Immune Dysfunction in Uremia 2020
Abstract
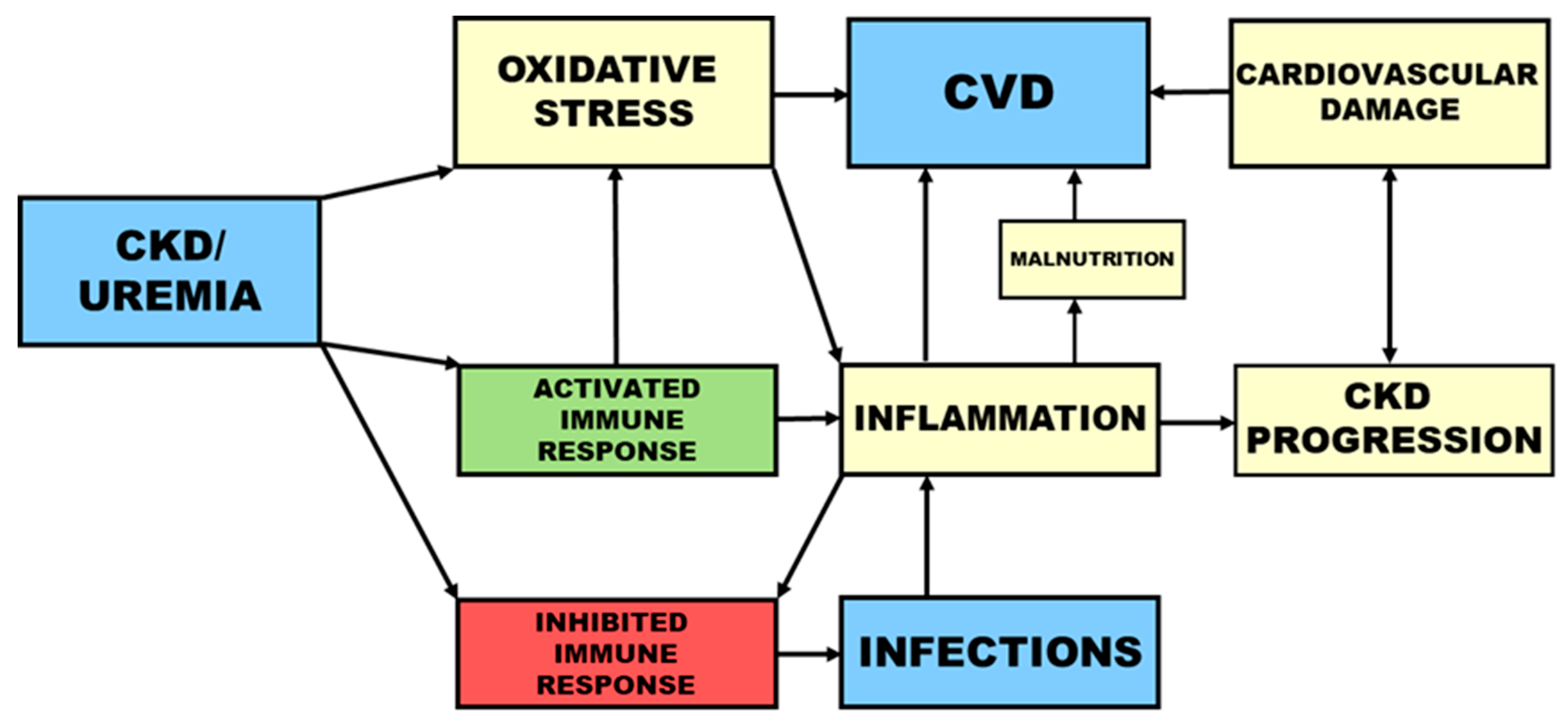
1. Cardiovascular Disease and Infections as the Main Causes of Death in Uremia
2. Immune Cells in Uremia
2.1. Polymorphonuclear Leukocytes (PMNLs)
2.1.1. Neutrophil Extracellular Traps
2.1.2. PMNL Priming
2.2. Monocytes
2.3. Dendritic Cells
2.4. Lymphocytes
3. Oxidative Stress and Inflammation
4. Toll-Like Receptors and Inflammasomes
5. Autophagy
6. Complement System
7. Metabolic Functions of the Kidney
7.1. Renin-Angiotensin-System (RAS)
7.2. Erythropoietin and Iron
Hepcidin
7.3. Vitamin D, Fibroblast Growth Factor 23, and Parathyroid Hormone
8. Uremic Toxins
8.1. Protein-Bound Solutes
8.2. Small Water-Soluble Compounds
8.3. Middle Molecules
8.3.1. Immunoglobulin Light Chains
8.3.2. Retinol-Binding Protein
8.3.3. Neuropeptides
8.3.4. Endothelin-1
8.3.5. Adipokines
8.4. Posttranslational Modifications
8.4.1. Carbamoylation
8.4.2. Advanced Glycation End Products (AGEs)
8.4.3. Oxidative Modifications
8.5. High-Density Lipoprotein (HDL)
8.6. Hydrogen Sulfide (H2S)
9. Conclusions
Funding
Conflicts of Interest
References
- Zemaitis, M.R.; Foris, L.A.; Chandra, S.; Bashir, K. Uremia; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Chapter 1: Definition and classification of CKD. Kidney Int. Suppl. 2013, 3, 19–62. [CrossRef] [PubMed]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global Prevalence of Chronic Kidney Disease—A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Zou, Z.; Cini, K.; Dong, B.; Ma, Y.; Ma, J.; Burgner, D.P.; Patton, G.C. Time Trends in Cardiovascular Disease Mortality Across the BRICS: An Age-Period-Cohort Analysis of Key Nations With Emerging Economies Using the Global Burden of Disease Study 2017. Circulation 2020, 141, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Gansevoort, R.T.; Correa-Rotter, R.; Hemmelgarn, B.R.; Jafar, T.H.; Heerspink, H.J.; Mann, J.F.; Matsushita, K.; Wen, C.P. Chronic kidney disease and cardiovascular risk: Epidemiology, mechanisms, and prevention. Lancet 2013, 382, 339–352. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Culleton, B.; House, A.; Rabbat, C.; Fok, M.; McAlister, F.; Garg, A.X. Chronic kidney disease and mortality risk: A systematic review. J. Am. Soc. Nephrol. 2006, 17, 2034–2047. [Google Scholar] [CrossRef]
- Kade, G.; Lubas, A.; Bodnar, L.; Szczylik, C.; Wankowicz, Z. Malignant tumors in patients with end stage renal failure undergoing renal replacement therapy. Contemp. Oncol. (Pozn) 2012, 16, 382–387. [Google Scholar] [CrossRef]
- Malyszko, J.; Kozlowski, L.; Kozlowska, K.; Malyszko, M.; Malyszko, J. Cancer and the kidney: Dangereoux liasons or price paid for the progress in medicine? Oncotarget 2017, 8, 66601–66619. [Google Scholar] [CrossRef]
- Maisonneuve, P.; Agodoa, L.; Gellert, R.; Stewart, J.H.; Buccianti, G.; Lowenfels, A.B.; Wolfe, R.A.; Jones, E.; Disney, A.P.; Briggs, D.; et al. Cancer in patients on dialysis for end-stage renal disease: An international collaborative study. Lancet 1999, 354, 93–99. [Google Scholar] [CrossRef]
- Thompson, S.; James, M.; Wiebe, N.; Hemmelgarn, B.; Manns, B.; Klarenbach, S.; Tonelli, M.; Alberta Kidney Disease, N. Cause of Death in Patients with Reduced Kidney Function. J. Am. Soc. Nephrol. 2015, 26, 2504–2511. [Google Scholar] [CrossRef] [PubMed]
- White, W.E.; Yaqoob, M.M.; Harwood, S.M. Aging and uremia: Is there cellular and molecular crossover? World J. Nephrol. 2015, 4, 19–30. [Google Scholar] [CrossRef]
- Kooman, J.P.; Broers, N.J.; Usvyat, L.; Thijssen, S.; van der Sande, F.M.; Cornelis, T.; Levin, N.W.; Leunissen, K.M.; Kotanko, P. Out of control: Accelerated aging in uremia. Nephrol. Dial. Transplant. 2013, 28, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Di Lullo, L.; House, A.; Gorini, A.; Santoboni, A.; Russo, D.; Ronco, C. Chronic kidney disease and cardiovascular complications. Heart Fail. Rev. 2015, 20, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.P.; Fisher, S.G.; Elder, J.L.; Winters, P.C.; Beckett, W.; Tacci, J.; Sloand, J.A. Increased Cardiovascular Risk Associated with Reduced Kidney Function. Am. J. Nephrol. 2009, 29, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Sarnak, M.J.; Jaber, B.L. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int. 2000, 58, 1758–1764. [Google Scholar] [CrossRef]
- Mansur, A.; Mulwande, E.; Steinau, M.; Bergmann, I.; Popov, A.F.; Ghadimi, M.; Beissbarth, T.; Bauer, M.; Hinz, J. Chronic kidney disease is associated with a higher 90-day mortality than other chronic medical conditions in patients with sepsis. Sci. Rep. 2015, 5, 10539. [Google Scholar] [CrossRef]
- Powe, N.R.; Jaar, B.; Furth, S.L.; Hermann, J.; Briggs, W. Septicemia in dialysis patients: Incidence, risk factors, and prognosis. Kidney Int. 1999, 55, 1081–1090. [Google Scholar] [CrossRef]
- James, M.T.; Laupland, K.B.; Tonelli, M.; Manns, B.J.; Culleton, B.F.; Hemmelgarn, B.R. Risk of bloodstream infection in patients with chronic kidney disease not treated with dialysis. Arch. Intern. Med. 2008, 168, 2333–2339. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Antoniadi, G.; Liakopoulos, V.; Kartsios, C.; Stefanidis, I. Disturbances of acquired immunity in hemodialysis patients. Semin. Dial. 2007, 20, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Kurts, C.; Panzer, U.; Anders, H.J.; Rees, A.J. The immune system and kidney disease: Basic concepts and clinical implications. Nat. Rev. Immunol. 2013, 13, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Lahoz-Beneytez, J.; Elemans, M.; Zhang, Y.; Ahmed, R.; Salam, A.; Block, M.; Niederalt, C.; Asquith, B.; Macallan, D. Human neutrophil kinetics: Modeling of stable isotope labeling data supports short blood neutrophil half-lives. Blood 2016, 127, 3431–3438. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Haag-Weber, M.; Hörl, W.H. Dysfunction of polymorphonuclear leukocytes in uremia. Semin. Nephrol. 1996, 16, 192–201. [Google Scholar] [PubMed]
- Chonchol, M. Neutrophil dysfunction and infection risk in end-stage renal disease. Semin. Dial. 2006, 19, 291–296. [Google Scholar] [CrossRef]
- Kim, J.K.; Hong, C.W.; Park, M.J.; Song, Y.R.; Kim, H.J.; Kim, S.G. Increased Neutrophil Extracellular Trap Formation in Uremia Is Associated with Chronic Inflammation and Prevalent Coronary Artery Disease. J. Immunol. Res. 2017, 2017, 8415179. [Google Scholar] [CrossRef]
- Bratton, D.L.; Henson, P.M. Neutrophil clearance: When the party is over, clean-up begins. Trends Immunol. 2011, 32, 350–357. [Google Scholar] [CrossRef]
- Filep, J.G.; El Kebir, D. Neutrophil apoptosis: A target for enhancing the resolution of inflammation. J. Cell Biochem. 2009, 108, 1039–1046. [Google Scholar] [CrossRef]
- Haag-Weber, M.; Hörl, W.H. Calcium-dependent neutrophil activation. Contrib. Nephrol. 1992, 100, 269–285. [Google Scholar]
- Hörl, W.H.; Haag-Weber, M.; Mai, B.; Massry, S.G. Verapamil reverses abnormal [Ca2+]i and carbohydrate metabolism of PMNL of dialysis patients. Kidney Int. 1995, 47, 1741–1745. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Diaz, P. Thapsigargin-induced calcium entry and apoptotic death of neutrophils are blocked by activation of protein kinase C. Pharmacology 2001, 63, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.H.; Bei, L.; Huang, Y.F.; Shen, X. The relationship between fMLP induced neutrophil respiratory burst and the apoptosis of neutrophil. Shi Yan Sheng Wu Xue Bao 1999, 32, 359–366. [Google Scholar] [PubMed]
- Kettritz, R.; Falk, R.J.; Jennette, J.C.; Gaido, M.L. Neutrophil superoxide release is required for spontaneous and FMLP-mediated but not for TNF alpha-mediated apoptosis. J. Am. Soc. Nephrol. 1997, 8, 1091–1100. [Google Scholar]
