Corn Flour Intake, Aflatoxin B1 Exposure, and Risk of Esophageal Precancerous Lesions in a High-Risk Area of Huai’an, China: A Case-Control Study
Abstract
1. Introduction
2. Results
2.1. Socio-Demographic Characteristics of the Subjects
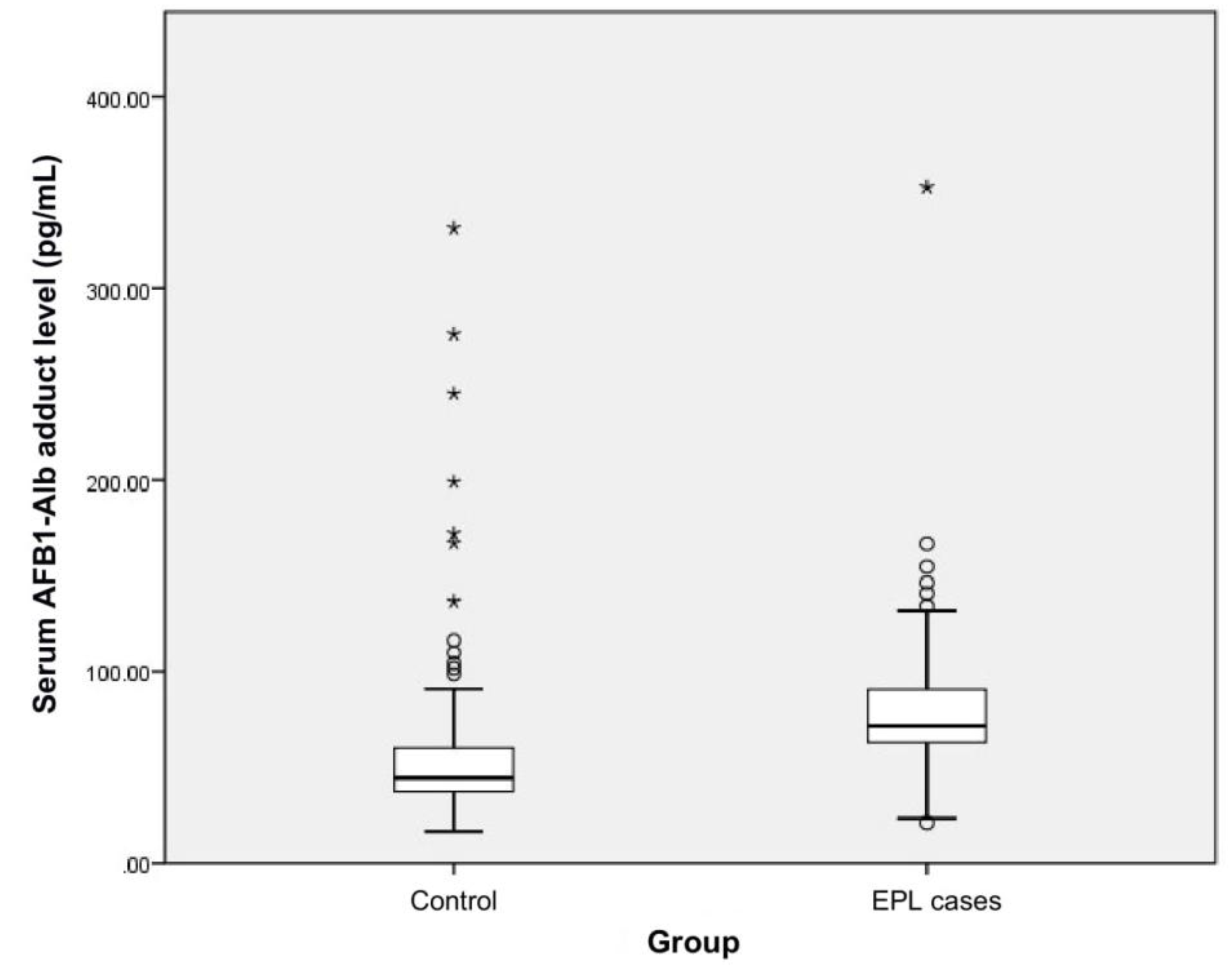
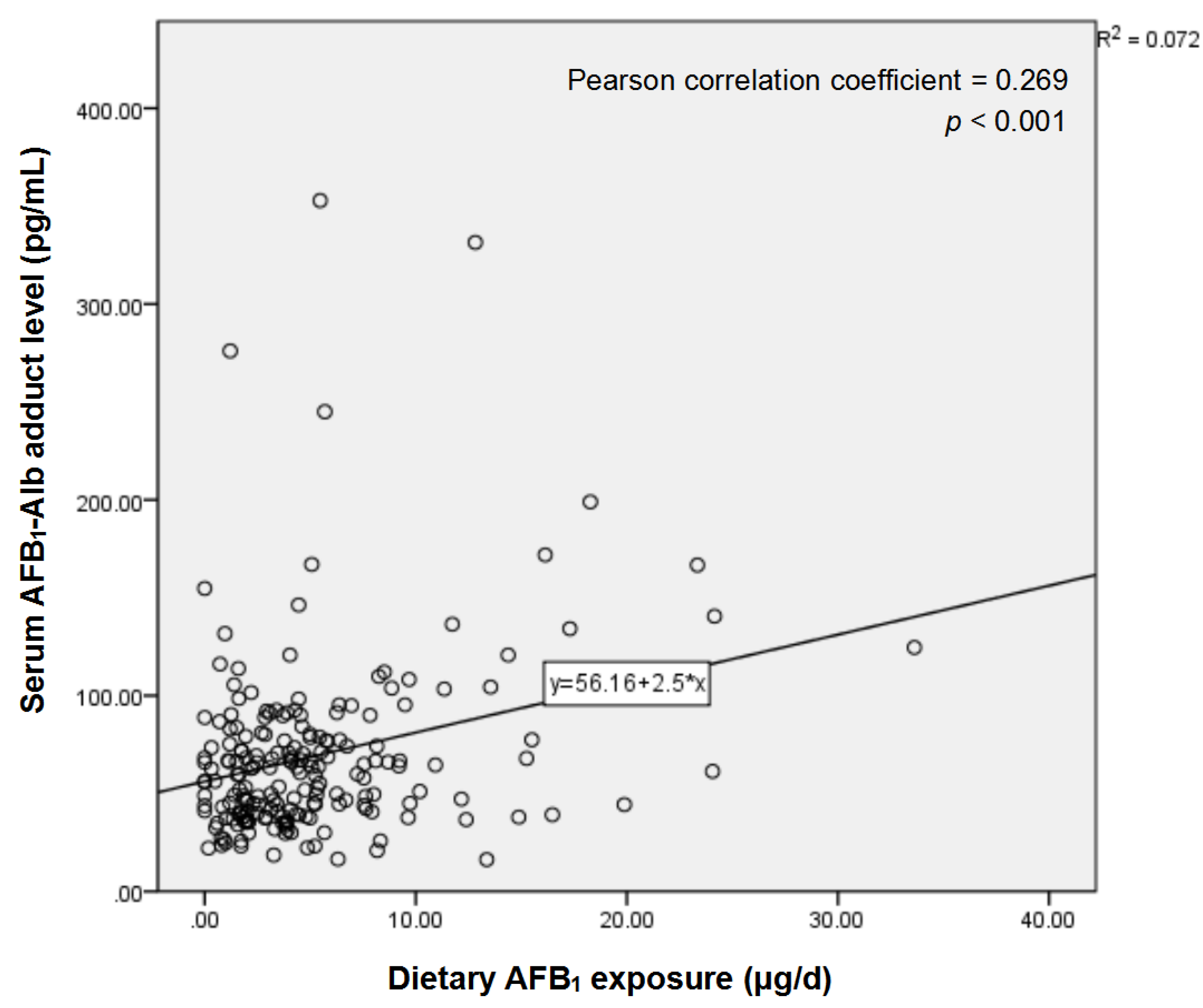
2.2. Serum AFB1-Alb Adduct Level and Dietary AFB1 Exposure of the Subjects
2.3. Association between AFB1-Related Variables and Risk of EPL
2.4. Association between Frequency of Corn Flour Intake and AFB1-Related Variables
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Study Population
5.2. EPL Diagnosis
5.3. Sample Collection
5.4. Determination of Dietary AFB1Exposure and Serum AFB1-Alb Adduct Level
5.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, A.K.; El-Serag, H.B. Esophageal carcinoma. N. Engl. J. Med. 2014, 371, 2499. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381. [Google Scholar] [CrossRef]
- Wang, G.; Abnet, C.; Shen, Q.; Lewin, K.; Sun, X.; Roth, M.; Qiao, Y.; Mark, S.; Dong, Z.; Taylor, P. Histological precursors of oesophageal squamous cell carcinoma: Results from a 13 year prospective follow up study in a high risk population. Gut 2005, 54, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Su, M.; Zhang, T.; Miao, C.; Fu, L.; Yang, L.; Song, G.; Raine, P.J.; Wang, S.; Sun, G. A Distinct Epidemiologic Pattern of Precancerous Lesions of Esophageal Squamous Cell Carcinoma in a High-risk Area of Huai’an, Jiangsu Province, China. Cancer Prev. Res. 2019, 12, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef]
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup report: Public health strategies for reducing aflatoxin exposure in developing countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef]
- European Food Safety Authority. Effects on public health of an increase of the levels for aflatoxin total from 4 µg/kg to 10 µg/kg for tree nuts other than almonds, hazelnuts and pistachios—Statement of the Panel on Contaminants in the Food Chain. Efsa J. 2009, 7, 1168. [Google Scholar]
