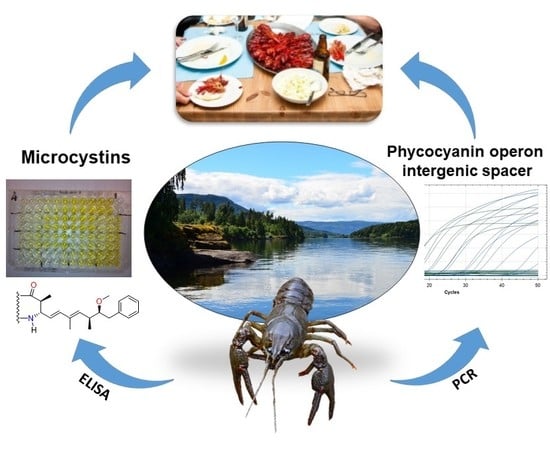
Microcystins in European Noble Crayfish Astacus astacus in Lake Steinsfjorden, a Planktothrix-Dominated Lake
Abstract
1. Introduction
2. Results and Discussion
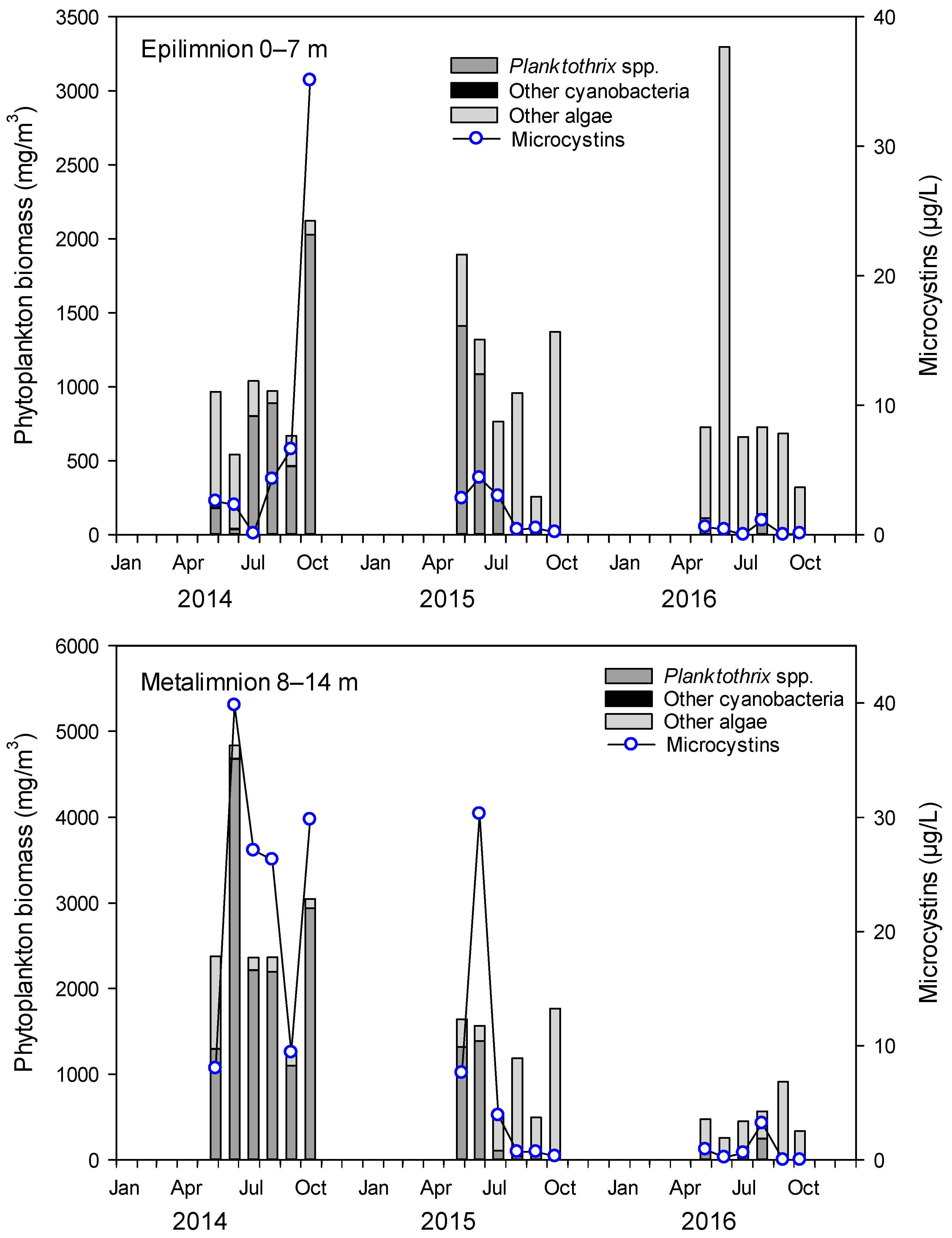
2.1. Cyanobacteria and MCs in Lake Steinsfjorden
2.2. Planktothrix in the Crayfish Diet
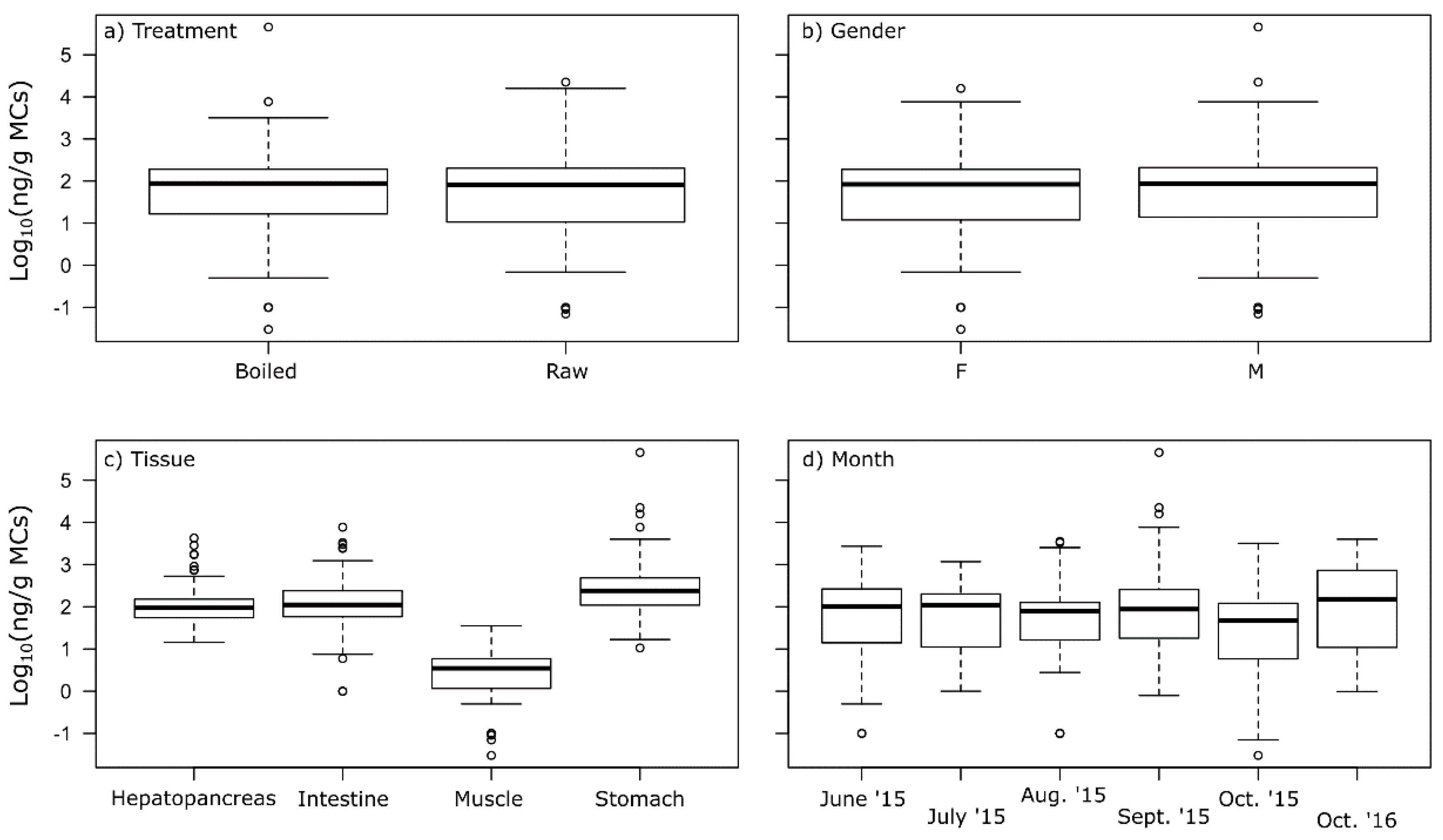