- Cohen, G.; Raupachova, J.; Wimmer, T.; Deicher, R.; Horl, W.H. The uraemic retention solute para-hydroxy-hippuric acid attenuates apoptosis of polymorphonuclear leukocytes from healthy subjects but not from haemodialysis patients. Nephrol. Dial. Transplant. 2008, 23, 2512–2519. [Google Scholar] [CrossRef]
- Massry, S.; Smogorzewski, M. Dysfunction of polymorphonuclear leukocytes in uremia: Role of parathyroid hormone. Kidney Int. Suppl. 2001, 78, S195–S196. [Google Scholar] [CrossRef]
- Hann, J.; Bueb, J.L.; Tolle, F.; Brechard, S. Calcium signaling and regulation of neutrophil functions: Still a long way to go. J. Leukoc. Biol. 2020, 107, 285–297. [Google Scholar] [CrossRef]
- Zawrotniak, M.; Rapala-Kozik, M. Neutrophil extracellular traps (NETs)—formation and implications. Acta Biochim. Pol. 2013, 60, 277–284. [Google Scholar] [CrossRef]
- Papayannopoulos, V.; Zychlinsky, A. NETs: A new strategy for using old weapons. Trends Immunol. 2009, 30, 513–521. [Google Scholar] [CrossRef]
- De Buhr, N.; von Kockritz-Blickwede, M. How Neutrophil Extracellular Traps Become Visible. J. Immunol. Res. 2016, 2016, 4604713. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, H.W.; Joo, N.; Lee, H.S.; Song, Y.R.; Kim, H.J.; Kim, S.G. Prognostic role of circulating neutrophil extracellular traps levels for long-term mortality in new end-stage renal disease patients. Clin. Immunol. 2019, 210, 108263. [Google Scholar] [CrossRef] [PubMed]
- Korabecna, M.; Tesar, V. NETosis provides the link between activation of neutrophils on hemodialysis membrane and comorbidities in dialyzed patients. Inflamm. Res. 2017, 66, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Darrah, E.; Andrade, F. NETs: The missing link between cell death and systemic autoimmune diseases? Front. Immunol. 2012, 3, 428. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.D.; Rohn, T.T.; Quinn, M.T. Neutrophil priming in host defense: Role of oxidants as priming agents. Antioxid. Redox Signal. 2002, 4, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Vogt, K.L.; Summers, C.; Chilvers, E.R.; Condliffe, A.M. Priming and de-priming of neutrophil responses in vitro and in vivo. Eur. J. Clin. Invest. 2018, 48 (Suppl. 2), e12967. [Google Scholar] [CrossRef] [PubMed]
- Klein, J.B.; McLeish, K.R.; Ward, R.A. Transplantation, not dialysis, corrects azotemia-dependent priming of the neutrophil oxidative burst. Am. J. Kidney Dis. 1999, 33, 483–491. [Google Scholar] [CrossRef]
- Chilvers, E.R.; Cadwallader, K.A.; Reed, B.J.; White, J.F.; Condliffe, A.M. The function and fate of neutrophils at the inflamed site: Prospects for therapeutic intervention. J. R. Coll. Physicians Lond. 2000, 34, 68–74. [Google Scholar]
- El-Benna, J.; Hurtado-Nedelec, M.; Marzaioli, V.; Marie, J.C.; Gougerot-Pocidalo, M.A.; Dang, P.M. Priming of the neutrophil respiratory burst: Role in host defense and inflammation. Immunol. Rev. 2016, 273, 180–193. [Google Scholar] [CrossRef]
- Sela, S.; Shurtz-Swirski, R.; Cohen-Mazor, M.; Mazor, R.; Chezar, J.; Shapiro, G.; Hassan, K.; Shkolnik, G.; Geron, R.; Kristal, B. Primed peripheral polymorphonuclear leukocyte: A culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J. Am. Soc. Nephrol. 2005, 16, 2431–2438. [Google Scholar] [CrossRef]
- Girndt, M.; Trojanowicz, B.; Ulrich, C. Monocytes in Uremia. Toxins (Basel) 2020, 12, 340. [Google Scholar] [CrossRef]
- Jeng, Y.; Lim, P.S.; Wu, M.Y.; Tseng, T.Y.; Chen, C.H.; Chen, H.P.; Wu, T.K. Proportions of Proinflammatory Monocytes Are Important Predictors of Mortality Risk in Hemodialysis Patients. Mediators Inflamm. 2017, 2017, 1070959. [Google Scholar] [CrossRef] [PubMed]
- Verkade, M.A.; van Druningen, C.J.; Vaessen, L.M.; Hesselink, D.A.; Weimar, W.; Betjes, M.G. Functional impairment of monocyte-derived dendritic cells in patients with severe chronic kidney disease. Nephrol. Dial. Transplant. 2007, 22, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.U.; Kim, M.; Kim, S.; Nguyen, T.T.; Kim, E.; Lee, S.; Kim, H. Dendritic Cell Dysfunction in Patients with End-stage Renal Disease. Immune Netw. 2017, 17, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R. Dendritic cells in progressive renal disease: Some answers, many questions. Nephrol. Dial. Transplant. 2014, 29, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Panzer, U.; Kurts, C. T cell cross-talk with kidney dendritic cells in glomerulonephritis. J. Mol. Med. 2010, 88, 19–26. [Google Scholar] [CrossRef]
- Betjes, M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013, 9, 255–265. [Google Scholar] [CrossRef]
- Betjes, M.G. Uremia-Associated Ageing of the Thymus and Adaptive Immune Responses. Toxins (Basel) 2020, 12, 224. [Google Scholar] [CrossRef]
- Pahl, M.V.; Gollapudi, S.; Sepassi, L.; Gollapudi, P.; Elahimehr, R.; Vaziri, N.D. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol. Dial. Transplant. 2010, 25, 205–212. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006, 48, 752–760. [Google Scholar] [CrossRef]
- Suvakov, S.; Jerotic, D.; Damjanovic, T.; Milic, N.; Pekmezovic, T.; Djukic, T.; Jelic-Ivanovic, Z.; Savic Radojevic, A.; Pljesa-Ercegovac, M.; Matic, M.; et al. Markers of Oxidative Stress and Endothelial Dysfunction Predict Haemodialysis Patients Survival. Am. J. Nephrol. 2019, 50, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Morena, M.; Cristol, J.P.; Senecal, L.; Leray-Moragues, H.; Krieter, D.; Canaud, B. Oxidative stress in hemodialysis patients: Is NADPH oxidase complex the culprit? Kidney Int. Suppl. 2002, 61, S109–S114. [Google Scholar] [CrossRef] [PubMed]
- Libetta, C.; Sepe, V.; Esposito, P.; Galli, F.; Dal Canton, A. Oxidative stress and inflammation: Implications in uremia and hemodialysis. Clin. Biochem. 2011, 44, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.A.; McLeish, K.R. Methylglyoxal: A stimulus to neutrophil oxygen radical production in chronic renal failure? Nephrol. Dial. Transplant. 2004, 19, 1702–1707. [Google Scholar] [CrossRef] [PubMed]
- Martini, S.; Nair, V.; Keller, B.J.; Eichinger, F.; Hawkins, J.J.; Randolph, A.; Boger, C.A.; Gadegbeku, C.A.; Fox, C.S.; Cohen, C.D.; et al. Integrative biology identifies shared transcriptional networks in CKD. J. Am. Soc. Nephrol. 2014, 25, 2559–2572. [Google Scholar] [CrossRef] [PubMed]
- Jerotic, D.; Matic, M.; Suvakov, S.; Vucicevic, K.; Damjanovic, T.; Savic-Radojevic, A.; Pljesa-Ercegovac, M.; Coric, V.; Stefanovic, A.; Ivanisevic, J.; et al. Association of Nrf2, SOD2 and GPX1 Polymorphisms with Biomarkers of Oxidative Distress and Survival in End-Stage Renal Disease Patients. Toxins (Basel) 2019, 11, 431. [Google Scholar] [CrossRef]
- Jofre, R.; Rodriguez-Benitez, P.; Lopez-Gomez, J.M.; Perez-Garcia, R. Inflammatory syndrome in patients on hemodialysis. J. Am. Soc. Nephrol. 2006, 17, S274–S280. [Google Scholar] [CrossRef]
- Wann, J.G.; Hsu, Y.H.; Yang, C.C.; Lin, C.S.; Tai, D.W.; Chen, J.S.; Hsiao, C.W.; Chen, C.F. Neutrophils in acidotic haemodialysed patients have lower intracellular pH and inflamed state. Nephrol. Dial. Transplant. 2007, 22, 2613–2622. [Google Scholar] [CrossRef]
- Jha, J.C.; Ho, F.; Dan, C.; Jandeleit-Dahm, K. A causal link between oxidative stress and inflammation in cardiovascular and renal complications of diabetes. Clin. Sci. (Lond.) 2018, 132, 1811–1836. [Google Scholar] [CrossRef]
- Jha, V.; Garcia-Garcia, G.; Iseki, K.; Li, Z.; Naicker, S.; Plattner, B.; Saran, R.; Wang, A.Y.; Yang, C.W. Chronic kidney disease: Global dimension and perspectives. Lancet 2013, 382, 260–272. [Google Scholar] [CrossRef]
