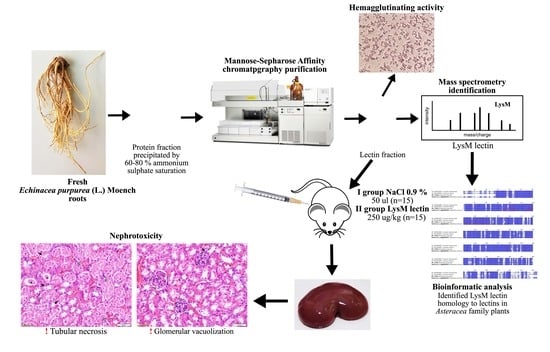
Identification of Echinacea Purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties
Abstract
1. Introduction
2. Results
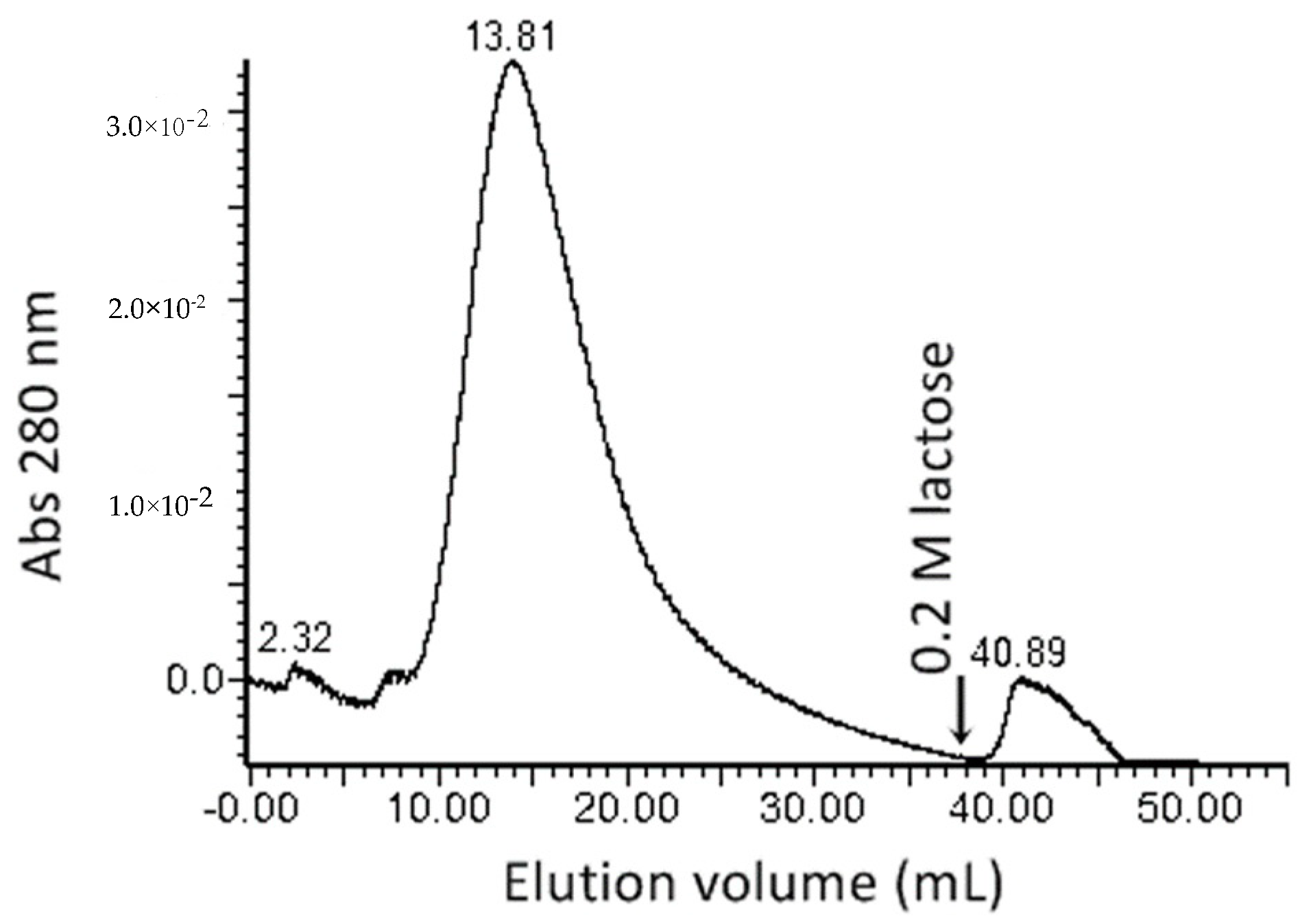
2.1. Protein Extraction, Lectin Purification, and Evaluation of Hemagglutinating Activity
2.2. Biochemical Characterisation of Purified Lectin
2.2.1. Hemagglutinating Activity Inhibition by Carbohydrates Test Results
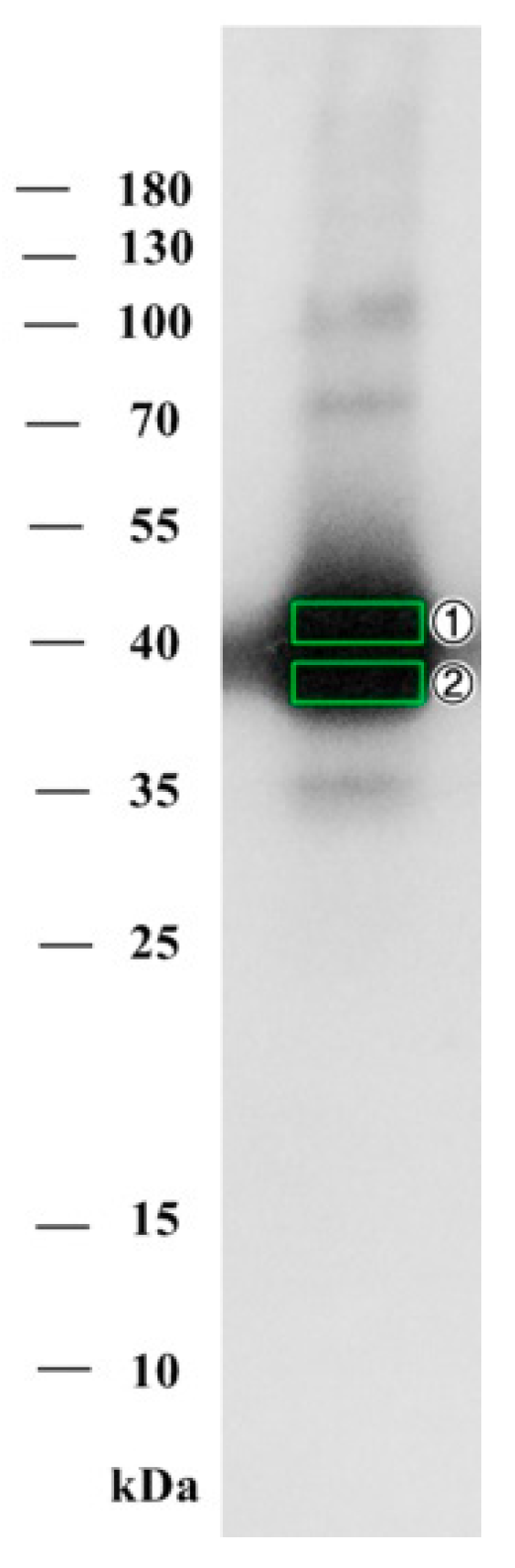
2.2.2. Purified Hemagglutinating Active Fraction Analysis by SDS-PAGE and Western Blot
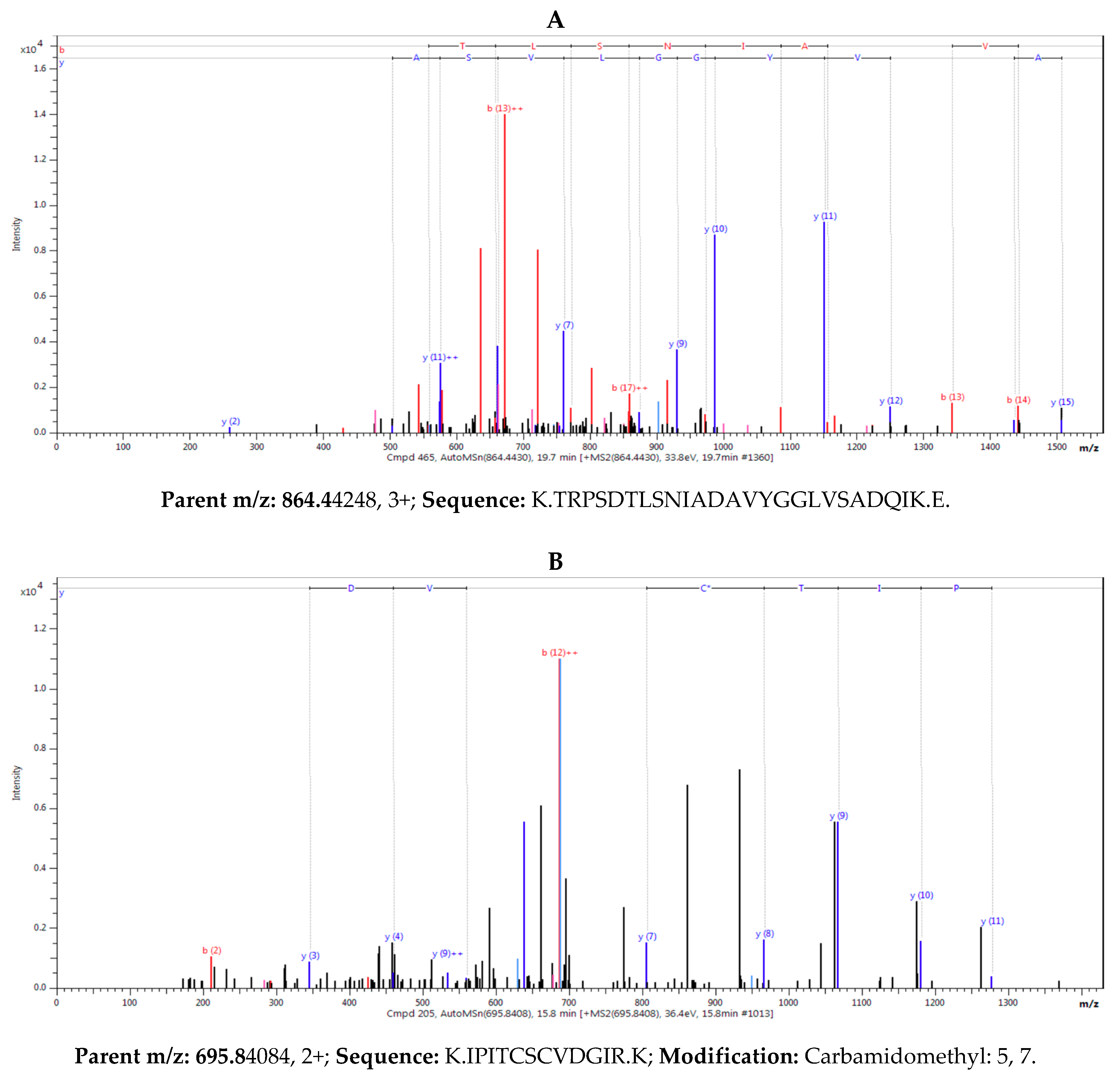
2.2.3. Lectin Identification
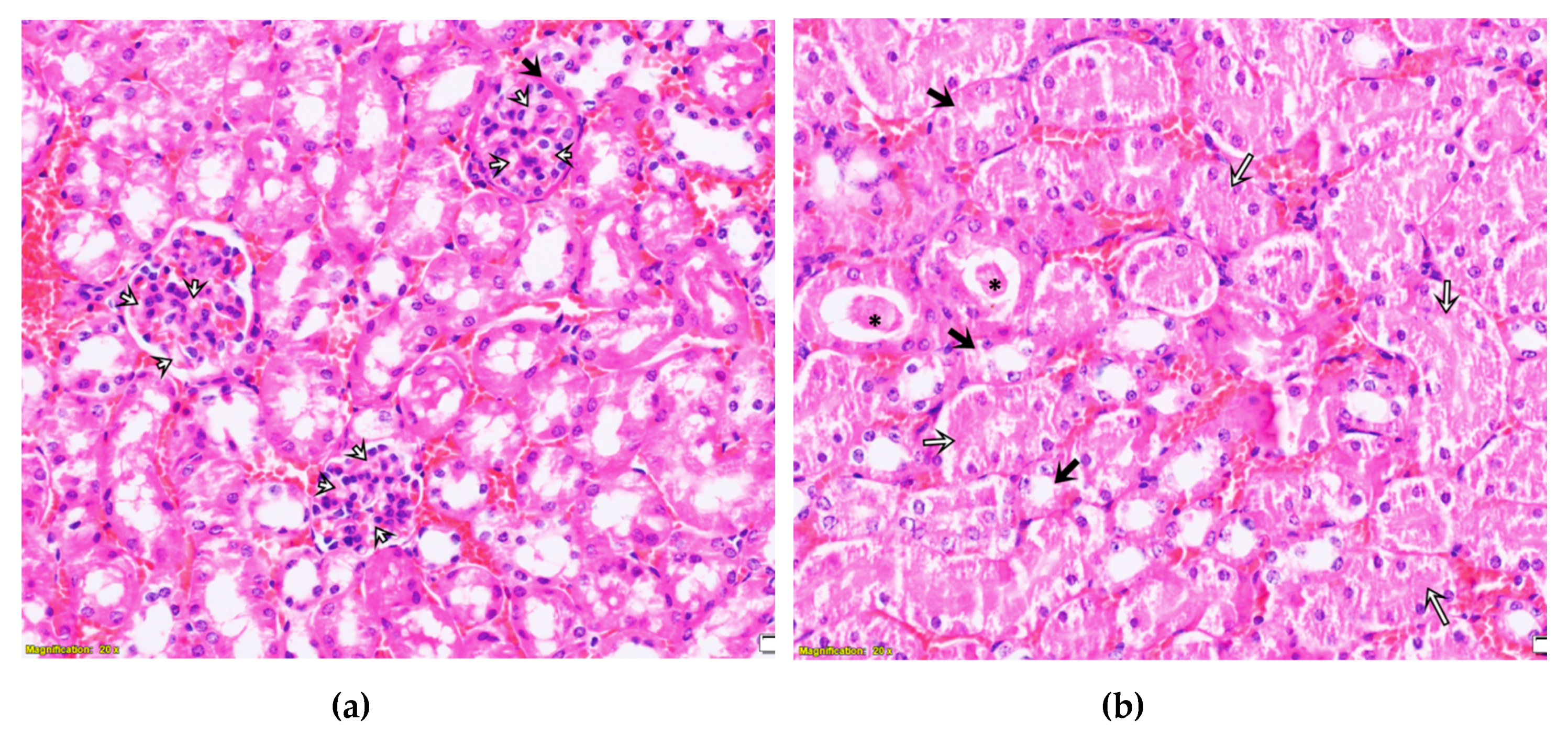
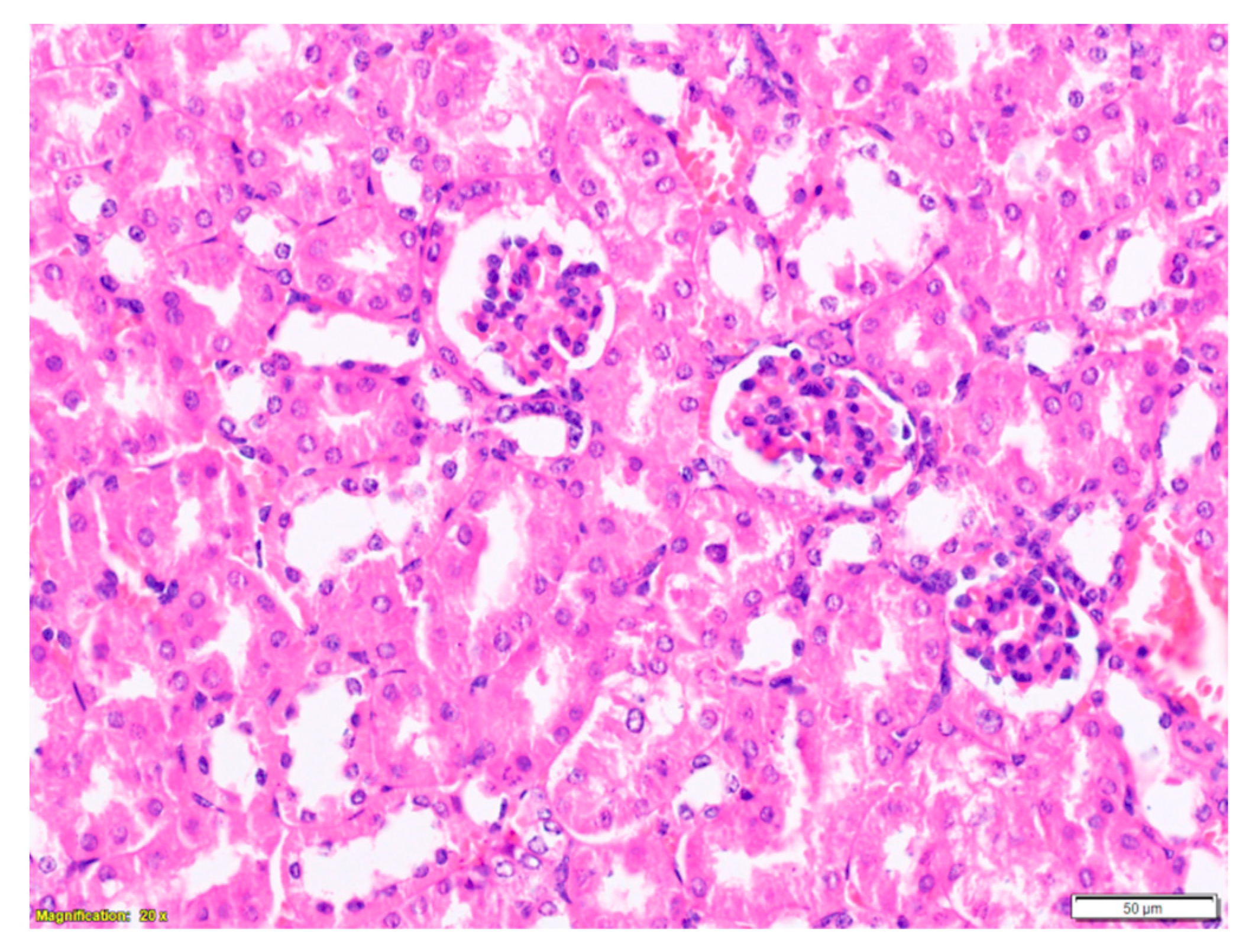
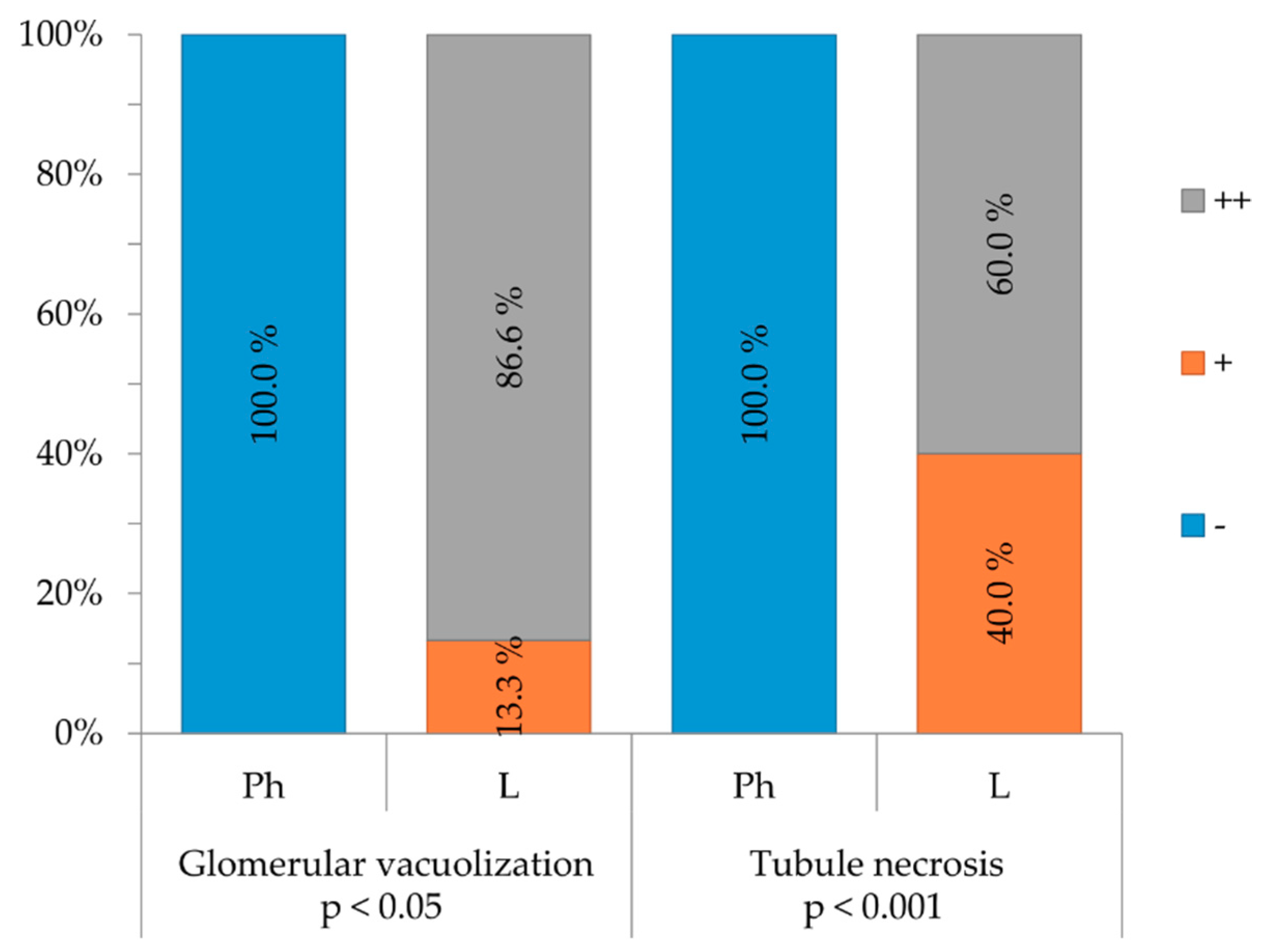
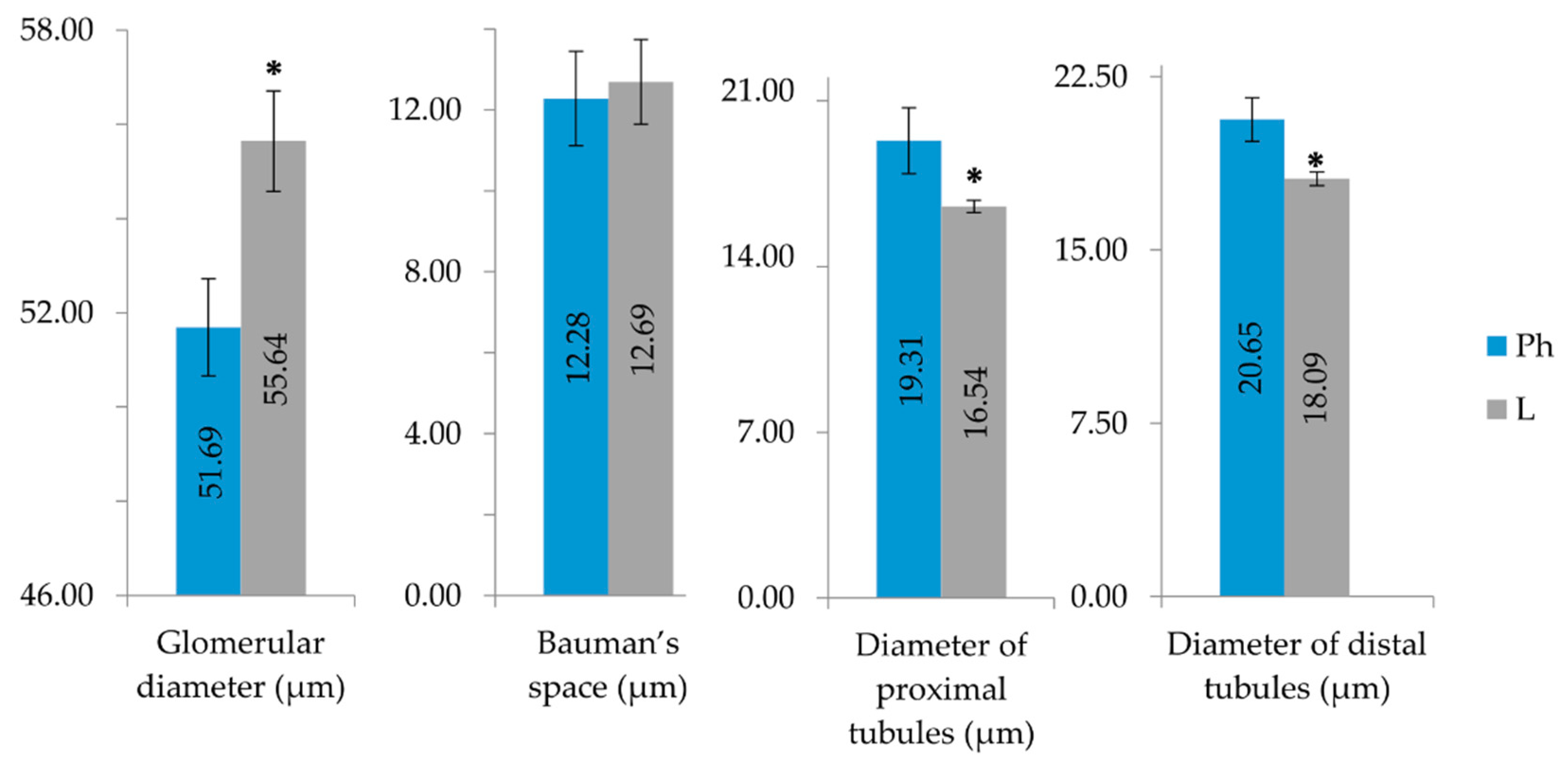
2.3. Purified LysM Lectin Impact to Kidney Morphology
3. Discussion
4. Materials and Methods
4.1. Herbal Material Preparation
4.2. Preparation of Crude Extract and Protein Precipitation
4.3. Lectin Separation by Affinity Chromatography
4.4. Biochemical Characterisation of Purified Lectin
4.4.1. Hemagglutination Assay
4.4.2. Hemagglutination Inhibition Assay
4.4.3. SDS-PAGE and Western Blotting
4.4.4. Protein In-Gel Digestion, Sample Preparation, and Mass Spectrometry Analysis
