Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections?
Abstract
1. Introduction
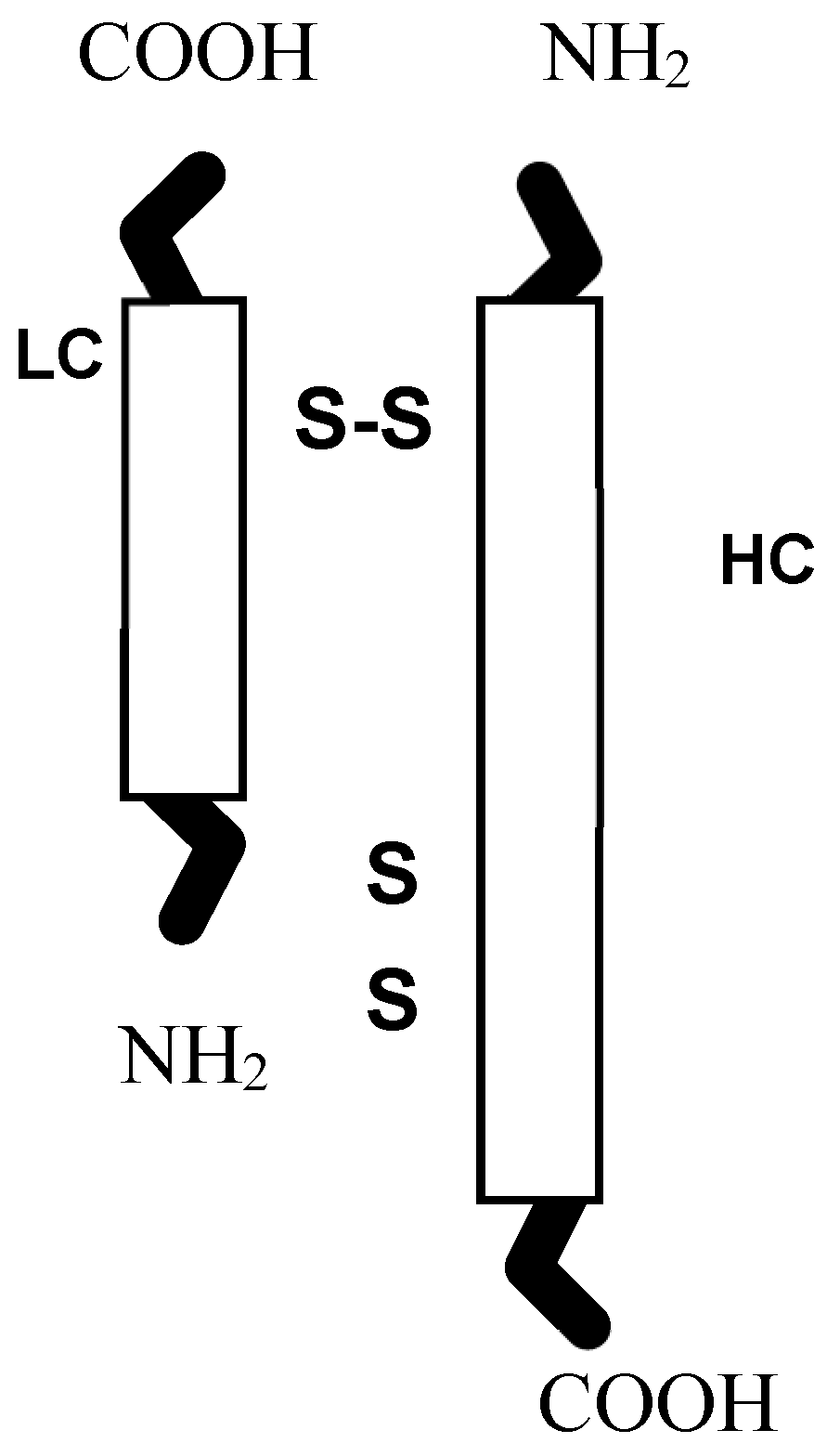
2. Site of Action: The Motor Endplate Zone
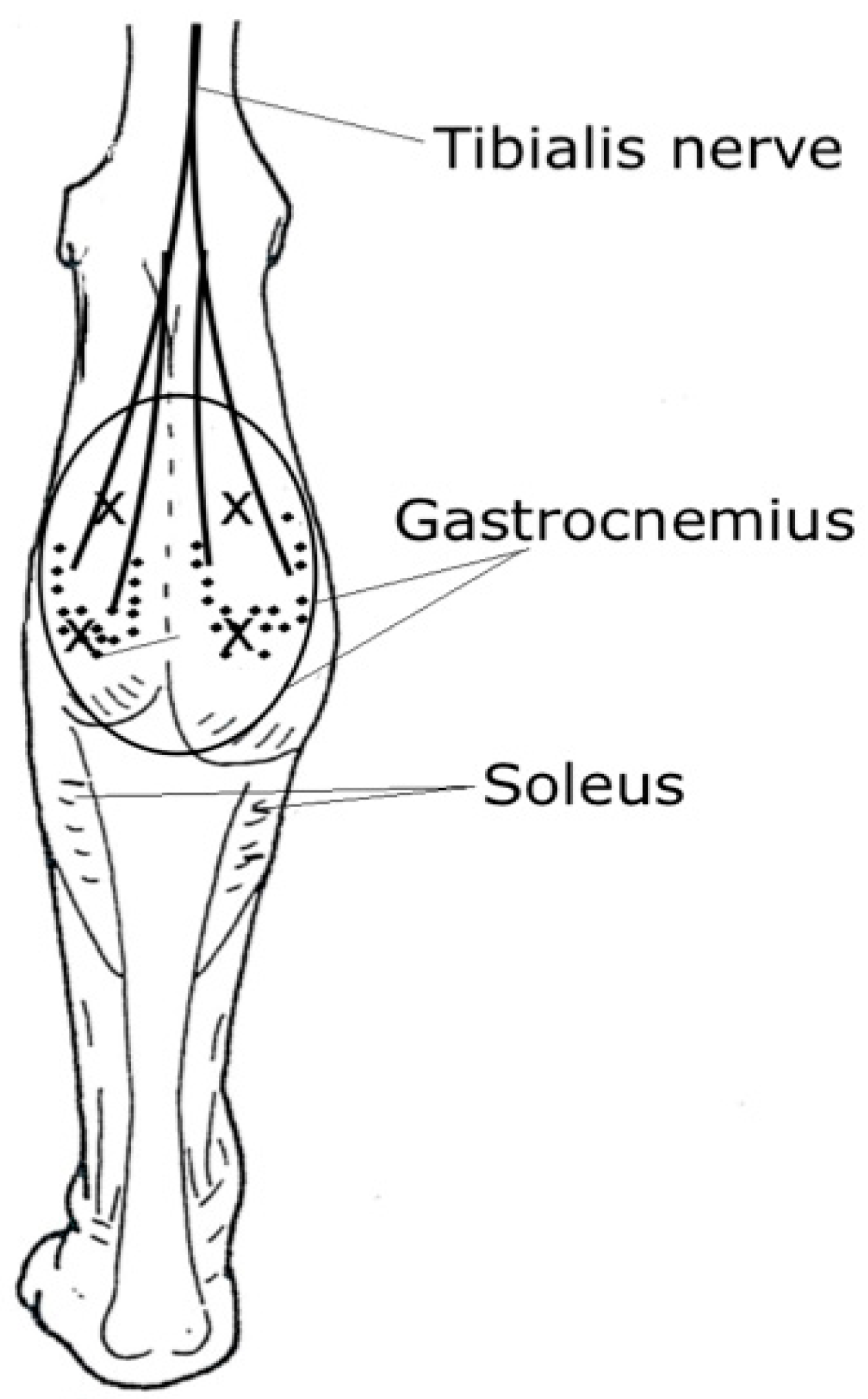
3. Site of Injections: Near the Motor Endplate Zone
4. Techniques for Localizing Muscles and Endplate Zones
5. Effect of Doses and Volumes in the Pediatric Setting
6. Recovery of Nerve Endings and Muscles
7. What About the Muscles of Children with CP?
8. Lessons Learned from the Repeated Treatment Studies
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Strobl, W.; Theologis, T.; Brunner, R.; Kocer, S.; Viehweger, E.; Pascual-Pascual, I.; Placzek, R. Best clinical practice in botulinum toxin treatment for children with cerebral palsy. Toxins 2015, 7, 1629–1648. [Google Scholar] [CrossRef]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum toxin in the management of children with cerebral palsy. Pediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef]
- Graham, H.K.; Aoki, K.R.; Autti-Rämö, I.; Boyd, R.N.; Delgado, M.R.; Gaebler-Spira, D.J.; Gormley, M.E., Jr.; Guyer, B.M.; Heinen, F.; Holton, A.F. Recommendations for the use of botulinum toxin type A in the management of cerebral palsy. Gait Posture 2000, 11, 67–79. [Google Scholar] [CrossRef]
- Gage, J.R.; Schwartz, M. Pathological gait and lever-arm dysfunction. In The Treatment of Gait Problems in Cerebral Palsy, 1st ed.; Gage, J.R., Ed.; MacKeith Press: London, UK, 2004; pp. 180–204. [Google Scholar]
- Autti-Rämö, I.; Larsen, A.; Taimo, A.; von Wendt, L. Management of the upper limb with botulinum toxin type A in children with spastic type cerebral palsy and acquired brain injury: Clinical implications. Eur. J. Neurol. 2001, 8 (Suppl. 5), 136–144. [Google Scholar] [CrossRef]
- Chin, T.Y.P.; Graham, H.K. Botulinum toxin A in the management of upper limb spasticity in cerebral palsy. Hand Clin. 2003, 19, 591–600. [Google Scholar] [CrossRef]
- Fehlings, D.; Rang, M.; Glazier, J.; Steele, C. Botulinum toxin type A injections in the spastic upper extremity of children with hemiplegia: Child characteristics that predict a positive outcome. Eur. J. Neurol. 2001, 8 (Suppl. 5), 145–149. [Google Scholar] [CrossRef]
- Koman, L.A.; Smith, B.P.; Balkrishnan, R. Spasticity associated with cerebral palsy in children. Guidelines for the use of botulinum A toxin. Pediatr. Drugs 2003, 5, 11–23. [Google Scholar] [CrossRef]