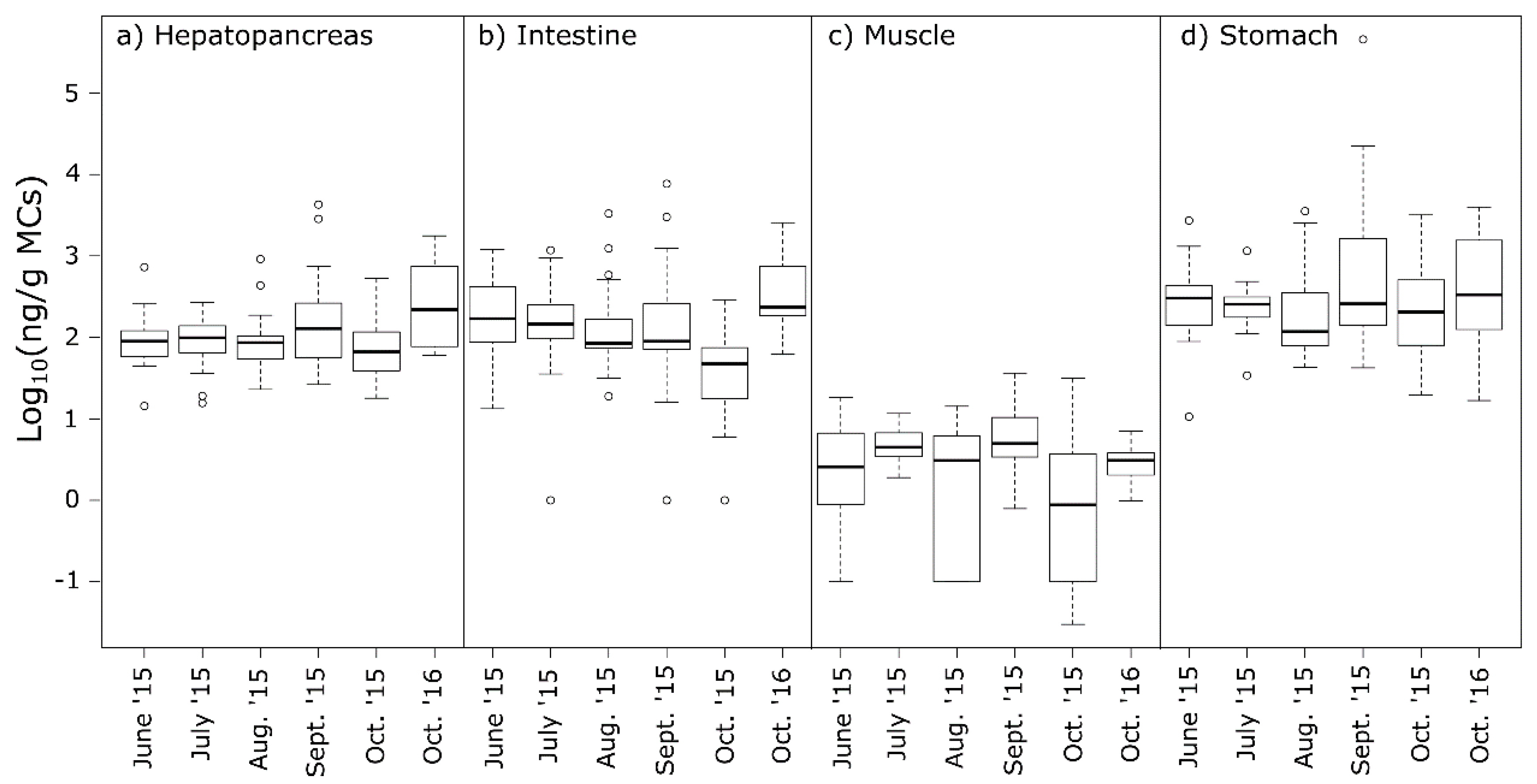
2.3. MCs in Crayfish Tissues
2.4. Food Safety
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Water samples
4.3. Phytoplankton
4.4. Noble Crayfish (A. astacus)
4.5. Adda-ELISA
4.6. Multihapten-ELISA
4.7. DNA Isolation
4.8. PCR
4.9. Statistics
4.10. TDI Calculations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Skurdal, J.; Garnås, E.; Taugbøl, T. Management strategies, yield and population development of the noble crayfish Astacus astacus in lake steinsfjorden. Bull. Fr. Pêche Piscic. 2002, 845–860. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Version 2019-3. Available online: http://www.iucnredlist.org (accessed on 26 February 2020).