- Bondoc, I. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part One: The Role of European Institutions in Laying Down and Passing Laws Specific to the Veterinary Sanitary and Food Safety Area. Universul Juridic Supliment. 2016, 12–15. Available online: http://revista.universuljuridic.ro/supliment/european-regulation-veterinary-sanitary-food-safety-area-component-european-policies-safety-food-products-protection-consumer-interests-2007-retrospective/ (accessed on 28 April 2020).
- Bondoc, I. European Regulation in the Veterinary Sanitary and Food Safety Area, a Component of the European Policies on the Safety of Food Products and the Protection of Consumer Interests: A 2007 Retrospective. Part Two: Regulations. Universul Jurid. Supliment. 2016, 16–19. Available online: http://revista.universuljuridic.ro/supliment/european-regulation-veterinary-sanitary-food-safety-area-component-european-policies-safety-food-products-protection-consumer-interests-2007-retrospective-2/ (accessed on 28 April 2020).
- Guzman de Pena, D. Exposure to aflatoxin B1 in experimental animals and its public health significance. Salud Publica Mex. 2007, 49, 227–235. [Google Scholar]
- Wang, J.S.; Groopman, J.D. DNA damage by mycotoxins. Mutat. Res. 1999, 424, 167–181. [Google Scholar] [CrossRef]
- Savic, Z.; Dudas, T.; Loc, M.; Grahovac, M.; Budakov, D.; Jajic, I.; Krstovic, S.; Barosevic, T.; Krska, R.; Sulyok, M.; et al. Biological Control of Aflatoxin in Maize Grown in Serbia. Toxins 2020, 12, 162. [Google Scholar] [CrossRef]
- Makarananda, K.; Pengpan, U.; Srisakulthong, M.; Yoovathaworn, K.; Sriwatanakul, K. Monitoring of aflatoxin exposure by biomarkers. J. Toxicol. Sci. 1998, 23 (Suppl. 2), 155–159. [Google Scholar] [CrossRef]
- Chu, Y.J.; Yang, H.I.; Wu, H.C.; Liu, J.; Wang, L.Y.; Lu, S.N.; Lee, M.H.; Jen, C.L.; You, S.L.; Santella, R.M.; et al. Aflatoxin B1 exposure increases the risk of cirrhosis and hepatocellular carcinoma in chronic hepatitis B virus carriers. Int. J. Cancer 2017, 141, 711–720. [Google Scholar] [CrossRef]
- Wild, C.P.; Hudson, G.J.; Sabbioni, G.; Chapot, B.; Hall, A.J.; Wogan, G.N.; Whittle, H.; Montesano, R.; Groopman, J.D. Dietary intake of aflatoxins and the level of albumin-bound aflatoxin in peripheral blood in The Gambia, West Africa. Cancer Epidemiol. Biomark. Prev. 1992, 1, 229–234. [Google Scholar]