- Desloovere, K.; Molenaers, G.; De Cat, J.; Pauwels, P.; Van Campenhout, A.; Ortibus, E.; Fabry, G.; De Cock, P. Motor function following multilevel botulinum toxin type A treatment in children with cerebral palsy. Dev. Med. Child Neurol. 2007, 49, 56–61. [Google Scholar] [CrossRef]
- Autti-Rämö, I.; Larsen, A.; Peltonen, J.; Taimo, A.; von Wendt, L. Botulinum toxin injection as an adjunct when planning hand surgery in children with spastic hemiplegia. Neuropediatrics 2000, 31, 4–8. [Google Scholar] [CrossRef]
- Russman, B.S.; Tilton, A.H.; Gormley, M.E. Cerebral palsy: A rational approach to a treatment protocol, and the role of botulinum toxin in treatment. In Spasticity: Etiology, Evaluation, Management and the Role of Botulinum Toxin; Mayer, N.H., Simpson, D.M., Eds.; Wiley: Hoboken, NJ, USA, 2002; pp. 134–142. [Google Scholar]
- Heinen, F.; Desloovere, K.; Schroeder, A.S.; Berweck, S.; Borggraefe, I.; van Campenhout, A.; Andersen, G.L.; Aydin, R.; Becher, J.G.; Bernert, G. The updated European Consensus 2009 on the use of botulinum toxin for children with cerebral palsy. Eur. J. Paediatr. Neurol. 2010, 14, 45–66. [Google Scholar] [CrossRef]
- Love, S.C.; Novak, I.; Kentish, M.; Desloovere, K.; Heinen, F.; Molenaers, G.; O’Flaherty, S.; Graham, H.K. Botulinum toxin assessment, intervention and aftercare for lower limb spasticity in children with cerebral palsy: International consensus statement. Eur. J. Neurol. 2010, 17 (Suppl. 2), 9–37. [Google Scholar] [CrossRef]
- Fehlings, D.; Novak, I.; Berweck, S.; Hoare, B.; Stott, N.S.; Russo, R.N. Botulinum toxin assessment, intervention and follow-up for paediatric upper limb hypertonicity: International consensus statement. Eur. J. Neurol. 2010, 17 (Suppl. 2), 38–56. [Google Scholar] [CrossRef]
- Schroeder, A.S.; Berweck, S.; Lee, S.H.; Heinen, F. Botulinum toxin treatment of children with cerebral palsy—A short review of different injection techniques. Neurotox. Res. 2006, 9, 189–196. [Google Scholar] [CrossRef]
- Sätilä, H. Botulinum Toxin A Treatment in Children with Spastic Cerebral Palsy: Studies on Injection Techniques and Doses. Ph.D. Thesis, Tampere University, Tampere, Finland, 2007. Available online: http://urn.fi/urn:isbn:978-951-44-7078-3 (accessed on 1 May 2020).