4.5. Evaluation of LysM Lectin Biological Activity In Vivo
4.5.1. Animals
4.5.2. Experimental Model
4.5.3. Histological Analysis of Kidney Samples
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| NCBI Blast Annotation | ||||||||
|---|---|---|---|---|---|---|---|---|
| Database Accession | MW, kDa | pI | Mascot Score | Number of Peptides | SC, % | RMS90, ppm | NCBI RefSeq Accession | Protein Name |
| Echinacea purpurea transcriptome | ||||||||
| epa_locus_7790_iso_1_len_1277_ver_2 | 40.1 | 4.5 | 184.6 | 2 | 9.5 | 6.21 | XP_023762850.1 | lysM domain-containing GPI-anchored protein 1-like |
| epa_locus_17753_iso_4_len_2582_ver_2 | 82.3 | 5 | 431.0 | 6 | 8.8 | 5.51 | XP_022020643.1 | glycerophosphodiester phosphodiesterase GDPDL3-like |
| Helianthus annus genome | ||||||||
| Ha412v1r1_08g009630 | 43.3 | 4.7 | 182.0 | 2 | 8.8 | 6.21 | XP_021976452.1 | lysM domain-containing GPI-anchored protein 1-like |
| Ha412v1r1_11g016880 | 37.6 | 6.3 | 259.2 | 2 | 5.4 | 4.09 | XP_021992913.1 | early nodulin-like protein 2 isoform X4 |
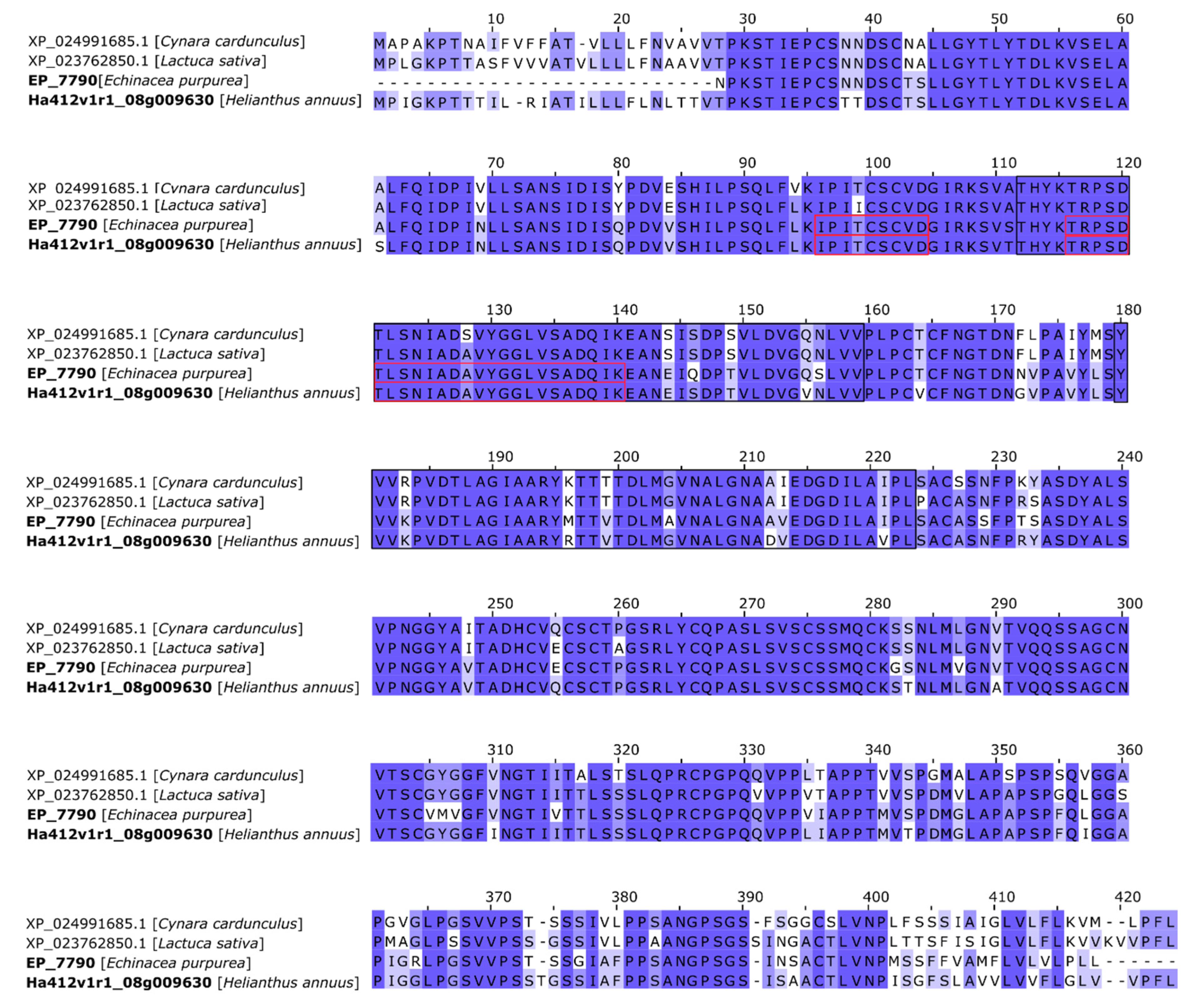
Appendix B
References
- Ribeiro, A.C.; Ferreira, R.; Freitas, R. Plant Lectins: Bioactivities and Bioapplications In Studies in Natural. Products Chemistry, 1st ed.; Rahman, A.U., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–42. [Google Scholar]