- Scholl, P.F.; Groopman, J.D. Long-term stability of human aflatoxin B1 albumin adducts assessed by isotope dilution mass spectrometry and high-performance liquid chromatography-fluorescence. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1436–1439. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Sun, G.; Wang, S.; Hu, X.; Su, J.; Zhang, Y.; Xie, Y.; Zhang, H.; Tang, L.; Wang, J.S. Co-contamination of aflatoxin B1 and fumonisin B1 in food and human dietary exposure in three areas of China. Food Addit. Contam. 2011, 28, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.S.; Tang, L.L.; Sun, G.J.; Wang, S.K.; Hu, X.; Wang, J.S. Mycotoxin exposure is associated with increased risk of esophageal squamous cell carcinoma in Huaian area, China. BMC Cancer 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–494. [Google Scholar] [CrossRef] [PubMed]
- Domingo, J.L.; Perello, G.; Gine Bordonaba, J. Dietary intake of metals by the population of Tarragona County (Catalonia, Spain): Results from a duplicate diet study. Biol. Trace Elem. Res. 2012, 146, 420–425. [Google Scholar] [CrossRef] [PubMed]
- IARC. Mycotoxin Control in Low- and Middle-income Countries; Wild, C.P., Miller, J.D., Groopman, J.D., Eds.; IARC: Lyon, France, 2015.
- Chu, F.S.; Li, G.Y. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 1994, 60, 847–852. [Google Scholar] [CrossRef]
- Ghasemi-Kebria, F.; Joshaghani, H.; Taheri, N.S.; Semnani, S.; Aarabi, M.; Salamat, F.; Roshandel, G. Aflatoxin contamination of wheat flour and the risk of esophageal cancer in a high risk area in Iran. Cancer Epidemiol. 2013, 37, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, N.J. Risk assessment for aflatoxin: I. Metabolism of aflatoxin B1 by different species. Risk Anal. 1990, 10, 539–559. [Google Scholar] [CrossRef] [PubMed]
- Benasutti, M.; Ejadi, S.; Whitlow, M.D.; Loechler, E.L. Mapping the binding site of aflatoxin B1 in DNA: Systematic analysis of the reactivity of aflatoxin B1 with guanines in different DNA sequences. Biochemistry 1988, 27, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Su, M.; Huang, G.; Luo, P.; Zhang, T.; Fu, L.; Wei, J.; Wang, S.; Sun, G. MTHFR C677T genetic polymorphism in combination with serum vitamin B2, B12 and aberrant DNA methylation of P16 and P53 genes in esophageal squamous cell carcinoma and esophageal precancerous lesions: A case-control study. Cancer Cell Int. 2019, 19, 288. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Tan, W.; Zhang, S. P53 gene codon 72 polymorphism and risk of esophageal squamous cell carcinoma: A case/control study in a Chinese population. Dis. Esophagus 2008, 21, 139–143. [Google Scholar] [CrossRef]