- Van Campenhout, A.; Molenaers, G. Localization of the motor endplate zone in human skeletal muscles of the lower limb: Anatomical guidelines for injection with botulinum toxin. Dev. Med. Child Neurol. 2011, 53, 108–119. [Google Scholar] [CrossRef]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharm. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef]
- Hamjian, J.A.; Walker, F.O. Serial neurophysiological studies of intramuscular botulinum-A toxin in humans. Muscle Nerve 1994, 17, 1385–1392. [Google Scholar] [CrossRef]
- Sloop, R.R.; Escutin, R.O.; Matus, J.A.; Cole, B.A.; Peterson, G.W. Dose-response curve of human extensor digitorum brevis muscle function to intramuscularly injected botulinum toxin type A. Neurology 1996, 46, 1382–1386. [Google Scholar] [CrossRef]
- Naumann, M.; Jost, W.H.; Toyka, K.V. Botulinum toxin in the treatment of neurological disorders of the autonomic nervous system. Arch. Neurol. 1999, 56, 914–916. [Google Scholar] [CrossRef]
- Christensen, E. Topography of terminal motor innervation in striated muscles from stillborn infants. Am. J. Phys. Med. 1959, 38, 65–78. [Google Scholar] [CrossRef]
- Coers, C.; Woolf, A.L. The Innervation of Muscle: A Biopsy Study; Charles C Thomas: Springfield, IL, USA, 1959. [Google Scholar]
- Deshpande, S.; Gormley, M.E.; Carey, J.R. Muscle fiber orientation in muscles commonly injected with botulinum toxin: An anatomical pilot study. Neurotox. Res. 2006, 9, 115–120. [Google Scholar] [CrossRef]
- Bickerton, L.E.; Agur, A.; Ashby, P. Flexor digitorum superficialis: Locations of individual muscle bellies for botulinum toxin injections. Muscle Nerve 1997, 20, 1041–1043. [Google Scholar] [CrossRef]
- Saitou, K.; Masuda, T.; Michikami, D.; Kojima, R.; Okada, M. Innervation zones of the upper and lower limb muscles estimated by using multichannel surface EMG. J. Hum. Ergol. 2000, 29, 35–52. [Google Scholar]
- Won, S.Y.; Hur, M.S.; Rha, D.W.; Park, H.D.; Hu, K.S.; Fontaine, C.; Kim, H.J. Extra- and intramuscular nerve distribution patterns of the muscles of the ventral compartment of the forearm. Am. J. Phys. Med. Rehabil. 2010, 89, 644–652. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, X.; Xie, X.; Yang, S.; Xu, Y.; Xie, P. Intramuscular nerve distribution patterns of anterior forearm muscles in children: A guide for botulinum toxin injection. Am. J. Transl. Res. 2016, 8, 5485–5493. [Google Scholar] [PubMed]
- Parratte, B.; Tatu, L.; Vuillier, F.; Diop, M.; Monnier, G. Intramuscular distribution of nerves in the human triceps surae muscle: Anatomical bases for treatment of spastic drop foot with botulinum toxin. Surg. Radiol. Anat. 2002, 24, 91–96. [Google Scholar] [CrossRef]
- Crystal, R.; Malone, A.A.; Eastwood, D.M. Motor points for neuromuscular blockade of the adductor muscle group. Clin. Orthopaed. Rel. Res. 2005, 437, 196–200. [Google Scholar] [CrossRef]
- Kim, M.W.; Kim, J.H.; Yang, Y.J.; Ko, Y.J. Anatomic localization of motor points in gastrocnemius and soleus muscles. Am. J. Phys. Med. Rehabil. 2005, 84, 680–683. [Google Scholar] [CrossRef]
- Oddy, M.J.; Brown, C.; Mistry, R.; Eastwood, D.M. Botulinum toxin injection site localization for the tibialis posterior muscle. J. Pediatr. Orthop. B 2006, 15, 414–417. [Google Scholar] [CrossRef]
- Sheverdin, V.A.; Hur, M.S.; Won, S.Y.; Song, W.C.; Hu, K.S.; Koh, K.S.; Kim, H.J. Extra- and intramuscular nerves distributions of the triceps surae muscle as a basis for muscle resection and botulinum toxin injections. Surg. Radiol. Anat. 2009, 31, 615–621. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Hubens, G.; Fagard, K.; Molenaers, G. Localization of motor nerve branches of the human psoas muscle. Muscle Nerve 2010, 42, 202–207. [Google Scholar] [CrossRef]
- Shaari, C.M.; Sanders, I. Quantifying how location and dose of botulinum toxin injections affect muscle paralysis. Muscle Nerve 1993, 16, 964–969. [Google Scholar] [CrossRef]
- Childers, M.K.; Kornegay, J.N.; Aoki, R.; Otaviani, L.; Bogan, D.J.; Petroski, G. Evaluating motor end-plate-targeted injections of botulinum toxin type A in a canine model. Muscle Nerve 1998, 21, 653–655. [Google Scholar] [CrossRef]
- Sätilä, H.; Iisalo, T.; Pietikäinen, T.; Seppänen, R.L.; Salo, M.; Koivikko, M.; Autti-Rämö, I.; Haataja, R. Botulinum toxin treatment of spastic equinus in cerebral palsy: A randomized trial comparing two injection sites. Am. J. Phys. Med. Rehabil. 2005, 84, 355–365. [Google Scholar]
- Van Campenhout, A.; Verhaegen, A.; Pans, S.; Molenaers, G. Botulinum toxin type A injections in the psoas muscle of children with cerebral palsy: Muscle atrophy after motor end plate-targeted injections. Res. Dev. Disabil. 2013, 34, 1052–1058. [Google Scholar] [CrossRef]
- Van Campenhout, A.; Bar-On, L.; Desloovere, K.; Huenaerts, C.; Molenaers, G. Motor endplate-targeted botulinum toxin injections of the gracilis muscle in children with cerebral palsy. Dev. Med. Child Neurol. 2015, 57, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Childers, M.K.; Stacy, M.; Cooke, D.L.; Stonnington, H.H. Comparison of two injection techniques using botulinum toxin in spastic hemiplegia. Am. J. Phys. Med. Rehabil. 1996, 75, 462–469. [Google Scholar] [CrossRef]