- Santos, A.F.S.; da Silva, M.D.C.; Napoleão, T.H.; Paiva, P.M.G.; Correia, M.T.; dos, S.; Coelho, L.C.B.B. Lectins: Function, structure, biological properties and potential applications. Curr. Top. Pept. Protein Res. 2014, 15, 41–62. [Google Scholar]
- Ishiguro, M.; Nakashima, H.; Tanabe, S.; Sakakibara, R. Biochemical Studies on Oral Toxicity of Ricin. Part III. Interaction of Toxic Lection Ricin with Epithelial Cells of Rat Small Intestine in Vitro. Chem. Pharm. Bull. 1992, 40, 441–445. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rani, R.; Tandon, A.; Wang, J.; Kumar, S.; Gandhi, C.R. Stellate Cells Orchestrate Concanavalin A–Induced Acute Liver Damage. Am. J. Pathol. 2017, 187, 2008–2019. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.M.; Oliveira, J.T. Antinutritional properties of plant lectins. Toxicon Off. J. Int. Soc. Toxinol. 2004, 44, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Lopez Nunez, O.F.; Pizon, A.F.; Tamama, K. Ricin Poisoning after Oral Ingestion of Castor Beans: A Case Report and Review of the Literature and Laboratory Testing. J. Emerg. Med. 2017, 53, e67–e71. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.S.; Procópio, T.F.; Napoleão, T.H.; Coelho, L.C.B.B.; Paiva, P.M.G. Antimicrobial lectin from Schinus terebinthifolius leaf. J. Appl. Microbiol. 2013, 114, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kanokwiroon, K.; Teanpaisan, R.; Wititsuwannakul, D.; Hooper, A.B.; Wititsuwannakul, R. Antimicrobial activity of a protein purified from the latex of Hevea brasiliensis on oral microorganisms. Mycoses 2008, 51, 301–307. [Google Scholar] [CrossRef]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.J.; Shirakura, M.; et al. Antiviral Lectins from Red and Blue-Green Algae Show Potent In Vitro and In Vivo Activity against Hepatitis C Virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef]
- Vandenborre, G.; Smagghe, G.; Van Damme, E.J. Plant lectins as defense proteins against phytophagous insects. Phytochemistry 2011, 72, 1538–1550. [Google Scholar] [CrossRef]
- Tomatsu, M.; Mujin, T.; Shibamoto, N.; Tashiro, F.; Ikuta, A. Production of Aralin, a Selective Cytotoxic Lectin against Human Transformed Cells, in Callus Culture of Aralia elata. Planta Medica 2004, 70, 469–471. [Google Scholar]
- Singh, R.S.; Bhari, R.; Rana, V.; Tiwary, A.K. Immunomodulatory and Therapeutic Potential of a Mycelial Lectin from Aspergillus nidulans. Appl. Biochem. Biotechnol. 2011, 165, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Li, Y.; Jiang, Z.; Sun, Y.; Zhu, L.; Ding, Z. Antiproliferation and apoptosis of human tumor cell lines by a lectin (AMML) of Astragalus mongholicus. Phytomedicine 2009, 16, 586–593. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Barre, A.; Mazard, A.-M.; Verhaert, P.; Horman, A.; Debray, H.; Rouge, P.; Peumans, W.J. Characterization and molecular cloning of the lectin from Helianthus tuberosus. JBIC J. Boil. Inorg. Chem. 1999, 259, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, T.; Oniszczuk, A.; Gondek, E.; Guz, L.; Puk, K.; Kocira, A.; Kusz, A.; Kasprzak, K.; Wójtowicz, A. Active polyphenolic compounds, nutrient contents and antioxidant capacity of extruded fish feed containing purple coneflower (Echinacea purpurea (L.) Moench.). Saudi J. Boil. Sci. 2016, 26, 24–30. [Google Scholar] [CrossRef]
- Qu, L.; Chen, Y.; Wang, X.; Scalzo, R.; Davis, J.M. Patterns of Variation in Alkamides and Cichoric Acid in Roots and Aboveground Parts of Echinacea purpurea (L.) Moench. HortScience 2005, 40, 1239–1242. [Google Scholar] [CrossRef]
- Classen, B.; Thude, S.; Blaschek, W.; Wack, M.; Bodinet, C. Immunomodulatory effects of arabinogalactan-proteins from Baptisia and Echinacea. Phytomedicine 2006, 13, 688–694. [Google Scholar] [CrossRef]