- Zhao, L.J.; Zhao, X.L.; Wu, X.M.; Tang, W.R. Association of p53 Arg72Pro Polymorphism with Esophageal Cancer: A Meta-Analysis Based on 14 Case-Control Studies. Genet. Test Mol. Biomark. 2013, 17, 721–726. [Google Scholar] [CrossRef]
- Piao, J.M.; Kim, H.N.; Song, H.R.; Kweon, S.S.; Choi, J.S.; Yoon, J.Y.; Chung, I.J.; Kim, S.H.; Shin, M.H. p53 codon 72 polymorphism and the risk of esophageal cancer: A Korean case-control study. Dis. Esophagus 2011, 24, 596–600. [Google Scholar] [CrossRef]
- Ghebranious, N.; Sell, S. The mouse equivalent of the human p53ser249 mutation p53ser246 enhances aflatoxin hepatocarcinogenesis in hepatitis B surface antigen transgenic and p53 heterozygous null mice. Hepatology 1998, 27, 967–973. [Google Scholar] [CrossRef]
- Tong, W.M.; Lee, M.K.; Galendo, D.; Wang, Z.Q.; Sabapathy, K. Aflatoxin-B exposure does not lead to p53 mutations but results in enhanced liver cancer of Hupki (human p53 knock-in) mice. Int. J. Cancer 2006, 119, 745–749. [Google Scholar] [CrossRef]
- Zhang, T.; Pan, D.; Su, M.; Fu, L.M.; Miao, C.Y.; Yan, Q.Y.; Wang, J.; Yang, L.G.; Wang, S.K.; Sun, G.J. Determination of dietary nitrite in patients with esophageal pre-cancerous lesion and normal people: A duplicate diet study. Food Addit. Contam. Part A 2018, 35, 2298–2308. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, Q.; Gu, Y.; Pan, E.; Sun, Z.; Zhang, H.; Zhou, J.; Du, Y.; Zhang, Y.; Feng, Y.; et al. Distribution of N-nitrosamines in drinking water and human urinary excretions in high incidence area of esophageal cancer in Huai’an, China. Chemosphere 2019, 235, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.L.; Yang, L.; Su, M.; Wang, S.K.; Yin, H.; Wang, J.S.; Sun, G.J. Vitamin D3 and beta-carotene deficiency is associated with risk of esophageal squamous cell carcinoma—Results of a case-control study in China. Asian Pac. J. Cancer Prev. 2014, 15, 819–823. [Google Scholar] [CrossRef]
- Huang, G.; Wang, S.; Su, M.; Wang, T.; Yin, H.; Sun, G. MTHFR C677T polymorphism and genetic susceptibility of esophageal cancer and esophageal precancerous lesions. J. Hyg. Res. 2014, 43, 254–258. [Google Scholar]