- Im, S.; Park, J.H.; Son, S.K.; Shin, J.E.; Cho, S.H.; Park, G.Y. Does botulinum toxin injection site determine outcome in post-stroke plantarflexion spasticity? Comparison study of two injection sites in the gastrocnemius muscle: A randomized double-blind controlled trial. Clin. Rehabil. 2014, 28, 604–613. [Google Scholar] [CrossRef]
- Gracies, J.M.; Lugassy, M.; Weisz, D.J.; Vecchio, M.; Flanagan, S.; Simpson, D.M. Botulinum toxin dilution and endplate targeting in spasticity: A double-blind controlled study. Arch. Phys. Med. Rehabil. 2009, 90, 9–16. [Google Scholar] [CrossRef]
- Lapatki, B.G.; van Dijk, J.P.; van der Warrenburg, B.P.; Zwarts, M.J. Botulinum toxin has an increased effect when targeted toward the muscle’s endplate zone: A high-density surface EMG guided study. Clin. Neurophysiol. 2011, 122, 1611–1616. [Google Scholar] [CrossRef]
- Borodic, G.E.; Cozzolino, D.; Ferrante, R.; Wiegner, A.W.; Young, R.R. Innervation zone of orbicularis oculi muscle and implications for botulinum toxin therapy. Ophthalmic Plast. Reconstr. Surg. 1991, 7, 54–60. [Google Scholar] [CrossRef]
- Borodic, G.E.; Pearce, L.B.; Smith, K.; Joseph, M. Botulinum A toxin for spasmodic torticollis: Multiple vs. single injection points per muscle. Head Neck 1992, 14, 33–37. [Google Scholar] [CrossRef]
- Mayer, N.H.; Whyte, J.; Wannstedt, G.; Ellis, C.A. Comparative impact of 2 botulinum toxin injection techniques for elbow flexor hypertonia. Arch. Phys. Med. Rehabil. 2008, 89, 982–987. [Google Scholar] [CrossRef]
- Sätilä, H.; Pietikäinen, T.; Iisalo, T.; Lehtonen-Räty, P.; Salo, M.; Haataja, R.; Koivikko, M.; Autti-Rämö, I. Botulinum toxin type A injections into the calf muscles for treatment of spastic equinus in cerebral palsy: A randomized trial comparing single and multiple injection sites. Am. J. Phys. Med. Rehabil. 2008, 87, 386–394. [Google Scholar] [CrossRef]
- Koman, L.A.; Mooney, J.F.; Smith, B.; Goodman, A.; Mulvaney, T. Management of cerebral palsy with botulinum-A toxin: Preliminary investigation. J. Pediatr. Orthop. 1993, 13, 489–495. [Google Scholar] [CrossRef]
- Cosgrove, A.P.; Corry, I.S.; Graham, H.K. Botulinum toxin in the management of the lower limb in cerebral palsy. Dev. Med. Child Neurol. 1994, 36, 386–396. [Google Scholar] [CrossRef]
- Fehlings, D.; Rang, M.; Glazier, J.; Steele, C. An evaluation of botulinum-A toxin injections to improve upper extremity function in children with cerebral palsy. J. Pediatr. 2000, 137, 331–337. [Google Scholar] [CrossRef]
- Chin, T.Y.P.; Nattrass, G.R.; Selber, P.; Graham, H.K. Accuracy of intramuscular injection of botulinum toxin A in juvenile cerebral palsy. A comparison between manual needle placement and placement guided by electrical stimulation. J. Pediatr. Orthop. 2005, 25, 286–291. [Google Scholar] [CrossRef]
- Berweck, S.; Feldkamp, A.; Francke, A.; Nehles, J.; Schwerin, A.; Heinen, F. Sonography-guided injection of botulinum toxin A in children with cerebral palsy. Neuropediatrics 2002, 33, 221–223. [Google Scholar] [CrossRef]
- Bayon-Mottu, B.; Gambart, G.; Deries, X.; Tessiot, C.; Richard, I.; Dinomais, M. Pain during injections of botulinum toxin in children: Influence of the localization technique. Ann. Phys. Rehabil. Med. 2014, 57, 578–586. [Google Scholar] [CrossRef]
- Yang, E.Y.; Rha, D.W.; Yoo, J.K.; Park, E.S. Accuracy of manual needle placement for gastrocnemius muscle in children with cerebral palsy checked against ultrasonography. Arch. Phys. Med. Rehabil. 2009, 90, 741–744. [Google Scholar] [CrossRef]
- Xu, K.; Yan, T.; Mai, J. A randomized controlled trial to compare two botulinum toxin injection techniques on the functional improvement of the leg of children with cerebral palsy. Clin. Rehabil. 2009, 23, 800–811. [Google Scholar]
- Kwon, J.Y.; Hwang, J.H.; Kim, J.S. Botulinum toxin A injection into calf muscles for treatment of spastic equinus in cerebral palsy: A controlled trial comparing sonography and electric stimulation-guided injection technique: A preliminary report. Am. J. Phys. Med. Rehabil. 2010, 89, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Ajax, T.; Ross, M.A.; Rodnitzky, R.L. The role of electromyography in guiding botulinum toxin injections for focal dystonia and spasticity. J. Neuro. Rehabil. 1998, 12, 1–4. [Google Scholar] [CrossRef]
- Molloy, F.M.; Schill, H.A.; Kaelin-Lang, A.; Karp, P.I. Accuracy of muscle localization without EMG: Implications for treatment of limb dystonia. Neurology 2002, 58, 805–807. [Google Scholar] [CrossRef]
- Picelli, A.; Bonetti, P.; Fontana, C.; Barausse, M.; Dambruoso, F.; Gajofatto, F.; Tamburin, S.; Girardi, P.; Gimigliano, R.; Smania, N. Accuracy of botulinum toxin type A injection into the gastrocnemius muscle of adults with spastic equinus: Manual needle placement and electrical stimulation guidance compared using ultrasonography. J. Rehabil. Med. 2012, 44, 450–452. [Google Scholar] [CrossRef]
- Picelli, A.; Roncari, L.; Baldessarelli, S.; Berto, G.; Lobba, D.; Santamato, A.; Fiore, P.; Smania, N. Accuracy of botulinum toxin type A injection into the forearm muscles of chronic stroke patients with spastic flexed wrist and clenched fist: Manual needle placement evaluated using ultrasonography. J. Rehabil. Med. 2014, 46, 1042–1045. [Google Scholar] [CrossRef]
- Schnitzler, A.; Roche, N.; Denormandie, P.; Lautridou, C.; Parratte, B.; Genet, F. Manual needle placement: Accuracy of botulinum toxin A injections. Muscle Nerve 2012, 46, 531–534. [Google Scholar] [CrossRef]