- Pires, C.; Martins, N.; Carvalho, A.M.; Barros, L.; Ferreira, I.C. Phytopharmacologic preparations as predictors of plant bioactivity: A particular approach to Echinacea purpurea (L.) Moench antioxidant properties. Nutrition 2016, 32, 834–839. [Google Scholar] [CrossRef]
- Dapas, B.; Dall’Acqua, S.; Bulla, R.; Agostinis, C.; Perissutti, B.; Invernizzi, S.; Grassi, G.; Voinovich, D. Immunomodulation mediated by a herbal syrup containing a standardized Echinacea root extract: A pilot study in healthy human subjects on cytokine gene expression. Phytomedicine 2014, 21, 1406–1410. [Google Scholar] [CrossRef]
- Borchers, A.T.; Keen, C.L.; Stern, J.S.; Gershwin, M.E. Inflammation and Native American medicine: The role of botanicals. Am. J. Clin. Nutr. 2000, 72, 339–347. [Google Scholar] [CrossRef]
- Medeiros, D.S.; Medeiros, T.L.; Ribeiro, J.K.; Monteiro, N.K.; Migliolo, L.; Uchôa, A.F.; Vasconcelos, I.M.; Oliveira, A.S.; De Sales, M.P.; Santos, E.A. A lactose specific lectin from the sponge Cinachyrella apion: Purification, characterization, N-terminal sequences alignment and agglutinating activity on Leishmania promastigotes. Comp. Biochem. Physiol. Part B Biochem. Mol. Boil. 2010, 155, 211–216. [Google Scholar] [CrossRef]