- Huang, G.L.; Wang, S.K.; Su, M.; Wang, T.T.; Cai, H.Z.; Yin, H.; Sun, G.J. Serum Folate, MTHFR C677T Polymorphism and Esophageal Squamous Cell Carcinoma Risk. Biomed. Environ. Sci. 2013, 26, 1008–1012. [Google Scholar] [PubMed]
- Wang, Z.; Tang, L.; Sun, G.; Tang, Y.; Yin, X.; Wang, S.; Xu, H.; Gao, W.; Cox, S.B.; Wang, J.S. Etiological study of esophageal squamous cell carcinoma in an endemic region: A population-based case control study in Huaian, China. BMC Cancer 2006, 6, 287. [Google Scholar] [CrossRef]
- Taylor, P.R.; Abnet, C.C.; Dawsey, S.M. Squamous dysplasia—The precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol. Biomark. Prev. 2013, 22, 540–552. [Google Scholar] [CrossRef]
- Szumilas, M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 227–229. [Google Scholar]
| AFB1-Related Varibles | Control (n = 100) | EPL (n = 100) | p Value * |
|---|---|---|---|
| Serum AFB1-Alb adduct level (pg/mL) | 44.48 (36.88 < 59.95) | 71.40 (62.80–91.07) | <0.001 |
| Dietary AFB1 exposure (μg/d) | 3.47 (1.86–7.46) | 4.19 (1.74–6.15) | 0.891 |
| AFB1-Related Varibles | No. of Cases/Controls | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) * | p Value |
|---|---|---|---|---|---|
| Serum AFB1-Alb adduct level (pg/mL) | |||||
| Tertile 1 (16.25–45.00) | 14/54 | 1.00 (reference) | – | 1.00 (reference) | – |
| Tertile 2 (45.01–71.32) | 36/30 | 5.05 (2.01–2.70) | 0.001 | 8.11 (2.56–25.71) | <0.001 |
| Tertile 3 (71.33–352.85) | 50/16 | 11.69 (4.39–31.11) | <0.001 | 25.12 (7.29–87.80) | <0.001 |
| p for trend | <0.001 | <0.001 | |||
| Dietary AFB1 exposure (μg/d) | |||||
| Tertile 1 (0.00–2.33) | 31/36 | 1.00 (reference) | – | 1.00 (reference) | – |
| Tertile 2 (2.34–5.24) | 35/32 | 1.32 (0.63–2.74) | 0.463 | 1.41 (0.64–3.09) | 0.391 |
| Tertile 3 (5.25–33.62) | 34/32 | 1.25 (0.63–2.49) | 0.527 | 1.25 (0.58–2.68) | 0.575 |
| p for trend | 0.593 | 0.648 | |||
| Frequency of corn flour intake | |||||
| Less than once a month | 22/38 | 1.00 (reference) | – | 1.00 (reference) | – |
| Once a month–less than 4 times a week | 26/25 | 1.81 (0.87–3.78) | 0.115 | 1.72 (0.75–3.95) | 0.197 |
| 4 times a week–less than twice a day | 27/19 | 2.65 (1.18–5.98) | 0.019 | 3.22 (1.29–8.02) | 0.012 |