- Henriksen, S.; Hilmo, O. Norsk Rødliste for Arter 2015. Artsdatabanken, Norge 2015. Available online: https://www.artsdatabanken.no/Rodliste/Resultater (accessed on 26 February 2020).
- Johnsen, S.I.; Vrålstad, T. Edelkreps (Astacus astacus)—Naturfaglig Utredning og Forslag til Samordning av Overvåkingsprogrammene for Edelkreps og Krepsepest; NINA: Trondheim, Norway, 2017; p. 39. Available online: https://brage.nina.no/nina-xmlui/handle/11250/2446549 (accessed on 26 February 2020).
- Johnsen, S.I. Nasjonal Overvåkning av Edelkreps−Presentasjon av Overvåkningsdata og Bestandstatus; NINA: Lillehammer, Norway, 2010; p. 100. Available online: http://www.nina.no/archive/nina/PppBasePdf/rapport/2010/492.pdf (accessed on 22 January 2018).
- Johnsen, S.I.; Skurdal, J.; Garnås, E. Status og Overvåkning av Krepsebestanden i Steinsfjorden i Buskerud 1979−2014; NINA: Lillehammer, Norway, 2015; p. 40. ISBN 978-82-426-2664-6. Available online: http://hdl.handle.net/11250/2397754 (accessed on 20 January 2018).
- Halstvedt, C.B.; Rohrlack, T.; Andersen, T.; Skulberg, O.; Edvardsen, B. Seasonal dynamics and depth distribution of Planktothrix spp. in Lake Steinsfjorden (Norway) related to environmental factors. J. Plankton Res. 2007, 29, 471–482. [Google Scholar] [CrossRef]
- Rohrlack, T.; Edvardsen, B.; Skulberg, R.; Halstvedt, C.B.; Utkilen, H.; Ptacnik, R.; Skulberg, O.M. Oligopeptide chemotypes of the toxic freshwater cyanobacterium Planktothrix can form subpopulations with dissimilar ecological traits. Limnol. Oceanogr. 2008, 53, 1279–1293. [Google Scholar] [CrossRef]
- Walsby, A.E.; Ng, G.; Dunn, C.; Davis, P.A. Comparison of the depth where Planktothrix rubescens stratifies and the depth where the daily insolation supports its neutral buoyancy. New Phytol. 2004, 162, 133–145. [Google Scholar] [CrossRef]
- Halstvedt, C.B.; Ptacnik, R.; Rohrlack, T.; Edvardsen, B. On the effect of abiotic environmental factors on production of bioactive oligopeptides in field populations of Planktothrix spp. (Cyanobacteria). J. Plankton Res. 2008, 30, 607–617. [Google Scholar] [CrossRef]
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural Diversity, Characterization and Toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef]
- Miles, C.O.; Sandvik, M.; Haande, S.; Nonga, H.; Ballot, A. LC-MS Analysis with Thiol Derivatization to Differentiate [Dhb7]- from [Mdha7]-Microcystins: Analysis of Cyanobacterial Blooms, Planktothrix Cultures and European Crayfish from Lake Steinsfjorden, Norway. Environ. Sci. Technol. 2013, 47, 4080–4087. [Google Scholar] [CrossRef]
- Bownik, A. Effects of cyanobacterial toxins, microcystins on freshwater invertebrates. Pol. J. Nat. Sci. 2013, 28, 185–195. [Google Scholar]