- Perkovic, V.; Agarwal, R.; Fioretto, P.; Hemmelgarn, B.R.; Levin, A.; Thomas, M.C.; Wanner, C.; Kasiske, B.L.; Wheeler, D.C.; Groop, P.H.; et al. Management of patients with diabetes and CKD: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2016, 90, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, M.; Cobo, G.; Lindholm, B.; Stenvinkel, P. Inflammation and Protein-Energy Wasting in the Uremic Milieu. Contrib Nephrol 2017, 191, 58–71. [Google Scholar] [PubMed]
- Yao, Q.; Axelsson, J.; Stenvinkel, P.; Lindholm, B. Chronic systemic inflammation in dialysis patients: An update on causes and consequences. Asaio J. 2004, 50, lii–lvii. [Google Scholar] [CrossRef] [PubMed]
- Helal, I.; Smaoui, W.; Hamida, F.B.; Ouniss, M.; Aderrahim, E.; Hedri, H.; Elyounsi, F.; Maiz, H.B.; Abdallah, T.B.; Kheder, A. Cardiovascular risk factors in hemodialysis and peritoneal dialysis patients. Saudi J. Kidney Dis. Transpl. 2010, 21, 59–62. [Google Scholar]
- Leemans, J.C.; Kors, L.; Anders, H.J.; Florquin, S. Pattern recognition receptors and the inflammasome in kidney disease. Nat. Rev. Nephrol. 2014, 10, 398–414. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Gollapudi, P.; Yoon, J.W.; Gollapudi, S.; Pahl, M.V.; Vaziri, N.D. Leukocyte Toll-Like Receptor Expression in End-Stage Kidney Disease. Am. J. Nephrol. 2010, 31, 247–254. [Google Scholar] [CrossRef]
- Baj, Z.; Zbrog, Z.; Szuflet, A.; Manka, S.; Bartnicki, P.; Majewska, E. Basic inflammatory indices and chosen neutrophil receptors expression in chronic haemodialysed patients. Cent. Eur. J. Immunol. 2018, 43, 168–173. [Google Scholar] [CrossRef]
- Shen, H.; Kreisel, D.; Goldstein, D.R. Processes of sterile inflammation. J. Immunol. 2013, 191, 2857–2863. [Google Scholar] [CrossRef]
- Komada, T.; Muruve, D.A. The role of inflammasomes in kidney disease. Nat. Rev. Nephrol. 2019, 15, 501–520. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immuno.l 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Kuo, K.L.; Peng, C.H.; Wu, C.H.; Lien, Y.C.; Wang, Y.C.; Tarng, D.C. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int. 2014, 85, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Moissl, U.; Chazot, C.; Mallamaci, F.; Tripepi, G.; Arkossy, O.; Wabel, P.; Stuard, S. Chronic Fluid Overload and Mortality in ESRD. J. Am. Soc. Nephrol. 2017, 28, 2491–2497. [Google Scholar] [CrossRef]
- Dekker, M.J.E.; van der Sande, F.M.; van den Berghe, F.; Leunissen, K.M.L.; Kooman, J.P. Fluid Overload and Inflammation Axis. Blood Purif. 2018, 45, 159–165. [Google Scholar] [CrossRef]
- Ulrich, C.; Wilke, A.; Schleicher, N.; Girndt, M.; Fiedler, R. Hypervolemia-Induced Immune Disturbances Do Not Involve IL-1ss but IL-6 and IL-10 Activation in Haemodialysis Patients. Toxins (Basel) 2020, 12, 159. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, M.J.; Lee, H.W.; Lee, H.S.; Choi, S.R.; Song, Y.R.; Kim, H.J.; Park, H.C.; Kim, S.G. The relationship between autophagy, increased neutrophil extracellular traps formation and endothelial dysfunction in chronic kidney disease. Clin. Immunol. 2018, 197, 189–197. [Google Scholar] [CrossRef]
- Deretic, V.; Kimura, T.; Timmins, G.; Moseley, P.; Chauhan, S.; Mandell, M. Immunologic manifestations of autophagy. J. Clin. Invest. 2015, 125, 75–84. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Shi, C.S.; Shenderov, K.; Huang, N.N.; Kabat, J.; Abu-Asab, M.; Fitzgerald, K.A.; Sher, A.; Kehrl, J.H. Activation of autophagy by inflammatory signals limits IL-1beta production by targeting ubiquitinated inflammasomes for destruction. Nat. Immunol. 2012, 13, 255–263. [Google Scholar] [CrossRef]
- Martinez, J.; Cunha, L.D.; Park, S.; Yang, M.; Lu, Q.; Orchard, R.; Li, Q.Z.; Yan, M.; Janke, L.; Guy, C.; et al. Noncanonical autophagy inhibits the autoinflammatory, lupus-like response to dying cells. Nature 2016, 533, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.K.; Lund, J.M.; Ramanathan, B.; Mizushima, N.; Iwasaki, A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science 2007, 315, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.A.; Wu, V.C.; Wang, C.Y. Autophagy in Chronic Kidney Diseases. Cells 2019, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Fougeray, S.; Pallet, N. Mechanisms and biological functions of autophagy in diseased and ageing kidneys. Nat. Rev. Nephrol. 2015, 11, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tanaka, A.; Choi, A.M.; Ryter, S.W. Autophagic proteins: New facets of the oxygen paradox. Autophagy 2012, 8, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.T.; Hung, K.C.; Wen, M.S.; Hsu, P.Y.; Chen, T.H.; Wang, H.D.; Fang, J.T.; Shie, S.S.; Wang, C.Y. Impaired leukocytes autophagy in chronic kidney disease patients. Cardiorenal. Med. 2013, 3, 254–264. [Google Scholar] [CrossRef]
- Ren, C.; Zhang, H.; Wu, T.T.; Yao, Y.M. Autophagy: A Potential Therapeutic Target for Reversing Sepsis-Induced Immunosuppression. Front. Immunol. 2017, 8, 1832. [Google Scholar] [CrossRef]
- Tecklenborg, J.; Clayton, D.; Siebert, S.; Coley, S.M. The role of the immune system in kidney disease. Clin. Exp. Immunol. 2018, 192, 142–150. [Google Scholar] [CrossRef]
- Willows, J.; Brown, M.; Sheerin, N.S. The role of complement in kidney disease. Clin. Med. (Lond.) 2020, 20, 156–160. [Google Scholar] [CrossRef]
- Riedl, M.; Noone, D.G.; Khan, M.A.; Pluthero, F.G.; Kahr, W.H.A.; Palaniyar, N.; Licht, C. Complement Activation Induces Neutrophil Adhesion and Neutrophil-Platelet Aggregate Formation on Vascular Endothelial Cells. Kidney Int. Rep. 2017, 2, 66–75. [Google Scholar] [CrossRef]
- Thurman, J.M. Complement in kidney disease: Core curriculum 2015. Am. J. Kidney Dis. 2015, 65, 156–168. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.W.; Renner, B.; Thurman, J.M. The role of the complement system in acute kidney injury. Semin. Nephrol. 2013, 33, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Franzin, R.; Stasi, A.; Fiorentino, M.; Stallone, G.; Cantaluppi, V.; Gesualdo, L.; Castellano, G. Inflammaging and Complement System: A Link Between Acute Kidney Injury and Chronic Graft Damage. Front. Immunol. 2020, 11, 734. [Google Scholar] [CrossRef] [PubMed]
- De Borst, M.H. Interaction between inflammation, mineral metabolism and the renin-angiotensin system: Implications for cardiorenal outcomes in chronic kidney disease. Nephrol. Dial. Transplant. 2019, 34, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Crowley, S.D.; Rudemiller, N.P. Immunologic Effects of the Renin-Angiotensin System. J. Am. Soc. Nephrol. 2017, 28, 1350–1361. [Google Scholar] [CrossRef]
- Trojanowicz, B.; Ulrich, C.; Kohler, F.; Bode, V.; Seibert, E.; Fiedler, R.; Girndt, M. Monocytic angiotensin-converting enzyme 2 relates to atherosclerosis in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2017, 32, 287–298. [Google Scholar] [CrossRef][Green Version]
- Ulrich, C.; Heine, G.H.; Garcia, P.; Reichart, B.; Georg, T.; Krause, M.; Kohler, H.; Girndt, M. Increased expression of monocytic angiotensin-converting enzyme in dialysis patients with cardiovascular disease. Nephrol. Dial. Transplant. 2006, 21, 1596–1602. [Google Scholar] [CrossRef]
- Trojanowicz, B.; Imdahl, T.; Ulrich, C.; Fiedler, R.; Girndt, M. Circulating miR-421 Targeting Leucocytic Angiotensin Converting Enzyme 2 Is Elevated in Patients with Chronic Kidney Disease. Nephron 2019, 141, 61–74. [Google Scholar] [CrossRef]
- Jurewicz, M.; McDermott, D.H.; Sechler, J.M.; Tinckam, K.; Takakura, A.; Carpenter, C.B.; Milford, E.; Abdi, R. Human T and natural killer cells possess a functional renin-angiotensin system: Further mechanisms of angiotensin II-induced inflammation. J. Am. Soc. Nephrol. 2007, 18, 1093–1102. [Google Scholar] [CrossRef]
- El Bekay, R.; Alvarez, M.; Monteseirin, J.; Alba, G.; Chacon, P.; Vega, A.; Martin-Nieto, J.; Jimenez, J.; Pintado, E.; Bedoya, F.J.; et al. Oxidative stress is a critical mediator of the angiotensin II signal in human neutrophils: Involvement of mitogen-activated protein kinase, calcineurin, and the transcription factor NF-kappaB. Blood 2003, 102, 662–671. [Google Scholar] [CrossRef]