- Ramirez-Castaneda, J.; Jankovic, J.; Comella, C.; Dashtipour, K.; Fernandez, H.; Mari, Z. Diffusion, spread, and migration of botulinum toxin. Mov. Disord. 2013, 28, 1775–1783. [Google Scholar] [CrossRef]
- Scaglione, F. Conversion ratio between Botox, Dysport, and Xeomin in clinical practice. Toxins 2016, 8, 65. [Google Scholar] [CrossRef]
- Hulst, J.B.; Minamoto, V.B.; Lim, M.B.; Bremner, S.N.; Ward, S.R.; Lieber, R.L. Systematic test of neurotoxin dose and volume on muscle function in a rat model. Muscle Nerve 2014, 49, 709–715. [Google Scholar] [CrossRef][Green Version]
- Borodic, G.E.; Joseph, M.; Fay, L.; Cozzolino, D.; Ferrante, R.J. Botulinum A toxin for the treatment of spasmodic torticollis: Dysphagia and regional toxin spread. Head Neck 1990, 12, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Borodic, G.E.; Ferrante, R.; Pearce, L.B.; Smith, K. Histologic assessment of dose-related diffusion and muscle fiber response after therapeutic botulinum A toxin injections. Mov. Disord. 1994, 9, 31–39. [Google Scholar] [CrossRef]
- Frasson, E.; DallÓra, E.; Bordignon, M.; Brigo, F.; Tocco, P.; Primon, D.; Didone, G.; Vicentini, S.; Fiaschi, A.; Bertolasi, L. Spread of botulinum neurotoxin type A at standard doses is inherent to the successful treatment of spastic equinus foot in cerebral palsy: Short-term neurophysiological and clinical study. J. Child Neurol. 2012, 27, 587–593. [Google Scholar] [CrossRef]
- Dressler, D.; Rothwell, J.C. Electromyographic quantification of the paralysing effect of botulinum toxin in the sternocleidomastoid muscle. Eur. Neurol. 2000, 43, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Koog, Y.H.; Min, B.I. Effects of botulinum toxin A on calf muscles in children with cerebral palsy: A systematic review. Clin. Rehabil. 2010, 24, 685–700. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Heinen, F.; Schenkel, A.; Doll, B.; Ebersbach, G.; Muller, J.; Poewe, W. Botulinum toxin A in the management of spastic gait disorders in children and young adults with cerebral palsy: A randomized, double-blind study of “high-dose” versus “low-dose” treatment. Neuropediatrics 1998, 30, 120–124. [Google Scholar] [CrossRef]
- Polak, F.; Morton, R.; Ward, C.; Wallace, W.A.; Doderlein, L.; Siebel, A. Double-blind comparison study of two doses of botulinum toxin A injected into calf muscles in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 2002, 44, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.; Jasinski, M.; Maciag-Tymecka, I.; Michalowska-Mrozek, J.; Bonikowski, M.; Carr, L.; MacLean, J.; Lin, J.-P.; Lynch, B.; Theologis, T. Botulinum toxin treatment of spasticity in diplegic cerebral palsy: A randomized, double-blind, placebo-controlled, dose-ranging study. Dev. Med. Child Neurol. 2002, 44, 666–675. [Google Scholar] [CrossRef]
- Delgado, M.R.; Tilton, A.; Russman, B.; Matthews, D.; Maciag-Tymecka, I.; Unlu, E.; Pham, E.; Tse, A.; Picaut, P. AbobotulinumtoxinA for equinus foot deformity in cerebral palsy: A randomized controlled trial. Pediatrics 2016, 137, e20152830. [Google Scholar] [CrossRef]
- Bakheit, A.M.O.; Severa, S.; Cosgrove, A.; Morton, R.; Roussonis, S.H.; Doderlein, L.; Lin, J.-P. Safety profile and efficacy of botulinum toxin A (Dysport) in children with muscle spasticity. Dev. Med. Child Neurol. 2001, 43, 234–238. [Google Scholar] [CrossRef]
- Sätilä, H.K.; Pietikäinen, T.; Lehtonen-Räty, P.; Koivikko, M.; Autti-Rämö, I. Treatment of spastic equinus gait with botulinum toxin A: Does dose matter? Analysis of a clinical cohort. Neuropediatrics 2006, 37, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Pascual, S.I.; Pascual-Castroviejo, I.; Garcia Ruiz, P.J. Treating spastic equinus foot from cerebral palsy with botulinum toxin type-A: What factors influence the results? An analysis of 189 consecutive cases. Am. J. Phys. Med. Rehabil. 2011, 90, 554–563. [Google Scholar] [CrossRef]
- Park, E.S.; Rha, D.W. Botulinum toxin type A injection for management of upper limb spasticity in children with cerebral palsy: A literature review. Yonsei Med. J. 2006, 47, 589–603. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, A.; Campbell, K.; Lam-Damji, S.; Fehlings, D. A randomized controlled trial comparing botulinum toxin A dosage in the upper extremity of children with spasticity. Dev. Med. Child Neurol. 2007, 49, 331–337. [Google Scholar] [CrossRef]
- Kim, H.S.; Hwang, J.H.; Jeong, S.T.; Lee, Y.T.; Lee, P.K.W.; Suh, Y.L.; Shim, J.S. Effect of muscle activity and botulinum toxin dilution volume on muscle paralysis. Dev. Med. Child Neurol. 2003, 45, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Francisco, G.E.; Boake, C.; Vaughn, A. Botulinum toxin in upper limb spasticity after acquired brain injury. Am. J. Phys. Med. Rehabil. 2002, 81, 355–363. [Google Scholar] [CrossRef]
- Lee, L.R.; Chuang, Y.C.; Yang, B.J.; Hsu, M.J.; Liu, Y.H. Botulinum toxin for lower limb spasticity in children with cerebral palsy: A single-blinded trial comparing dilution techniques. Am. J. Phys. Med. Rehabil. 2004, 83, 766–773. [Google Scholar] [CrossRef]
- Lee, J.H.; Sung, I.Y.; Yoo, J.Y.; Park, E.H.; Park, S.R. Effects of different dilutions of botulinum toxin type A treatment for children with cerebral palsy with spastic ankle plantarflexor: A randomized controlled trial. J. Rehabil. Med. 2009, 41, 740–745. [Google Scholar] [CrossRef]