- Vinagre, T.M.; Alciati, J.C.; Yunes, J.S.; Richards, J.; Bianchini, A.; Monserrat, J.M. Effects of Extracts from the Cyanobacterium Microcystis aeruginosa on Ion Regulation and Gill Na+,K+-ATPase and K+-Dependent Phosphatase Activities of the Estuarine Crab Chasmagnathus granulata (Decapoda, Grapsidae). Physiol. Biochem. Zool. 2002, 75, 600–608. [Google Scholar] [CrossRef]
- Lirås, V.; Lindberg, M.; Nyström, P.; Annadotter, H.; Lawton, L.; Graf, B. Can ingested cyanobacteria be harmful to the signal crayfish (Pacifastacus leniusculus)? Freshw. Boil. 1998, 39, 233–242. [Google Scholar] [CrossRef]
- Tricarico, E.; Bertocchi, S.; Brusconi, S.; Casalone, E.; Gherardi, F.; Giorgi, G.; Mastromei, G.; Parisi, G. Depuration of microcystin-LR from the red swamp crayfish Procambarus clarkii with assessment of its food quality. Aquaculture 2008, 285, 90–95. [Google Scholar] [CrossRef]
- Gutiérrez-Praena, D.; Jos, A.; Pichardo, S.; Moreno, I.M.; Cameán, A.M. Presence and bioaccumulation of microcystins and cylindrospermopsin in food and the effectiveness of some cooking techniques at decreasing their concentrations: A review. Food Chem. Toxicol. 2013, 53, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, T.; Kagalou, I.; Stalikas, C.; Pilidis, G.; Leonardos, I.D. Assessment of microcystin distribution and biomagnification in tissues of aquatic food web compartments from a shallow lake and evaluation of potential risks to public health. Ecotoxicology 2012, 21, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Aune, T.; Ramstad, H.; Skulberg, O.M.; Underdal, B.; Yndestad, M.; Østensvik, Ø. Cyanobakterier og Edelkreps–Toksinproduserende Blågrønnalger i Steinsfjorden Sommeren 1997; Norges veterinærhøgskole, Institutt for farmakologi, mikrobiologi og næringsmiddelhygiene and Norsk institutt for vannforskning: Oslo, Norway, 1997. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; Available online: http://whqlibdoc.who.int/publications/2011/9789241548151_eng.pdf (accessed on 20 February 2020).
- Garcia, A.C.; Bargu, S.; Dash, P.; Rabalais, N.N.; Sutor, M.; Morrison, W.; Walker, N. Evaluating the potential risk of microcystins to blue crab (Callinectes sapidus) fisheries and human health in a eutrophic estuary. Harmful Algae 2010, 9, 134–143. [Google Scholar] [CrossRef]
- Haande, S. Overvåking av Steinsfjorden 2015; NIVA: Oslo, Norway, 2015; p. 14. Available online: https://www.hole.kommune.no/getfile.php/4385704.2423.pmsmustbqzbssj/STEINSFJORDEN_NOTAT_2015.pdf (accessed on 13 November 2019).
- Ballot, A. Overvåking av Steinsfjorden 2016; NIVA: Oslo, Norway, 2016; p. 14. Available online: https://www.hole.kommune.no/getfile.php/3882432.2423.napbsujuwmwlnq/STEINSFJORDEN_NOTAT_2016.pdf (accessed on 13 November 2019).
- Johnsen, S.I.; Strand, D.; Rusch, J.; Vrålstad, T. Nasjonal Overvåking av Edelkreps og Spredning av Signalkreps-Presentasjon av Overvåkingsdata og Bestandsstatus; NINA Rapport 1590. 114 s; NINA: Trondheim, Norway, 2019; p. 114. Available online: https://brage.nina.no/nina-xmlui/handle/11250/2589548 (accessed on 12 March 2020).