- Martinez, F.; Pallet, N. When erythropoietin meddles in immune affairs. J. Am. Soc. Nephrol. 2014, 25, 1887–1889. [Google Scholar] [CrossRef][Green Version]
- Gavish, R.; Watad, S.; Ben-Califa, N.; Goldberg, O.J.; Haskin, O.; Davidovits, M.; Koren, G.; Falush, Y.; Neumann, D.; Krause, I. Response to erythropoietin in pediatric patients with chronic kidney disease: Insights from an in vitro bioassay. Pediatr. Nephrol. 2018, 33, 2123–2129. [Google Scholar] [CrossRef] [PubMed]
- Eschbach, J.W.; Egrie, J.C.; Downing, M.R.; Browne, J.K.; Adamson, J.W. Correction of the anemia of end-stage renal disease with recombinant human erythropoietin. Results of a combined phase I and II clinical trial. N. Engl. J. Med. 1987, 316, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Macdougall, I.C.; Cooper, A.C. Erythropoietin resistance: The role of inflammation and pro-inflammatory cytokines. Nephrol. Dial. Transplant. 2002, 17 (Suppl. 11), 39–43. [Google Scholar] [CrossRef]
- Patruta, S.I.; Hörl, W.H. Iron and infection. Kidney Int. Suppl. 1999, 69, S125–S130. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.L.; Lin, Y.S.; Lu, Y.A.; Lee, H.F.; Lee, C.C.; Tung, Y.C.; Kuo, G.; Wu, L.S.; Tian, Y.C.; Chu, P.H. Intravenous iron supplementation does not increase infectious disease risk in hemodialysis patients: A nationwide cohort-based case-crossover study. BMC Nephrol. 2019, 20, 327. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Jasiulewicz, A.; Bryl, E.; Witkowski, J.M. Erythropoietin as an Immunomodulating Agent. Nephro-Urol Mon. 2011, 3, 208–212. [Google Scholar]
- Sela, S.; Shurtz-Swirski, R.; Sharon, R.; Manaster, J.; Chezar, J.; Shkolnik, G.; Shapiro, G.; Shasha, S.M.; Merchav, S.; Kristal, B. The polymorphonuclear leukocyte--a new target for erythropoietin. Nephron 2001, 88, 205–210. [Google Scholar] [CrossRef]
- Lisowska, K.A.; Debska-Slizien, A.; Bryl, E.; Rutkowski, B.; Witkowski, J.M. Erythropoietin receptor is expressed on human peripheral blood T and B lymphocytes and monocytes and is modulated by recombinant human erythropoietin treatment. Artif. Organs 2010, 34, 654–662. [Google Scholar] [CrossRef]
- Rocchetta, F.; Solini, S.; Mister, M.; Mele, C.; Cassis, P.; Noris, M.; Remuzzi, G.; Aiello, S. Erythropoietin enhances immunostimulatory properties of immature dendritic cells. Clin. Exp. Immunol. 2011, 165, 202–210. [Google Scholar] [CrossRef]
- Cravedi, P.; Manrique, J.; Hanlon, K.E.; Reid-Adam, J.; Brody, J.; Prathuangsuk, P.; Mehrotra, A.; Heeger, P.S. Immunosuppressive effects of erythropoietin on human alloreactive T cells. J. Am. Soc. Nephrol. 2014, 25, 2003–2015. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Latunde-Dada, G.O. Anemia of Inflammation with An Emphasis on Chronic Kidney Disease. Nutrients 2019, 11, 2424. [Google Scholar] [CrossRef] [PubMed]
- Atanasiu, V.; Manolescu, B.; Stoian, I. Hepcidin the link between inflammation and anemia in chronic renal failure. Rom. J. Intern. Med. 2006, 44, 25–33. [Google Scholar] [PubMed]
- Deicher, R.; Horl, W.H. New insights into the regulation of iron homeostasis. Eur. J. Clin. Invest. 2006, 36, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Saneela, S.; Iqbal, R.; Raza, A.; Qamar, M.F. Hepcidin: A key regulator of iron. J. Pak. Med. Assoc. 2019, 69, 1170–1175. [Google Scholar] [PubMed]
- Ashby, D.R.; Gale, D.P.; Busbridge, M.; Murphy, K.G.; Duncan, N.D.; Cairns, T.D.; Taube, D.H.; Bloom, S.R.; Tam, F.W.; Chapman, R.S.; et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009, 75, 976–981. [Google Scholar] [CrossRef]
- Lee, S.W.; Kim, J.M.; Lim, H.J.; Hwang, Y.H.; Kim, S.W.; Chung, W.; Oh, K.H.; Ahn, C.; Lee, K.B.; Sung, S.A. Serum hepcidin may be a novel uremic toxin, which might be related to erythropoietin resistance. Sci. Rep. 2017, 7, 4260. [Google Scholar] [CrossRef]
- Reichel, H.; Recker, A.; Deppisch, R.; Stier, E.; Ritz, E. 25-Hydroxyvitamin D3 metabolism in vitro by mononuclear cells from hemodialysis patients. Nephron 1992, 62, 404–412. [Google Scholar] [CrossRef]
- Schomig, M.; Ritz, E. Management of disturbed calcium metabolism in uraemic patients: 1. Use of vitamin D metabolites. Nephrol. Dial. Transplant. 2000, 15 (Suppl. 5), 18–24. [Google Scholar] [CrossRef]
- Glorieux, G.; Vanholder, R. Blunted response to vitamin D in uremia. Kidney Int Suppl 2001, 78, S182–S185. [Google Scholar] [CrossRef]
- Lang, C.L.; Wang, M.H.; Chiang, C.K.; Lu, K.C. Vitamin D and the Immune System from the Nephrologist’s Viewpoint. ISRN Endocrinol. 2014, 2014, 105456. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.; Wan, M.; Rees, L. Can vitamin D slow down the progression of chronic kidney disease? Pediatr. Nephrol. 2011, 27, 2167–2173. [Google Scholar] [CrossRef] [PubMed]
- Szeto, F.L.; Reardon, C.A.; Yoon, D.; Wang, Y.; Wong, K.E.; Chen, Y.; Kong, J.; Liu, S.Q.; Thadhani, R.; Getz, G.S.; et al. Vitamin d receptor signaling inhibits atherosclerosis in mice. Mol. Endocrinol. 2012, 26, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.T.G.; Schneider, M.; Cuppari, L.; Grabulosa, C.C.; Danilo, T.A.; Redublo, B.M.Q.; Marcelo, C.B.; Cendoroglo, M.; Maria Moyses, R.; Dalboni, M.A. Cholecalciferol decreases inflammation and improves vitamin D regulatory enzymes in lymphocytes in the uremic environment: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0179540. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, J.; Kumar, J. Vitamin D and cardiovascular disease in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 2509–2522. [Google Scholar] [CrossRef]
- Wahl, P.; Wolf, M. FGF23 in chronic kidney disease. Adv. Exp. Med. Biol. 2012, 728, 107–125. [Google Scholar]
- Vogt, I.; Haffner, D.; Leifheit-Nestler, M. FGF23 and Phosphate-Cardiovascular Toxins in CKD. Toxins (Basel) 2019, 11, 647. [Google Scholar] [CrossRef]
- Czaya, B.; Faul, C. The Role of Fibroblast Growth Factor 23 in Inflammation and Anemia. Int. J. Mol. Sci. 2019, 20, 4195. [Google Scholar] [CrossRef]
- Isakova, T.; Cai, X.; Lee, J.; Xie, D.; Wang, X.; Mehta, R.; Allen, N.B.; Scialla, J.J.; Pencina, M.J.; Anderson, A.H.; et al. Longitudinal FGF23 Trajectories and Mortality in Patients with CKD. J. Am. Soc. Nephrol. 2018, 29, 579–590. [Google Scholar] [CrossRef]
- Pichler, G.; Haller, M.C.; Kainz, A.; Wolf, M.; Redon, J.; Oberbauer, R. Prognostic value of bone- and vascular-derived molecular biomarkers in hemodialysis and renal transplant patients: A systematic review and meta-analysis. Nephrol. Dial. Transplant. 2017, 32, 1566–1578. [Google Scholar] [CrossRef]
- Takashi, Y.; Wakino, S.; Minakuchi, H.; Ishizu, M.; Kuroda, A.; Shima, H.; Tashiro, M.; Miya, K.; Okada, K.; Minakuchi, J.; et al. Circulating FGF23 is not associated with cardiac dysfunction, atherosclerosis, infection or inflammation in hemodialysis patients. J Bone Miner. Metab. 2020, 38, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Memmos, E.; Sarafidis, P.; Pateinakis, P.; Tsiantoulas, A.; Faitatzidou, D.; Giamalis, P.; Vasilikos, V.; Papagianni, A. Soluble Klotho is associated with mortality and cardiovascular events in hemodialysis. BMC Nephrol. 2019, 20, 217. [Google Scholar] [CrossRef]
- Block, G.A.; Kilpatrick, R.D.; Lowe, K.A.; Wang, W.; Danese, M.D. CKD-mineral and bone disorder and risk of death and cardiovascular hospitalization in patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 2132–2140. [Google Scholar] [CrossRef] [PubMed]
- Duque, E.J.; Elias, R.M.; Moyses, R.M.A. Parathyroid Hormone: A Uremic Toxin. Toxins (Basel) 2020, 12, 189. [Google Scholar] [CrossRef]