| Twice a day or more | 25/18 | 3.26 (1.23–8.61) | 0.017 | 3.56 (1.23–10.34) | 0.019 |
| p for trend | 0.024 | 0.017 | |||
| Mildew of stored grains | |||||
| No | 90/98 | 1.00 (reference) | – | 1.00 (reference) | – |
| Yes | 10/2 | 5.00 (1.10–22.82) | 0.038 | 10.28 (1.44–73.22) | 0.020 |
| AFB1-Related Varibles | Frequency of Corn Flour Intake | |||
|---|---|---|---|---|
| Less Than Once a Month | Once a Month–Less Than 4 Times a Week | 4 Times a Week–Less Than Twice a Day | Twice a Day or More | |
| Serum AFB1-Alb adduct level * | ||||
| No. of ≤60.97/>60.97 pg/mL | 36/24 | 25/26 | 24/22 | 15/28 |
| Adjusted OR (95% CI) † | 1.00 (reference) | 1.26 (0.54–2.90) | 1.59 (0.68–3.69) | 3.72 (1.54–9.04) |
| p value | 0.594 | 0.282 | 0.004 | |
| p for trend | 0.003 | |||
| Dietary AFB1 exposure * | ||||
| No. of ≤3.92/>3.92 μg/d | 38/22 | 32/19 | 19/27 | 11/32 |
| Adjusted OR (95% CI) † | 1.00 (reference) | 1.01 (0.42–2.42) | 3.04 (1.26–7.34) | 7.35 (2.85–19.01) |
| p value | 0.982 | 0.013 | <0.001 | |
| p for trend | <0.001 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Pan, D.; Zhang, T.; Su, M.; Sun, G.; Wei, J.; Guo, Z.; Wang, K.; Song, G.; Yan, Q. Corn Flour Intake, Aflatoxin B1 Exposure, and Risk of Esophageal Precancerous Lesions in a High-Risk Area of Huai’an, China: A Case-Control Study. Toxins 2020, 12, 299. https://doi.org/10.3390/toxins12050299
Wang S, Pan D, Zhang T, Su M, Sun G, Wei J, Guo Z, Wang K, Song G, Yan Q. Corn Flour Intake, Aflatoxin B1 Exposure, and Risk of Esophageal Precancerous Lesions in a High-Risk Area of Huai’an, China: A Case-Control Study. Toxins. 2020; 12(5):299. https://doi.org/10.3390/toxins12050299
Chicago/Turabian StyleWang, Shaokang, Da Pan, Ting Zhang, Ming Su, Guiju Sun, Jie Wei, Ziqi Guo, Kai Wang, Guang Song, and Qingyang Yan. 2020. "Corn Flour Intake, Aflatoxin B1 Exposure, and Risk of Esophageal Precancerous Lesions in a High-Risk Area of Huai’an, China: A Case-Control Study" Toxins 12, no. 5: 299. https://doi.org/10.3390/toxins12050299
APA StyleWang, S., Pan, D., Zhang, T., Su, M., Sun, G., Wei, J., Guo, Z., Wang, K., Song, G., & Yan, Q. (2020). Corn Flour Intake, Aflatoxin B1 Exposure, and Risk of Esophageal Precancerous Lesions in a High-Risk Area of Huai’an, China: A Case-Control Study. Toxins, 12(5), 299. https://doi.org/10.3390/toxins12050299