- Pinedo, M.; Regente, M.; Elizalde, M.; Quiroga, I.Y.; Pagnussat, L.A.; Jorrin-Novo, J.; Maldonado, A.; De La Canal, L. Extracellular sunflower proteins: Evidence on non-classical secretion of a jacalin-related lectin. Protein Pept. Lett. 2012, 19, 270–276. [Google Scholar] [CrossRef]
- Van Damme, E.J.M.; Lannoo, N.; Peumans, W.J. Plant Lectins. Adv. Bot Res. 2008, 48, 107–209. [Google Scholar]
- Lannoo, N.; Van Damme, E.J.M. Lectin domains at the frontiers of plant defense. Front. Plant Sci. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.; Radutoiu, S.; Stougaard, J. Legume LysM receptors mediate symbiotic and pathogenic signalling. Curr. Opin. Plant Boil. 2017, 39, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Gust, A.A.; Willmann, R.; Desaki, Y.; Grabherr, H.M.; Nürnberger, T. Plant LysM proteins: Modules mediating symbiosis and immunity. Trends Plant Sci. 2012, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Buist, G.; Steen, A.; Kok, J.; Kuipers, O.P. LysM, a widely distributed protein motif for binding to (peptido)glycans. Mol. Microbiol. 2008, 68, 838–847. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Cannon, S.B.; Stacey, G. Evolutionary genomics of LysM genes in land plants. BMC Evol. Boil. 2009, 9, 183. [Google Scholar] [CrossRef]