- Vasconcelos, V.; Oliveira, S.; Teles, L.O. Impact of a toxic and a non-toxic strain of Microcystis aeruginosa on the crayfish Procambarus clarkii. Toxicon 2001, 39, 1461–1470. [Google Scholar] [CrossRef]
- Magalhães, V.; Marinho, M.M.; Domingos, P.; Oliveira, A.C.; Costa, S.M.; Azevedo, L.O.; Azevedo, S.M.F.O. Microcystins (cyanobacteria hepatotoxins) bioaccumulation in fish and crustaceans from Sepetiba Bay (Brasil, RJ). Toxicon 2003, 42, 289–295. [Google Scholar] [CrossRef]
- Gaget, V.; Humpage, A.R.; Huang, Q.; Monis, P.T.; Brookes, J.D. Benthic cyanobacteria: A source of cylindrospermopsin and microcystin in Australian drinking water reservoirs. Water Res. 2017, 124, 454–464. [Google Scholar] [CrossRef]
- Uriza, E.A.C.; Asencio, A.D.; Aboal, M. Are We Underestimating Benthic Cyanotoxins? Extensive Sampling Results from Spain. Toxins 2017, 9, 385. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Verein. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier/Spektrum: Heidelberg, Germany, 2005; Volume 13/2, p. 759. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer: Jena-Stuttgart-Lübeck-Ulm, Germany, 1998; Volume 19/1, p. 548. [Google Scholar]
- Komárek, J. Cyanoprokaryota 3: Heterocytous genera. In Süsswasserflora von Mitteleuropa 19/3; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier/Spektrum: Heidelberg, Germany, 2013; Volume 19/3, p. 1130. [Google Scholar]
- Büdel, B.; Gärtner, G.; Krienitz, L.; Schagerl, M. Süsswasserflora von Mitteleuropa, Freshwater Flora of Central Europe Volume 1–19; Spektrum Akademischer Verlag: Heidelberg, Germany, 1975−2015.
- Samdal, I.A.; Ballot, A.; Løvberg, K.E.; Miles, C.O. Multihapten Approach Leading to a Sensitive ELISA with Broad Cross-Reactivity to Microcystins and Nodularin. Environ. Sci. Technol. 2014, 48, 8035–8043. [Google Scholar] [CrossRef]
- Kalendar, R.; Khassenov, B.; Ramankulov, Y.; Samuilova, O.; Ivanov, K.; Ramanculov, Y.M. FastPCR: An in silico tool for fast primer and probe design and advanced sequence analysis. Genomics 2017, 109, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Agersnap, S.; Larsen, W.B.; Knudsen, S.; Strand, D.; Thomsen, P.F.; Hesselsøe, M.; Mortensen, P.B.; Vrålstad, T.; Møller, P.R. Monitoring of noble, signal and narrow-clawed crayfish using environmental DNA from freshwater samples. PLoS ONE 2017, 12, e0179261. [Google Scholar] [CrossRef] [PubMed]
- R Studio Team. Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2016; Available online: https://www.R-project.org/ (accessed on 26 February 2020).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 26 February 2019).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-137. R Core Team 2018. Available online: https://cran.r-project.org/package=nlme (accessed on 26 February 2019).