- Deicher, R.; Kirsch, B.; Mullner, M.; Kaczirek, K.; Niederle, B.; Horl, W.H. Impact of parathyroidectomy on neutrophil cytosolic calcium in chronic kidney disease patients: A prospective parallel group trial. J. Intern. Med. 2005, 258, 67–76. [Google Scholar] [CrossRef]
- Smogorzewski, M.; Massry, S.G. Defects in B-cell function and metabolism in uremia: Role of parathyroid hormone. Kidney Int. Suppl. 2001, 78, S186–S189. [Google Scholar] [CrossRef] [PubMed]
- Griveas, I.; Visvardis, G.; Papadopoulou, D.; Mitsopoulos, E.; Kyriklidou, P.; Manou, E.; Meimaridou, D.; Ginikopoulou, E.; Sakellariou, G.; Fleva, A.; et al. Cellular immunity and levels of parathyroid hormone in uremic patients receiving hemodialysis. Ren. Fail. 2005, 27, 275–278. [Google Scholar] [CrossRef]
- Vanholder, R.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; Descamps-Latscha, B.; Henle, T.; et al. Uremic toxicity: Present state of the art. Int. J. Artif. Organs 2001, 24, 695–725. [Google Scholar] [CrossRef] [PubMed]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argiles, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Schepers, E.; Glorieux, G.; Vanholder, R. The gut: The forgotten organ in uremia? Blood Purif. 2010, 29, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Meijers, B.; Glorieux, G.; Poesen, R.; Bakker, S.J. Nonextracorporeal methods for decreasing uremic solute concentration: A future way to go? Semin. Nephrol. 2014, 34, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Gryp, T.; Perna, A. Gut-Derived Metabolites and Their Role in Immune Dysfunction in Chronic Kidney Disease. Toxins (Basel) 2020, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Glorieux, G.; Zacchia, M.; Trepiccione, F.; Capolongo, G.; Vigorito, C.; Anishchenko, E.; Ingrosso, D. The role of the intestinal microbiota in uremic solute accumulation: A focus on sulfur compounds. J. Nephrol. 2019, 32, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.L.; Elson, C.O.; Hatton, R.D.; Weaver, C.T. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012, 489, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, S.; Li, S.; Zhao, L.; Hao, Y.; Qin, J.; Zhang, L.; Zhang, C.; Bian, W.; Zuo, L.; et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut 2020. [Google Scholar] [CrossRef]
- Ramezani, A.; Massy, Z.A.; Meijers, B.; Evenepoel, P.; Vanholder, R.; Raj, D.S. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. Am. J. Kidney Dis. 2016, 67, 483–498. [Google Scholar] [CrossRef]
- Popkov, V.A.; Silachev, D.N.; Zalevsky, A.O.; Zorov, D.B.; Plotnikov, E.Y. Mitochondria as a Source and a Target for Uremic Toxins. Int. J. Mol. Sci. 2019, 20, 3094. [Google Scholar] [CrossRef]
- Neirynck, N.; Vanholder, R.; Schepers, E.; Eloot, S.; Pletinck, A.; Glorieux, G. An update on uremic toxins. Int. Urol. Nephrol. 2013, 45, 139–150. [Google Scholar] [CrossRef]
- Vanholder, R.; Pletinck, A.; Schepers, E.; Glorieux, G. Biochemical and Clinical Impact of Organic Uremic Retention Solutes: A Comprehensive Update. Toxins (Basel) 2018, 10, 33. [Google Scholar] [CrossRef]
- Rroji, M.; Eloot, S.; Dhondt, A.; Van Biesen, W.; Glorieux, G.; Neirynck, N.; Vandennoortgate, N.; Liabeuf, S.; Massy, Z.; Vanholder, R. Association of advanced age with concentrations of uraemic toxins in CKD. J. Nephrol. 2016, 29, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Espi, M.; Koppe, L.; Fouque, D.; Thaunat, O. Chronic Kidney Disease-Associated Immune Dysfunctions: Impact of Protein-Bound Uremic Retention Solutes on Immune Cells. Toxins (Basel) 2020, 12, 300. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoo, T.H.; Hwang, Y.; Lee, G.H.; Kim, B.; Jang, J.; Yu, H.T.; Kim, M.C.; Cho, J.Y.; Lee, C.J.; et al. Indoxyl sulfate (IS)-mediated immune dysfunction provokes endothelial damage in patients with end-stage renal disease (ESRD). Sci. Rep. 2017, 7, 3057. [Google Scholar] [CrossRef]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; Van Landschoot, M.; Eloot, S.; Rops, A.; Van de Voorde, J.; De Vriese, A.; et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J. Am. Soc. Nephrol. 2013, 24, 1981–1994. [Google Scholar] [CrossRef]
- Schepers, E.; Meert, N.; Glorieux, G.; Goeman, J.; Van der Eycken, J.; Vanholder, R. P-cresylsulphate, the main in vivo metabolite of p-cresol, activates leucocyte free radical production. Nephrol. Dial. Transplant. 2007, 22, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.L.; Bonan, N.B.; Dias, G.; Brehm, F.; Steiner, T.M.; Souza, W.M.; Stinghen, A.E.; Barreto, F.C.; Elifio-Esposito, S.; Pecoits-Filho, R.; et al. p-Cresyl sulfate affects the oxidative burst, phagocytosis process, and antigen presentation of monocyte-derived macrophages. Toxicol. Lett. 2016, 263, 1–5. [Google Scholar] [CrossRef]
- Pawlak, K.; Domaniewski, T.; Mysliwiec, M.; Pawlak, D. The kynurenines are associated with oxidative stress, inflammation and the prevalence of cardiovascular disease in patients with end-stage renal disease. Atherosclerosis 2009, 204, 309–314. [Google Scholar] [CrossRef]
- Barth, M.C.; Ahluwalia, N.; Anderson, T.J.; Hardy, G.J.; Sinha, S.; Alvarez-Cardona, J.A.; Pruitt, I.E.; Rhee, E.P.; Colvin, R.A.; Gerszten, R.E. Kynurenic acid triggers firm arrest of leukocytes to vascular endothelium under flow conditions. J. Biol. Chem. 2009, 284, 19189–19195. [Google Scholar] [CrossRef]
- Schepers, E.; Glorieux, G.; Dou, L.; Cerini, C.; Gayrard, N.; Louvet, L.; Maugard, C.; Preus, P.; Rodriguez-Ortiz, M.; Argiles, A.; et al. Guanidino compounds as cause of cardiovascular damage in chronic kidney disease: An in vitro evaluation. Blood Purif. 2010, 30, 277–287. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Toxic Dimethylarginines: Asymmetric Dimethylarginine (ADMA) and Symmetric Dimethylarginine (SDMA). Toxins (Basel) 2017, 9, 92. [Google Scholar] [CrossRef]
- Shafi, T.; Hostetter, T.H.; Meyer, T.W.; Hwang, S.; Hai, X.; Melamed, M.L.; Banerjee, T.; Coresh, J.; Powe, N.R. Serum Asymmetric and Symmetric Dimethylarginine and Morbidity and Mortality in Hemodialysis Patients. Am. J. Kidney Dis. 2017, 70, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Glorieux, G.; Hsu, C.H.; de Smet, R.; Dhondt, A.; van Kaer, J.; Vogeleere, P.; Lameire, N.; Vanholder, R. Inhibition of calcitriol-induced monocyte CD14 expression by uremic toxins: Role of purines. J. Am. Soc. Nephrol. 1998, 9, 1826–1831. [Google Scholar] [PubMed]
- Cohen, G.; Raupachova, J.; Horl, W.H. The uraemic toxin phenylacetic acid contributes to inflammation by priming polymorphonuclear leucocytes. Nephrol. Dial. Transplant. 2013, 28, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Schepers, E.; Glorieux, G.; Jankowski, V.; Dhondt, A.; Jankowski, J.; Vanholder, R. Dinucleoside polyphosphates: Newly detected uraemic compounds with an impact on leucocyte oxidative burst. Nephrol. Dial. Transplant. 2010, 25, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Okado, A.; Kawasaki, Y.; Hasuike, Y.; Takahashi, M.; Teshima, T.; Fujii, J.; Taniguchi, N. Induction of apoptotic cell death by methylglyoxal and 3-deoxyglucosone in macrophage-derived cell lines. Biochem. Biophys. Res. Commun. 1996, 225, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nakayama, K.; Zhu, W.J.; Shirota, Y.; Terawaki, H.; Sato, T.; Kohno, M.; Ito, S. Polymorphonuclear leukocyte injury by methylglyoxal and hydrogen peroxide: A possible pathological role for enhanced oxidative stress in chronic kidney disease. Nephrol. Dial. Transplant. 2008, 23, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Hannam-Harris, A.C.; Gordon, J.; Smith, J.L. Immunoglobulin synthesis by neoplastic B lymphocytes: Free light chain synthesis as a marker of B cell differentiation. J. Immunol. 1980, 125, 2177–2181. [Google Scholar]