- Hu, G.C.; Chuang, Y.C.; Liu, J.P.; Chien, K.L.; Chen, Y.M.; Chen, Y.F. Botulinum toxin (Dysport) treatment of the spastic gastrocnemius muscle in children with cerebral palsy: A randomized trial comparing two injection volumes. Clin. Rehabil. 2009, 23, 64–71. [Google Scholar] [CrossRef]
- Holds, J.B.; Alderson, K.; Fogg, S.G.; Anderson, R.L. Motor nerve sprouting in human orbicularis muscle after botulinum A injection. Investig. Ophthalmol. Vis. Sci. 1990, 31, 964–967. [Google Scholar]
- De Paiva, A.; Meunier, F.A.; Molgo, J.; Aoki, K.R.; Dolly, J.O. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: Biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc. Natl. Acad. Sci. USA 1999, 96, 3200–3205. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.L.; Selsby, J.; Payne, A.; Judge, A.; Dott, C. Botulinum neurotoxin type A causes shifts in myosin heavy chain composition in muscle. Toxicon 2005, 46, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ma, J.; Lee, C.; Smith, B.P.; Smith, T.L.; Tan, K.H.; Koman, A. How muscles recover from paresis and atrophy after intramuscular injection of botulinum toxin A: Study in juvenile rats. J. Orthop. Res. 2006, 24, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, V.B.; Suzuki, K.P.; Bremner, S.N.; Lieber, R.L.; Ward, S.R. Dramatic changes in muscle contractile and structural properties after two botulinum toxin injections. Muscle Nerve 2015, 52, 649–657. [Google Scholar] [CrossRef]
- Ward, S.R.; Minamoto, V.B.; Suzuki, K.P.; Hulst, J.B.; Bremner, S.N.; Lieber, R.L. Recovery of rat muscle size but not function more than one-year after a single botulinum toxin injection. Muscle Nerve 2018, 57, 435–441. [Google Scholar] [CrossRef]
- Thacker, B.E.; Tomiya, A.; Hulst, J.B.; Suzuki, K.P.; Bremner, S.N.; Gastwirt, R.F.; Greaser, M.L.; Lieber, R.L.; Ward, S.R. Passive mechanical properties and related proteins change with botulinum neurotoxin A injection of normal skeletal muscle. J. Orthop. Res. 2012, 30, 497–502. [Google Scholar] [CrossRef]
- Mukund, K.; Mathewson, M.; Minamoto, V.; Ward, S.R.; Subramaniam, S.; Lieber, R.L. Systems analysis of transcriptional data provides insights into muscle’s biological response to botulinum toxin. Muscle Nerve 2014, 50, 744–758. [Google Scholar] [CrossRef]
- Fortuna, R.; Vaz, M.A.; Youssef, A.R.; Longino, D.; Herzog, W. Changes in contractile properties of muscles receiving repeat injections of botulinum toxin (Botox). J. Biomech. 2011, 44, 39–44. [Google Scholar] [CrossRef]
- Fortuna, R.; Horisberger, M.; Vaz, M.A.; Herzog, W. Do skeletal muscle properties recover following repeat onabotulinum toxin A injections? J. Biomech. 2013, 46, 2426–2433. [Google Scholar] [CrossRef]
- Fortuna, R.; Horisberger, M.; Vaz, M.A.; Van der Marel, R.; Herzog, W. The effects of electrical stimulation exercise on muscles injected with botulinum toxin type-A (Botox). J. Biomech. 2013, 46, 36–42. [Google Scholar] [CrossRef]
- Harris, C.P.; Alderson, K.; Nebeker, J.; Holds, J.B.; Anderson, R.L. Histologic features of human orbicularis oculi treated with botulinum A toxin. Arch. Ophthalmol. 1991, 109, 393–395. [Google Scholar] [CrossRef]
- Borodic, G.E.; Ferrante, R. Effects of repeated botulinum toxin injections on orbicularis oculi muscle. J. Clin. Neuro-Ophthalmol. 1992, 12, 121–127. [Google Scholar]
- Schroeder, A.S.; Ertl-Wagner, B.; Britsch, S.; Schröder, J.M.; Nikolin, S.; Weis, J.; Muller-Felber, W.; Koerte, I.; Stehr, M.; Berweck, S.; et al. Muscle biopsy substantiates long-term MRI alterations one year after a single dose of botulinum toxin injected into the lateral gastrocnemius muscle of healthy volunteers. Mov. Disord. 2009, 24, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, G.; Wagner, P. Development of spasticity with age in a total population of children with cerebral palsy. BMC Musculoskelet. Disord. 2008, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Willerslev-Olsen, M.; Lorentzen, J.; Sinkjaer, T.; Nielsen, J.B. Passive muscle properties are altered in children with cerebral palsy before the age of 3 years and are difficult to distinguish clinically from spasticity. Dev. Med. Child Neurol. 2013, 55, 617–623. [Google Scholar] [CrossRef]
- Willerslev-Olsen, M.; Choe Lund, M.; Lorentzen, J.; Barber, L.; Kofoed Hansen, M.; Nielsen, J.B. Impaired muscle growth precedes development of increased stiffness of the triceps surae musculo-tendinous unit in children with cerebral palsy. Dev. Med. Child Neurol. 2018, 60, 672–679. [Google Scholar] [CrossRef]
- Barret, R.S.; Lichtwark, G.A. Gross muscle morphology and structure in spastic cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2010, 52, 794–804. [Google Scholar] [CrossRef]
- Hoare, B.J.; Wallen, M.A.; Imms, C.; Villanueva, E.; Rawicki, H.B.; Carey, L. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE). Cochrane Database Syst. Rev. 2010, 1, CD003469. [Google Scholar] [CrossRef]
- Blumetti, F.C.; Belloti, J.C.; Tamaoki, M.J.S.; Pinto, J.A. Botulinum toxin type A in the treatment of lower limb spasticity in children with cerebral palsy. Cochrane Database Syst. Rev. 2019, 10, CD001408. [Google Scholar] [CrossRef]
