
| Dried longans | AFB1 | 15 | ND | ND | ND | |||||||
| AFB2 | 6.7 | 0.7 | 0.7 ± 0.01 | |||||||||
| AFG1 | ND | ND | ND | |||||||||
| AFG2 | 40 | 0.1–2.9 | 1.5 ± 0.07 |
| Province | Product | Year | Origin of the Sample | Analytical Method | Mycotoxin | Detection Limit (µg/kg) | Total Samples (n) | Incidences (%) | Range of Positive Samples/Maximum Value (µg/kg) | Mean ± SEM of Positive Samples/Mean (μg/kg) | Level Contamination above Chinese Regulatory Limit (%) (µg/kg or L) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ten provinces (Heilongjiang, Inner Mongolia Beijing, Tianjin Ningxia, Hebei, Shanxi, Shandong, North Shanghai, Guangdong, South) | Dairy cow feeds | 2013 | Dairy farms | HPLC | AFB1 | 200 | 42 | 0.05–3.53 | 0.31 | <10 | [91] | |
| AFB2 | 200 | 36 | 0.03–0.84 | 0.14 | - | |||||||
| AFB1 + AFB2 | 200 | 24.5 | 0.05–3.53 | 0.34 | - | |||||||
| Milk | Dairy farms | ELISA | AFM1 | 0.005 | 200 | 32.5% | 5.2–59.6 ng/L | 0.0153 | <0.5 | |||
| Beijing | Feed and feedstuffs | 2012 | Animal farms | HPLC | AFB1 | - | 22 | 50 | 59 | 6.0 | <Chinese limit | [92] |
| AFB2 | 9.1 | 12 | 0.6 | |||||||||
| AFG1 | 4.5 | 0.5 | 0.0 | |||||||||
| AFG2 | 9.1 | 0.5 | 0.0 | |||||||||
| 31 provinces | Yoghurt | 2013 | Retail store and supermarkets | ELISA | AFM1 | 0.05 μg/kg | 178 | 4.49 | - | 27.10 | <0.5 | [93] |
| Milk | 0.005 μg/kg | 233 | 48.07 | - | 21.49 | <0.5 | ||||||
| Tangshan region of China | Milk | 2012–2014 | Milk stations | HPLC-MS/MS | AFM1 | 530 | 52.8% | 10–200 ng/L | 73.0 ng/L | <0.5 | [94] | |
| China | Feed | Company and livestock farms | Eu-Nano-TRFIA | Total AFs | 0.16 μg/kg | 397 | 78.3% | 0.50–145.30 μg/kg | [95] | |||
| Northern China | Raw milk | 2019/2020 | Shops, distributors, farms | ELISA | AFM1 | - | 84 | 10–430 ng/L | 110 ng/kg | 34.5% exceeds EU limits | [96] | |
| Commercial milk | AFM1 | 69 | ||||||||||
| Total mixed rations (TMR) | HPLC | AFB1 | 0.03 μg/kg | 22 | 30–370 ng/L | 4.16 μg/kg | 31.8% exceeds EU limits | |||||
| Central China | Feed | 2016/2017 | HPLC | AFB1 | 0.03 μg/kg | 174 | 35.1% | 2.3% (30) | [97] | |||
| UHT milk | ELISA | AFM1 | 0.005 μg/kg | 111 | 73.6% | - | 100.0 ng/L | All below 0.5 | ||||
| Pasteurized milk | ELISA | AFM1 | 131 | - | ||||||||
| China (Beijing and Shanghai) | UHT milk | 2010 | super- markets | ELISA | AFM1 | - | 153 | 54.9% | 0.006–0.160 mg/L | - | All below 0.5 | [98] |
| Pasteurized milk | - | 26 | 96.2% | 0.023–0.154 mg/L | - | 20.3% of UHT milk samples and 65.4% of pasteurized milk samples exceed the EU limit |
| Location | Product | Number of Samples | Source | Sampling Season | Incidences of Fungi, or Aspergillus spp. or Aspergillus Section Flavi | Incidences of A. flavus Species | Toxin Production of A. flavus Strains | Nature Biosynthetic Genes | Morphological Nature | Reference | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Liaoning Province (Northeast) | Maize | 120 | Household stored (1–3 years) | 2003 | 55.8% (Aspergillus section Flavi) | 98.5% | - | - | 64%-L 36%-S | [39] | ||
| Guangxi province | Maize | 89 | 2016/2017 | 195 (Aspergillus section Flavi) | 98.5% | 86.6% (30) were aflatoxin and CPA positive | - | Fluorescence and pink color observed in carbon added PDA | [139] | |||
| 19 provinces, 1 autonomous region and 1 municipality | Peanut, maize, rice | - | - | 2013/2014 | 724 A.flavus species isolated | >95% | 32% (229) atoxigenic | 10.4% atoxigenic strains found to have lost aflR, fas-1 and aflJ genes | 51% S-type (229) 34% L- type (229)15% NS (229) | [40] | ||
| 14 provinces | Peanut pod | 1106 | 2013 | 265 Aspergillus spp. | 262 (98.9%) | 18.8% A.flavus atoxigenic | 38.0% atoxigenic strains lost nor-1, ver-1, aflR, omtA genes | - | [140] | |||
| 2015 | 257 Aspergillus spp. | 254 (98.8%) | ||||||||||
| 12 provinces | Rice | - | - | - | - | 127 A.flavus | 47(37%) toxigenic | - | - | [141] | ||
| Provinces | Liaoning | Peanut-cropped soils | - | Field | 2013 | 343 fungi isolated | 9 | 323 | 76 Atoxigenic | [46] | 97% of atoxigenic strains lost one of the aflT, nor-1, aflR, hypB genes | |
| Shandong | 73 | |||||||||||
| Hubei | 125 | |||||||||||
| Guangdong | 116 | |||||||||||
| Different provinces of China | Peanut cropped soil | - | Field | - | - | 56 A.flavus | 35 atoxigenic | 11 A. flavus isolates had 5 deletion patterns for 12 genes | 21 atoxigenic strains were either L- or S-type | [45] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamo, F.T.; Abate, B.A.; Zheng, Y.; Nie, C.; He, M.; Liu, Y. Distribution of Aspergillus Fungi and Recent Aflatoxin Reports, Health Risks, and Advances in Developments of Biological Mitigation Strategies in China. Toxins 2021, 13, 678. https://doi.org/10.3390/toxins13100678
Mamo FT, Abate BA, Zheng Y, Nie C, He M, Liu Y. Distribution of Aspergillus Fungi and Recent Aflatoxin Reports, Health Risks, and Advances in Developments of Biological Mitigation Strategies in China. Toxins. 2021; 13(10):678. https://doi.org/10.3390/toxins13100678
Chicago/Turabian StyleMamo, Firew Tafesse, Birhan Addisie Abate, Yougquan Zheng, Chengrong Nie, Mingjun He, and Yang Liu. 2021. "Distribution of Aspergillus Fungi and Recent Aflatoxin Reports, Health Risks, and Advances in Developments of Biological Mitigation Strategies in China" Toxins 13, no. 10: 678. https://doi.org/10.3390/toxins13100678
APA StyleMamo, F. T., Abate, B. A., Zheng, Y., Nie, C., He, M., & Liu, Y. (2021). Distribution of Aspergillus Fungi and Recent Aflatoxin Reports, Health Risks, and Advances in Developments of Biological Mitigation Strategies in China. Toxins, 13(10), 678. https://doi.org/10.3390/toxins13100678