- Hutchison, C.A.; Harding, S.; Hewins, P.; Mead, G.P.; Townsend, J.; Bradwell, A.R.; Cockwell, P. Quantitative assessment of serum and urinary polyclonal free light chains in patients with chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1684–1690. [Google Scholar] [CrossRef]
- Cohen, G.; Rudnicki, M.; Schmaldienst, S.; Horl, W.H. Effect of dialysis on serum/plasma levels of free immunoglobulin light chains in end-stage renal disease patients. Nephrol. Dial. Transplant. 2002, 17, 879–883. [Google Scholar] [CrossRef]
- Hutchison, C.A.; Cockwell, P.; Reid, S.; Chandler, K.; Mead, G.P.; Harrison, J.; Hattersley, J.; Evans, N.D.; Chappell, M.J.; Cook, M.; et al. Efficient removal of immunoglobulin free light chains by hemodialysis for multiple myeloma: In vitro and in vivo studies. J. Am. Soc. Nephrol. 2007, 18, 886–895. [Google Scholar] [CrossRef]
- Kirsch, A.H.; Lyko, R.; Nilsson, L.G.; Beck, W.; Amdahl, M.; Lechner, P.; Schneider, A.; Wanner, C.; Rosenkranz, A.R.; Krieter, D.H. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol. Dial. Transplant. 2017, 32, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Haag-Weber, M.; Mai, B.; Deicher, R.; Horl, W.H. Effect of immunoglobulin light chains from hemodialysis and continuous ambulatory peritoneal dialysis patients on polymorphonuclear leukocyte functions. J. Am. Soc. Nephrol. 1995, 6, 1592–1599. [Google Scholar] [PubMed]
- Cohen, G.; Rudnicki, M.; Deicher, R.; Horl, W.H. Immunoglobulin light chains modulate polymorphonuclear leucocyte apoptosis. Eur. J. Clin. Invest. 2003, 33, 669–676. [Google Scholar] [CrossRef]
- Frey, S.K.; Nagl, B.; Henze, A.; Raila, J.; Schlosser, B.; Berg, T.; Tepel, M.; Zidek, W.; Weickert, M.O.; Pfeiffer, A.F.; et al. Isoforms of retinol binding protein 4 (RBP4) are increased in chronic diseases of the kidney but not of the liver. Lipids Health Dis. 2008, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Hsieh, T.J.; Lin, K.D.; Lin, H.Y.; Lee, M.Y.; Hung, W.W.; Hsiao, P.J.; Shin, S.J. Increased unbound retinol-binding protein 4 concentration induces apoptosis through receptor-mediated signaling. J. Biol. Chem. 2012, 287, 9694–9707. [Google Scholar] [CrossRef] [PubMed]
- Farjo, K.M.; Farjo, R.A.; Halsey, S.; Moiseyev, G.; Ma, J.X. Retinol-binding protein 4 induces inflammation in human endothelial cells by an NADPH oxidase- and nuclear factor kappa B-dependent and retinol-independent mechanism. Mol. Cell. Biol. 2012, 32, 5103–5115. [Google Scholar] [CrossRef]
- Cohen, G.; Horl, W.H. Retinol binding protein isolated from acute renal failure patients inhibits polymorphonuclear leucocyte functions. Eur. J. Clin. Invest. 2004, 34, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Barke, R.A.; Ma, J.; Charboneau, R.; Roy, S. Opiate abuse, innate immunity, and bacterial infectious diseases. Arch. Immunol. Ther. Exp. (Warsz) 2008, 56, 299–309. [Google Scholar] [CrossRef]
- Zoccali, C.; Ciccarelli, M.; Mallamaci, F.; Maggiore, Q.; Lotti, M.; Zucchelli, G.C. Plasma met-enkephalin and leu-enkephalin in chronic renal failure. Nephrol. Dial. Transplant. 1987, 1, 219–222. [Google Scholar]
- Pasnik, J.; Tchorzewski, H.; Baj, Z.; Luciak, M.; Tchorzewski, M. Priming effect of met-enkephalin and beta-endorphin on chemiluminescence, chemotaxis and CD11b molecule expression on human neutrophils in vitro. Immunol. Lett. 1999, 67, 77–83. [Google Scholar] [CrossRef]
- Wieder, L. Effects of Met-Enkephalin, Neuropeptide-Y and Endothelin-1 on Essential Functions of Polymorphonuclear Leukocytes (PMNLs). Ph.D. Thesis, Medical University of Vienna, Vienna, Austria, 2018. [Google Scholar]
- Deshmukh, S.; Phillips, B.G.; O’Dorisio, T.; Flanigan, M.J.; Lim, V.S. Hormonal responses to fasting and refeeding in chronic renal failure patients. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E47–E55. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Tripepi, G.; Benedetto, F.A.; Parlongo, S.; Cutrupi, S.; Iellamo, D.; Bonanno, G.; Rapisarda, F.; Fatuzzo, P.; et al. Prospective study of neuropeptide y as an adverse cardiovascular risk factor in end-stage renal disease. J. Am. Soc. Nephrol. 2003, 14, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Reichmann, F.; Holzer, P. The homeostatic role of neuropeptide Y in immune function and its impact on mood and behaviour. Acta Physiol. (Oxf.) 2015, 213, 603–627. [Google Scholar] [CrossRef] [PubMed]
- Bedoui, S.; Kromer, A.; Gebhardt, T.; Jacobs, R.; Raber, K.; Dimitrijevic, M.; Heine, J.; von Horsten, S. Neuropeptide Y receptor-specifically modulates human neutrophil function. J. Neuroimmunol. 2008, 195, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Dimitrijevic, M.; Stanojevic, S. The intriguing mission of neuropeptide Y in the immune system. Amino Acids 2013, 45, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Deray, G.; Carayon, A.; Maistre, G.; Benhmida, M.; Masson, F.; Barthelemy, C.; Petitclerc, T.; Jacobs, C. Endothelin in chronic renal failure. Nephrol. Dial. Transplant. 1992, 7, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Zarpelon, A.C.; Pinto, L.G.; Cunha, T.M.; Vieira, S.M.; Carregaro, V.; Souza, G.R.; Silva, J.S.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Endothelin-1 induces neutrophil recruitment in adaptive inflammation via TNFalpha and CXCL1/CXCR2 in mice. Can. J. Physiol. Pharmacol. 2012, 90, 187–199. [Google Scholar] [CrossRef]
- Freeman, B.D.; Machado, F.S.; Tanowitz, H.B.; Desruisseaux, M.S. Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 2014, 118, 110–119. [Google Scholar] [CrossRef]
- Zouki, C.; Baron, C.; Fournier, A.; Filep, J.G. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: Role of ET(A) receptors and platelet-activating factor. Br. J. Pharmacol. 1999, 127, 969–979. [Google Scholar] [CrossRef]
- Sessa, W.C.; Kaw, S.; Hecker, M.; Vane, J.R. The biosynthesis of endothelin-1 by human polymorphonuclear leukocytes. Biochem. Biophys. Res. Commun. 1991, 174, 613–618. [Google Scholar] [CrossRef]
- Mencarelli, M.; Pecorelli, A.; Carbotti, P.; Valacchi, G.; Grasso, G.; Muscettola, M. Endothelin receptor A expression in human inflammatory cells. Regul. Pept. 2009, 158, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ishida, K.; Takeshige, K.; Minakami, S. Endothelin-1 enhances superoxide generation of human neutrophils stimulated by the chemotactic peptide N-formyl-methionyl-leucyl-phenylalanine. Biochem. Biophys. Res. Commun. 1990, 173, 496–500. [Google Scholar] [CrossRef]
- Teta, D. Adipokines as uremic toxins. J. Ren. Nutr. 2012, 22, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Kopp, A.; Buechler, C.; Neumeier, M.; Weigert, J.; Aslanidis, C.; Scholmerich, J.; Schaffler, A. Innate immunity and adipocyte function: Ligand-specific activation of multiple Toll-like receptors modulates cytokine, adipokine, and chemokine secretion in adipocytes. Obes. (Silver Spring) 2009, 17, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, S. The complex effects of adipokines in the patients with kidney disease. J. Res. Med. Sci. 2018, 23, 60. [Google Scholar] [CrossRef]
- Zhu, Q.; Scherer, P.E. Immunologic and endocrine functions of adipose tissue: Implications for kidney disease. Nat. Rev. Nephrol. 2018, 14, 105–120. [Google Scholar] [CrossRef]
- Chan, C.C.; Damen, M.; Alarcon, P.C.; Sanchez-Gurmaches, J.; Divanovic, S. Inflammation and Immunity: From an Adipocyte’s Perspective. J. Interferon Cytokine Res. 2019, 39, 459–471. [Google Scholar] [CrossRef]
- Aminzadeh, M.A.; Pahl, M.V.; Barton, C.H.; Doctor, N.S.; Vaziri, N.D. Human uraemic plasma stimulates release of leptin and uptake of tumour necrosis factor-alpha in visceral adipocytes. Nephrol. Dial. Transplant. 2009, 24, 3626–3631. [Google Scholar] [CrossRef]