- Alhusaini, A.A.A.; Crosbie, J.; Shepherd, R.B.; Dean, C.M.; Scheinberg, A. No change in calf muscle passive stiffness after botulinum toxin injection in children with cerebral palsy. Dev. Med. Child Neurol. 2011, 53, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Eames, N.; Baker, R.; Hill, N.; Graham, K.; Taylor, T.; Cosgrove, A. The effect of botulinum toxin A on gastrocnemius length: Magnitude and duration of response. Dev. Med. Child Neurol. 1999, 41, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Sim, E.; Rha, D.W.; Jung, S. Architectural changes of the gastrocnemius muscle after botulinum toxin type A injection in children with cerebral palsy. Yonsei Med. J. 2014, 55, 1406–1412. [Google Scholar] [CrossRef][Green Version]
- Kawano, A.; Yanagizono, T.; Kadouchi, I.; Umezaki, T.; Chosa, E. Ultrasonographic evaluation of changes in the muscle architecture of the gastrocnemius with botulinum toxin treatment for lower extremity spasticity in children with cerebral palsy. J. Orthop. Sci. 2018, 23, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Stannage, K.; Fabian, V.; Ellis, K.; Reid, S.; Pitcher, C.; Elliot, C. Muscle histopathology in children with spastic cerebral palsy receiving botulinum toxin type A. Muscle Nerve 2016, 53, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Ponten, E.; Hedström, Y.; Ward, S.R.; Chambers, H.G.; Subramaniam, S.; Lieber, R.L. Novel transcriptional profile in wrist muscles from cerebral palsy patients. BMC Med. Genom. 2009, 2, 44. [Google Scholar] [CrossRef]
- Fry, N.R.; Gough, M.; McNee, A.; Shortland, A.P. Changes in the volume and length of the medial gastrocnemius after surgical recession in children with spastic diplegic cerebral palsy. J. Pediatr. Orthop. 2007, 27, 769–774. [Google Scholar] [CrossRef]
- Campbell, M.; Varley-Campbell, J.; Fulford, J.; Taylor, B.; Mileva, K.N.; Bowtell, J. Effect of immobilization on neuromuscular function in vivo in humans: A systematic review. Sports Med. 2019, 49, 931–950. [Google Scholar] [CrossRef]
- Williams, S.A.; Reid, S.; Elliot, C.; Shipman, P.; Valentine, J. Muscle volume alterations in spastic muscles immediately following botulinum toxin type-A treatment in children with cerebral palsy. Dev. Med. Child Neurol. 2013, 55, 813–820. [Google Scholar] [CrossRef]
- Alexander, C.; Elliot, C.; Valentine, J.; Stannage, K.; Bear, N.; Donnelly, C.J.; Shipman, P.; Reid, S. Muscle volume alterations after first botulinum neurotoxin A treatment in children with cerebral palsy: A 6-month prospective cohort study. Dev. Med. Child Neurol. 2018, 60, 1165–1171. [Google Scholar] [CrossRef]
- Schroeder, A.S.; Koerte, I.; Berweck, S.; Ertl-Wagner, B.; Heinen, F. How doctors think—and treat with botulinum toxin. Dev. Med. Child Neurol. 2010, 52, 875–876. [Google Scholar] [CrossRef]
- Barber, L.; Hastings-Ison, T.; Baker, R.; Graham, H.K.; Barrett, R.; Lichtwark, G. The effects of botulinum toxin injection frequency on calf muscle growth in young children with spastic cerebral palsy: A 12-month prospective study. J. Child Orthop. 2013, 7, 425–433. [Google Scholar] [CrossRef]
- Schless, S.-H.; Cenni, F.; Bar-On, L.; Hanssen, B.; Kalkman, B.; O’Brien, T.; Aertbelien, E.; Van Campenhout, A.; Molenaers, G.; Desloovere, K. Medial gastrocnemius volume and echo-intensity after botulinum neurotoxin A interventions in children with spastic cerebral palsy. Dev. Med. Child Neurol. 2019, 61, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Dodd, K.J.; Taylor, N.F.; Damiano, D.L. A systematic review of the effectiveness of strength-training programs for people with cerebral palsy. Arch. Phys. Med. Rehabil. 2002, 83, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Verschuren, O.; Ada, L.; Maltais, D.B.; Gorter, J.W.; Scianni, A.; Ketelaar, M. Muscle strengthening in children and adolescents with spastic cerebral palsy: Considerations for future resistance training protocols. Phys. Ther. 2011, 81, 1130–1139. [Google Scholar] [CrossRef]
- Park, E.-Y.; Kim, W.-H. Meta-analysis of the effect of strengthening interventions in individuals with cerebral palsy. Res. Dev. Disabil. 2014, 35, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Gillett, J.G.; Boyd, R.N.; Carty, C.P.; Barber, L.A. The impact of strength training on skeletal muscle morphology and architecture in children and adolescents with cerebral palsy: A systematic review. Res. Dev. Disabil. 2016, 56, 183–196. [Google Scholar] [CrossRef]
- Elvrum, A.-K.G.; Braendvik, S.M.; Saether, R.; Lamvik, T.; Vereijken, B.; Roeleveld, K. Effectiveness of resistance training in combination with botulinum toxin-A on hand and arm use in children with cerebral palsy: A pre-post intervention study. BMC Pediatr. 2012, 12, 91. [Google Scholar] [CrossRef]
- Bandholm, T.; Jensen, B.R.; Nielsen, L.M.; Rasmussen, H.; Bencke, J.; Curtis, D.; Pedersen, S.A.; Sonne-Holm, S. Neurorehabilitation with versus without resistance training after botulinum toxin treatment in children with cerebral palsy: A randomized pilot study. Neurorehabilitation 2012, 30, 277–286. [Google Scholar] [CrossRef]
- Williams, S.A.; Elliot, C.; Valentine, J.; Gubbay, A.; Shipman, P.; Reid, S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: The impact on muscle morphology and strength. Disabil. Rehabil. 2013, 35, 596–605. [Google Scholar] [CrossRef]
- Cosgrove, A.P.; Graham, H.K. Botulinum toxin A prevents the development of contractures in the hereditary spastic mouse. Dev. Med. Child Neurol. 1994, 36, 379–385. [Google Scholar] [CrossRef]