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; Volume XXII, p. 574. [Google Scholar]


| Crayfish sample | Location | Month | Year | Planktothrix PC-IGS | A. astacus COI | MCs by ELISA (ng/g) |
|---|---|---|---|---|---|---|
| S74 | L. Steinsfjorden | June | 2015 | + | + | 362 |
| S75 | L. Steinsfjorden | June | 2015 | + | + | 1310 |
| S66 | L. Steinsfjorden | June | 2015 | + | + | 89 |
| S62 | L. Steinsfjorden | June | 2015 | + | + | 292 |
| S50 | L. Steinsfjorden | June | 2015 | + | + | 111 |
| S96 | L. Steinsfjorden | Sept | 2015 | + | + | 42 |
| S91 | L. Steinsfjorden | Sept | 2015 | − | + | 140 |
| S87 | L. Steinsfjorden | Sept | 2015 | − | + | 15856 |
| S95 | L. Steinsfjorden | Sept | 2015 | + | + | 69 |
| S97 | L. Steinsfjorden | Sept | 2015 | + | + | 22434 |
| Ex1 | L. Steinsfjorden | Oct | 2016 | − | + | 1555 |
| Ex2 | L. Steinsfjorden | Oct | 2016 | − | + | 25 1 |
| Ex3 | L. Steinsfjorden | Oct | 2016 | − | + | 3873 |
| Ex4 | L. Steinsfjorden | Oct | 2016 | − | + | 345 |
| Ex5 | L. Steinsfjorden | Oct | 2016 | − | + | 322 |
| Ex6 | L. Steinsfjorden | Oct | 2016 | − | + | 258 |
| Ex7 | L. Steinsfjorden | Oct | 2016 | − | + | 3982 |
| Ex8 | L. Steinsfjorden | Oct | 2016 | − | + | 932 |
| Ex9 | L. Steinsfjorden | Oct | 2016 | − | + | 171 |
| Ex10 | L. Steinsfjorden | Oct | 2016 | − | + | 125 |
| 1A1 | Control (Kasa) | 2016 | − | + | n.a. 2 | |
| 1B1 | Control (Kasa) | 2016 | − | + | n.a. 2 | |
| 4A1 | Control (Kasa) | 2016 | − | + | n.a. 2 | |
| 4B1 | Control (Kasa) | 2016 | − | + | n.a. 2 |
| Value | Std. error | p-value | |
|---|---|---|---|
| Intercept (Hepatopancreas) | 2.052 | 0.064 | <0.001 |
| Month (October_2015) | −0.203 | 0.151 | 0.180 |
| Intestine | 0.120 | 0.063 | 0.058 |
| Muscle | −1.589 | 0.063 | <0.001 |
| Stomach | 0.419 | 0.063 | <0.001 |
| Month (October_2015)*Intestine | −0.430 | 0.149 | 0.004 |
| Month (October_2015)*Muscle | −0.339 | 0.149 | 0.023 |
| Month (October_2015)*Stomach | 0.048 | 0.149 | 0.747 |
| Month | Weight of Tail Muscle (g) | Number of Noble Crayfish 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| w/intestine | w/o intestine | w/intestine | w/o intestine | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| June | 165 | 152 | 484 | 417 | 41 | 38 | 121 | 104 |
| July | 175 | 186 | 451 | 822 | 44 | 46 | 113 | 206 |
| August | 155 | 83 | 629 | 621 | 39 | 21 | 157 | 155 |
| September | 76 | 35 | 283 | 280 | 19 | 9 | 71 | 70 |
| October | 462 | 264 | 766 | 349 | 115 | 66 | 192 | 87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samdal, I.A.; Strand, D.A.; Ballot, A.; Rusch, J.C.; Haande, S.; Løvberg, K.L.E.; Miles, C.O.; Vrålstad, T. Microcystins in European Noble Crayfish Astacus astacus in Lake Steinsfjorden, a Planktothrix-Dominated Lake. Toxins 2020, 12, 298. https://doi.org/10.3390/toxins12050298
Samdal IA, Strand DA, Ballot A, Rusch JC, Haande S, Løvberg KLE, Miles CO, Vrålstad T. Microcystins in European Noble Crayfish Astacus astacus in Lake Steinsfjorden, a Planktothrix-Dominated Lake. Toxins. 2020; 12(5):298. https://doi.org/10.3390/toxins12050298
Chicago/Turabian StyleSamdal, Ingunn Anita, David Allan Strand, Andreas Ballot, Johannes Christopher Rusch, Sigrid Haande, Kjersti Liv Eriksen Løvberg, Christopher Owen Miles, and Trude Vrålstad. 2020. "Microcystins in European Noble Crayfish Astacus astacus in Lake Steinsfjorden, a Planktothrix-Dominated Lake" Toxins 12, no. 5: 298. https://doi.org/10.3390/toxins12050298
APA StyleSamdal, I. A., Strand, D. A., Ballot, A., Rusch, J. C., Haande, S., Løvberg, K. L. E., Miles, C. O., & Vrålstad, T. (2020). Microcystins in European Noble Crayfish Astacus astacus in Lake Steinsfjorden, a Planktothrix-Dominated Lake. Toxins, 12(5), 298. https://doi.org/10.3390/toxins12050298