- Alix, P.M.; Guebre-Egziabher, F.; Soulage, C.O. Leptin as an uremic toxin: Deleterious role of leptin in chronic kidney disease. Biochimie 2014, 105, 12–21. [Google Scholar] [CrossRef]
- Peng, D.Z.; Liu, X.W.; Huang, L.; Zhu, X.F.; Zheng, Y.Q.; Wang, L.X. Relationship between leptin and chronic inflammatory state in uremic patients. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2882–2885. [Google Scholar]
- Cohen, G.; Raupachova, J.; Ilic, D.; Werzowa, J.; Horl, W.H. Effect of leptin on polymorphonuclear leucocyte functions in healthy subjects and haemodialysis patients. Nephrol. Dial. Transplant. 2011, 26, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Curat, C.A.; Wegner, V.; Sengenes, C.; Miranville, A.; Tonus, C.; Busse, R.; Bouloumie, A. Macrophages in human visceral adipose tissue: Increased accumulation in obesity and a source of resistin and visfatin. Diabetologia 2006, 49, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Kunnari, A.M.; Savolainen, E.R.; Ukkola, O.H.; Kesaniemi, Y.A.; Jokela, M.A. The expression of human resistin in different leucocyte lineages is modulated by LPS and TNFalpha. Regul. Pept. 2009, 157, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.; Singbartl, K.; Chroneos, Z.C.; Ruiz-Velasco, V.; Lang, C.H.; Bonavia, A. Resistin directly inhibits bacterial killing in neutrophils. Intensive Care Med. Exp. 2019, 7, 30. [Google Scholar] [CrossRef]
- Cohen, G.; Ilic, D.; Raupachova, J.; Hörl, W.H. Resistin inhibits essential functions of polymorphonuclear leukocytes. J. Immunol. 2008, 181, 3761–3768. [Google Scholar] [CrossRef]
- Bostrom, E.A.; Tarkowski, A.; Bokarewa, M. Resistin is stored in neutrophil granules being released upon challenge with inflammatory stimuli. Biochim. Biophys. Acta 2009, 1793, 1894–1900. [Google Scholar] [CrossRef]
- Walcher, D.; Hess, K.; Berger, R.; Aleksic, M.; Heinz, P.; Bach, H.; Durst, R.; Hausauer, A.; Hombach, V.; Marx, N. Resistin: A newly identified chemokine for human CD4-positive lymphocytes. Cardiovasc. Res. 2010, 85, 167–174. [Google Scholar] [CrossRef]
- Delanghe, S.; Delanghe, J.R.; Speeckaert, R.; Van Biesen, W.; Speeckaert, M.M. Mechanisms and consequences of carbamoylation. Nat. Rev. Nephrol. 2017, 13, 580–593. [Google Scholar] [CrossRef]
- Koeth, R.A.; Kalantar-Zadeh, K.; Wang, Z.; Fu, X.; Tang, W.H.; Hazen, S.L. Protein carbamylation predicts mortality in ESRD. J. Am. Soc. Nephrol. 2013, 24, 853–861. [Google Scholar] [CrossRef]
- Kraus, L.M.; Elberger, A.J.; Handorf, C.R.; Pabst, M.J.; Kraus, A.P., Jr. Urea-derived cyanate forms epsilon-amino-carbamoyl-lysine (homocitrulline) in leukocyte proteins in patients with end-stage renal disease on peritoneal dialysis. J. Lab. Clin. Med. 1994, 123, 882–891. [Google Scholar]
- Jaisson, S.; Lorimier, S.; Ricard-Blum, S.; Sockalingum, G.D.; Delevallee-Forte, C.; Kegelaer, G.; Manfait, M.; Garnotel, R.; Gillery, P. Impact of carbamylation on type I collagen conformational structure and its ability to activate human polymorphonuclear neutrophils. Chem. Biol. 2006, 13, 149–159. [Google Scholar] [CrossRef]
- Pavone, B.; Sirolli, V.; Giardinelli, A.; Bucci, S.; Forli, F.; Di Cesare, M.; Sacchetta, P.; Di Pietro, N.; Pandolfi, A.; Urbani, A.; et al. Plasma protein carbonylation in chronic uremia. J. Nephrol. 2011, 24, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Makita, Z.; Radoff, S.; Rayfield, E.J.; Yang, Z.; Skolnik, E.; Delaney, V.; Friedman, E.A.; Cerami, A.; Vlassara, H. Advanced glycosylation end products in patients with diabetic nephropathy. N. Engl. J. Med. 1991, 325, 836–842. [Google Scholar] [CrossRef] [PubMed]
- Stinghen, A.E.; Massy, Z.A.; Vlassara, H.; Striker, G.E.; Boullier, A. Uremic Toxicity of Advanced Glycation End Products in CKD. J. Am. Soc. Nephrol. 2016, 27, 354–370. [Google Scholar] [CrossRef] [PubMed]
- Kislinger, T.; Tanji, N.; Wendt, T.; Qu, W.; Lu, Y.; Ferran, L.J., Jr.; Taguchi, A.; Olson, K.; Bucciarelli, L.; Goova, M.; et al. Receptor for advanced glycation end products mediates inflammation and enhanced expression of tissue factor in vasculature of diabetic apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Rudnicki, M.; Walter, F.; Niwa, T.; Horl, W.H. Glucose-modified proteins modulate essential functions and apoptosis of polymorphonuclear leukocytes. J. Am. Soc. Nephrol. 2001, 12, 1264–1271. [Google Scholar]
- Glorieux, G.; Helling, R.; Henle, T.; Brunet, P.; Deppisch, R.; Lameire, N.; Vanholder, R. In vitro evidence for immune activating effect of specific AGE structures retained in uremia. Kidney Int. 2004, 66, 1873–1880. [Google Scholar] [CrossRef]
- Kirstein, M.; Brett, J.; Radoff, S.; Ogawa, S.; Stern, D.; Vlassara, H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: Role in vascular disease of diabetes and aging. Proc. Natl. Acad. Sci. USA 1990, 87, 9010–9014. [Google Scholar] [CrossRef]
- Rashid, G.; Korzets, Z.; Bernheim, J. Advanced glycation end products stimulate tumor necrosis factor-alpha and interleukin-1 beta secretion by peritoneal macrophages in patients on continuous ambulatory peritoneal dialysis. Isr. Med. Assoc. J. 2006, 8, 36–39. [Google Scholar]
- Toure, F.; Zahm, J.M.; Garnotel, R.; Lambert, E.; Bonnet, N.; Schmidt, A.M.; Vitry, F.; Chanard, J.; Gillery, P.; Rieu, P. Receptor for advanced glycation end-products (RAGE) modulates neutrophil adhesion and migration on glycoxidated extracellular matrix. Biochem. J. 2008, 416, 255–261. [Google Scholar] [CrossRef]
- D’Agati, V.; Schmidt, A.M. RAGE and the pathogenesis of chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Descamps-Latscha, B. Are advanced oxidation protein products potential uremic toxins? Kidney Int. Suppl. 2003, 63, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Capeillere-Blandin, C.; Gausson, V.; Nguyen, A.T.; Descamps-Latscha, B.; Drueke, T.; Witko-Sarsat, V. Respective role of uraemic toxins and myeloperoxidase in the uraemic state. Nephrol. Dial. Transplant. 2006, 21, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Körmöczi, G.F.; Wolfel, U.M.; Rosenkranz, A.R.; Hörl, W.H.; Oberbauer, R.; Zlabinger, G.J. Serum proteins modified by neutrophil-derived oxidants as mediators of neutrophil stimulation. J. Immunol. 2001, 167, 451–460. [Google Scholar] [CrossRef]
- Jurek, A.; Turyna, B.; Kubit, P.; Klein, A. The ability of HDL to inhibit VCAM-1 expression and oxidized LDL uptake is impaired in renal patients. Clin. Biochem. 2008, 41, 1015–1018. [Google Scholar] [CrossRef]
- Meier, P.; Golshayan, D.; Blanc, E.; Pascual, M.; Burnier, M. Oxidized LDL modulates apoptosis of regulatory T cells in patients with ESRD. J. Am. Soc. Nephrol. 2009, 20, 1368–1384. [Google Scholar] [CrossRef]
- Donadio, C.; Tognotti, D.; Donadio, E. Albumin modification and fragmentation in renal disease. Clin. Chim. Acta 2012, 413, 391–395. [Google Scholar] [CrossRef]
- Mera, K.; Anraku, M.; Kitamura, K.; Nakajou, K.; Maruyama, T.; Tomita, K.; Otagiri, M. Oxidation and carboxy methyl lysine-modification of albumin: Possible involvement in the progression of oxidative stress in hemodialysis patients. Hypertens Res. 2005, 28, 973–980. [Google Scholar] [CrossRef]
- Gordon, S.M.; Hofmann, S.; Askew, D.S.; Davidson, W.S. High density lipoprotein: it’s not just about lipid transport anymore. Trends Endocrinol. Metab. 2011, 22, 9–15. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Brewer, H.B., Jr.; Davidson, W.S.; Fayad, Z.A.; Fuster, V.; Goldstein, J.; Hellerstein, M.; Jiang, X.C.; Phillips, M.C.; Rader, D.J.; et al. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation 2012, 125, 1905–1919. [Google Scholar] [CrossRef]
- Navab, M.; Reddy, S.T.; Van Lenten, B.J.; Fogelman, A.M. HDL and cardiovascular disease: Atherogenic and atheroprotective mechanisms. Nat. Rev. Cardiol. 2011, 8, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Rye, K.A.; Barter, P.J. Cardioprotective functions of HDLs. J. Lipid Res. 2014, 55, 168–179. [Google Scholar] [CrossRef] [PubMed]