- Kahraman, A.; Seyhan, K.; Deger, U.; Kutluturk, S.; Mutlu, A. Should botulinum toxin A injections be repeated in children with cerebral palsy? A systematic review. Dev. Med. Child Neurol. 2016, 58, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Kanovsky, P.; Bares, M.; Severa, S.; Richardson, A.; The Dysport Paediatric Limb Spasticity Study Group. Long-term efficacy and tolerability of 4-monthly versus yearly botulinum toxin type A treatment for lower-limb spasticity in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Hastings-Ison, T.; Blackburn, C.; Rawicki, B.; Fahey, M.; Simpson, P.; Baker, R.; Graham, K. Injection frequency of botulinum toxin A for spastic equinus: A randomized clinical trial. Dev. Med. Child Neurol. 2016, 58, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Tedroff, K.; Granath, F.; Forssberg, H.; Haglund-Akerlind, Y. Long-term effects of botulinum toxin A in children with cerebral palsy. Dev. Med. Child Neurol. 2009, 51, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Davidson, S.A.; Bear, N.; Blair, E.; Ward, R.; Thornton, A.; Stannage, K.; Watson, L.; Forbes, D.; Elliot, C. Botulinum toxin and surgical intervention in children and adolescents with cerebral palsy: Who, when and why do we treat? Disabil. Rehabil. 2019, 15, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valentine, J.; Davidson, S.A.; Bear, N.; Blair, E.; Paterson, L.; Ward, R.; Forbes, D.; Elliot, C. A prospective study investigating gross motor function of children with cerebral palsy and GMFCS level II after long-term botulinum toxin type A use. BMC Pediatr. 2020, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Hägglund, G.; Andersson, S.; Duppe, H.; Pedertsen, H.L.; Nordmark, E.; Westbom, L. Prevention of severe contractures might replace multilevel surgery in cerebral palsy: Results of a population-based health care programme and new techniques to reduce spasticity. J. Pediatr. Orthop. B 2005, 14, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Molenaers, G.; Desloovere, K.; Fabry, G.; De Cock, P. The effects of quantitative gait assessment and botulinum toxin A on musculoskeletal surgery in children with cerebral palsy. J. Bone Jt. Surg. (Am.) 2006, 88-A, 161–170. [Google Scholar]
- Miller, S.D.; Juricic, M.; Hesketh, K.; McClean, L.; Magnuson, S.; Gasior, S.; Schaeffer, E.; O’Donnell, M.; Mulpuri, K. Prevention of hip displacement in children with cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2017, 59, 1130–1139. [Google Scholar] [CrossRef]
- Grigoriu, A.-I.; Dinomais, M.; Remy-Neris, O.; Brochard, S. Impact of injection-guiding techniques on the effectiveness of botulinum toxin for the treatment of focal spasticity and dystonia: A systematic review. Arch. Phys. Med. Rehabil. 2015, 96, 2067–2078. [Google Scholar] [CrossRef]
- Naidu, K.; Smith, K.; Sheedy, M.; Adair, B.; Yu, X.; Graham, H.K. Systemic adverse events following botulinum toxin A therapy in children with cerebral palsy. Dev. Med. Child Neurol. 2010, 52, 139–144. [Google Scholar] [CrossRef] [PubMed]
| Base for Dose Calculation: |
| Total units per treatment session |
| Total units per kg body weight per session |
| Units per muscle |
| Units per injection site |
| Units per kg body weight per muscle (U/kg/muscle) |
| Dose Modifiers: |
| Weight of the patient |
| Number of muscles needing treatment |
| Size and activity of the muscle |
| Knowledge of the muscle configuration and MEP distribution |
| Severity of spasticity/dystonia |
| The goal of the treatment |
| Comorbidities (e.g., dysphagia, aspiration, and breathing problems) |
| Dynamic vs fibrotic muscle |
| Experience from previous injections |
| Muscle | Dose Range U/kg of OnabotulinumtoxinA | Dose Range U/kg of AbobotulinumtoxinA | ||
|---|---|---|---|---|
| Gastrocnemius mediale | 1–3 | 3–6 | ||
| Gastrocnemius laterale | 1–3 | total max 4–6 U/kg | 3–6 | total max 10–15 U/kg |
| Soleus | 1–2 | 2–4 | ||
| Tibialis posterior | 1–2 | -- | ||
| Semitendinosus | 1–3 | 10–15 | ||
| Semimembranosus | 1–3 | 10–15 | ||
| Biceps femoris | 1–3 | -- | ||
| Adductor longus/magnus | 1–4 | 20–30 | ||
| Gracilis | 1–2 | -- | ||
| Flexor carpi radialis | 0.5–1.5(2) | 5–10 | ||
| Flexor carpi ulnaris | 0.5–1.5(2) | 5–10 | ||
| Pronator teres | 0.75–1(2) | 5–10 | ||
| Brachioradialis | 0.75–1(2) | 5–10 | ||
| Flexor digitorum superficialis 1 | (0.5)1–1.5(2) | 5–10 | ||
| Flexor digitorum profundus 1 | (0.5)1–1.5(2) | 5–10 | ||
| Biceps brachii/Brachialis | 1–2 (2–3) | 5–10 | ||
| Adductor pollicis 1 | 0.3–1; Total max 10–15 U | 3–5; total max 45 (−75) U | ||
| Flexor pollicis brevis 1 | 0.3–1; Total max 10–15 U | 3–5; total max 45 (−75) U | ||
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sätilä, H. Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections? Toxins 2020, 12, 440. https://doi.org/10.3390/toxins12070440
Sätilä H. Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections? Toxins. 2020; 12(7):440. https://doi.org/10.3390/toxins12070440
Chicago/Turabian StyleSätilä, Heli. 2020. "Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections?" Toxins 12, no. 7: 440. https://doi.org/10.3390/toxins12070440
APA StyleSätilä, H. (2020). Over 25 Years of Pediatric Botulinum Toxin Treatments: What Have We Learned from Injection Techniques, Doses, Dilutions, and Recovery of Repeated Injections? Toxins, 12(7), 440. https://doi.org/10.3390/toxins12070440