- Creasy, K.T.; Kane, J.P.; Malloy, M.J. Emerging roles of HDL in immune function. Curr. Opin. Lipidol. 2018, 29, 486–487. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Ammirati, E.; Catapano, A.L. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 2012, 220, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, G.; Wulf, G.; Bruning, T.; Assmann, G. Flow-cytometric determination of high-density-lipoprotein binding sites on human leukocytes. Clin. Chem. 1987, 33, 2195–2203. [Google Scholar] [CrossRef]
- Blackburn, W.D., Jr.; Dohlman, J.G.; Venkatachalapathi, Y.V.; Pillion, D.J.; Koopman, W.J.; Segrest, J.P.; Anantharamaiah, G.M. Apolipoprotein A-I decreases neutrophil degranulation and superoxide production. J. Lipid Res. 1991, 32, 1911–1918. [Google Scholar]
- Murphy, A.J.; Woollard, K.J.; Suhartoyo, A.; Stirzaker, R.A.; Shaw, J.; Sviridov, D.; Chin-Dusting, J.P. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1333–1341. [Google Scholar] [CrossRef]
- Murphy, A.J.; Woollard, K.J.; Hoang, A.; Mukhamedova, N.; Stirzaker, R.A.; McCormick, S.P.; Remaley, A.T.; Sviridov, D.; Chin-Dusting, J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 2071–2077. [Google Scholar] [CrossRef]
- Liao, X.L.; Lou, B.; Ma, J.; Wu, M.P. Neutrophils activation can be diminished by apolipoprotein A-I. Life Sci. 2005, 77, 325–335. [Google Scholar] [CrossRef]
- Westerterp, M.; Fotakis, P.; Ouimet, M.; Bochem, A.E.; Zhang, H.; Molusky, M.M.; Wang, W.; Abramowicz, S.; la Bastide-van Gemert, S.; Wang, N.; et al. Cholesterol Efflux Pathways Suppress Inflammasome Activation, NETosis, and Atherogenesis. Circulation 2018, 138, 898–912. [Google Scholar] [CrossRef]
- Spirig, R.; Schaub, A.; Kropf, A.; Miescher, S.; Spycher, M.O.; Rieben, R. Reconstituted high-density lipoprotein modulates activation of human leukocytes. PLoS ONE 2013, 8, e71235. [Google Scholar] [CrossRef] [PubMed]
- Pirillo, A.; Catapano, A.L.; Norata, G.D. Biological consequences of dysfunctional HDL. Curr. Med. Chem. 2018, 26, 1644–1664. [Google Scholar] [CrossRef] [PubMed]
- Marsche, G.; Saemann, M.D.; Heinemann, A.; Holzer, M. Inflammation alters HDL composition and function: Implications for HDL-raising therapies. Pharmacol. Ther. 2013, 137, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Saemann, M.D.; Poglitsch, M.; Kopecky, C.; Haidinger, M.; Horl, W.H.; Weichhart, T. The versatility of HDL: A crucial anti-inflammatory regulator. Eur. J. Clin. Invest. 2010, 40, 1131–1143. [Google Scholar] [CrossRef] [PubMed]
- Zewinger, S.; Kleber, M.E.; Rohrer, L.; Lehmann, M.; Triem, S.; Jennings, R.T.; Petrakis, I.; Dressel, A.; Lepper, P.M.; Scharnagl, H.; et al. Symmetric dimethylarginine, high-density lipoproteins and cardiovascular disease. Eur. Heart J. 2017, 38, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Kopecky, C.; Kubicek, M.; Haidinger, M.; Doller, D.; Katholnig, K.; Suarna, C.; Eller, P.; Tolle, M.; Gerner, C.; et al. Serum Amyloid A in Uremic HDL Promotes Inflammation. J. Am. Soc. Nephrol. 2012, 23, 934–947. [Google Scholar] [CrossRef] [PubMed]
- Florens, N.; Calzada, C.; Lyasko, E.; Juillard, L.; Soulage, C.O. Modified Lipids and Lipoproteins in Chronic Kidney Disease: A New Class of Uremic Toxins. Toxins (Basel) 2016, 8, 376. [Google Scholar] [CrossRef]
- Tolle, M.; Huang, T.; Schuchardt, M.; Jankowski, V.; Prufer, N.; Jankowski, J.; Tietge, U.J.; Zidek, W.; van der Giet, M. High-density lipoprotein loses its anti-inflammatory capacity by accumulation of pro-inflammatory-serum amyloid A. Cardiovasc. Res. 2012, 94, 154–162. [Google Scholar] [CrossRef]
- Raupachova, J.; Kopecky, C.; Cohen, G. High-Density Lipoprotein from Chronic Kidney Disease Patients Modulates Polymorphonuclear Leukocytes. Toxins (Basel) 2019, 11, 73. [Google Scholar] [CrossRef]
- Askari, H.; Seifi, B.; Kadkhodaee, M.; Sanadgol, N.; Elshiekh, M.; Ranjbaran, M.; Ahghari, P. Protective effects of hydrogen sulfide on chronic kidney disease by reducing oxidative stress, inflammation and apoptosis. EXCLI J. 2018, 17, 14–23. [Google Scholar]
- Cao, X.; Bian, J.S. The Role of Hydrogen Sulfide in Renal System. Front. Pharmacol. 2016, 7, 385. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Luciano, M.G.; Ingrosso, D.; Raiola, I.; Pulzella, P.; Sepe, I.; Lanza, D.; Violetti, E.; Capasso, R.; Lombardi, C.; et al. Hydrogen Sulfide, the Third Gaseous Signaling Molecule With Cardiovascular Properties, Is Decreased in Hemodialysis Patients. J. Renal Nutr. 2010, 20, S11–S14. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.F.; Sepe, I.; Lanza, D.; Ingrosso, D. Hydrogen sulfide increases after a single hemodialysis session. Kidney Int. 2011, 80, 1108–1109. [Google Scholar] [CrossRef] [PubMed]
- Vigorito, C.; Anishchenko, E.; Mele, L.; Capolongo, G.; Trepiccione, F.; Zacchia, M.; Lombari, P.; Capasso, R.; Ingrosso, D.; Perna, A.F. Uremic Toxin Lanthionine Interferes with the Transsulfuration Pathway, Angiogenetic Signaling and Increases Intracellular Calcium. Int. J. Mol. Sci. 2019, 20, 2269. [Google Scholar] [CrossRef] [PubMed]
- Lo Faro, M.L.; Fox, B.; Whatmore, J.L.; Winyard, P.G.; Whiteman, M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide 2014, 41, 38–47. [Google Scholar] [CrossRef]
- Collin, M.; Anuar, F.B.; Murch, O.; Bhatia, M.; Moore, P.K.; Thiemermann, C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br. J. Pharmacol. 2005, 146, 498–505. [Google Scholar] [CrossRef]
- Zhang, H.; Zhi, L.; Moochhala, S.; Moore, P.K.; Bhatia, M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L960–L971. [Google Scholar] [CrossRef]
- Mok, Y.Y.; Atan, M.S.; Yoke Ping, C.; Zhong Jing, W.; Bhatia, M.; Moochhala, S.; Moore, P.K. Role of hydrogen sulphide in haemorrhagic shock in the rat: Protective effect of inhibitors of hydrogen sulphide biosynthesis. Br. J. Pharmacol. 2004, 143, 881–889. [Google Scholar] [CrossRef]
- Rinaldi, L.; Gobbi, G.; Pambianco, M.; Micheloni, C.; Mirandola, P.; Vitale, M. Hydrogen sulfide prevents apoptosis of human PMN via inhibition of p38 and caspase 3. Lab. Invest. 2006, 86, 391–397. [Google Scholar] [CrossRef]
- Spiller, F.; Orrico, M.I.; Nascimento, D.C.; Czaikoski, P.G.; Souto, F.O.; Alves-Filho, J.C.; Freitas, A.; Carlos, D.; Montenegro, M.F.; Neto, A.F.; et al. Hydrogen sulfide improves neutrophil migration and survival in sepsis via K+ATP channel activation. Am. J. Respir. Crit. Care Med. 2010, 182, 360–368. [Google Scholar] [CrossRef]
- Wallace, J.L.; Ferraz, J.G.; Muscara, M.N. Hydrogen sulfide: An endogenous mediator of resolution of inflammation and injury. Antioxid. Redox Signal. 2012, 17, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Zanardo, R.C.; Brancaleone, V.; Distrutti, E.; Fiorucci, S.; Cirino, G.; Wallace, J.L. Hydrogen sulfide is an endogenous modulator of leukocyte-mediated inflammation. Faseb. J. 2006, 20, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Faller, S.; Hausler, F.; Goeft, A.; von Itter, M.A.; Gyllenram, V.; Hoetzel, A.; Spassov, S.G. Hydrogen sulfide limits neutrophil transmigration, inflamm.ation, and oxidative burst in lipopolysaccharide-induced acute lung injury. Sci. Rep. 2018, 8, 14676. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. https://doi.org/10.3390/toxins12070439
Cohen G. Immune Dysfunction in Uremia 2020. Toxins. 2020; 12(7):439. https://doi.org/10.3390/toxins12070439
Chicago/Turabian StyleCohen, Gerald. 2020. "Immune Dysfunction in Uremia 2020" Toxins 12, no. 7: 439. https://doi.org/10.3390/toxins12070439
APA StyleCohen, G. (2020). Immune Dysfunction in Uremia 2020. Toxins, 12(7), 439. https://doi.org/10.3390/toxins12070439