- Bateman, A.; Bycroft, M. The structure of a LysM domain from E. coli membrane-bound lytic murein transglycosylase D (MltD) 1 1Edited by P. E. Wight. J. Mol. Boil. 2000, 299, 1113–1119. [Google Scholar] [CrossRef]
- Mulder, L.; Lefebvre, B.; Cullimore, J.; Imberty, A. LysM domains of Medicago truncatula NFP protein involved in Nod factor perception. Glycosylation state, molecular modeling and docking of chitooligosaccharides and Nod factors. Glycobiology 2006, 16, 801–809. [Google Scholar] [CrossRef]
- Wan, J. Diverse roles of lysin-motif (LysM) proteins in mediating plant-microbe interactions. Walailak J. Sci. Technol. 2015, 12, 631–641. [Google Scholar]
- Ohnuma, T.; Onaga, S.; Murata, K.; Taira, T.; Katoh, E. LysM Domains from Pteris ryukyuensis Chitinase-A: A stability study and characterization of the chitin binding. J. Biol. Chem. 2008, 283, 5178–5187. [Google Scholar] [CrossRef]
- Iizasa, E.; Mitsutomi, M.; Nagano, Y. Direct Binding of a Plant LysM Receptor-like Kinase, LysM RLK1/CERK1, to Chitin in Vitro. J. Biol. Chem. 2010, 285, 2996–3004. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.E.M.M.; Midtgaard, S.R.; Gysel, K.; Thygesen, M.B.; Sørensen, K.K.; Jensen, K.J.; Stougaard, J.; Thirup, S.; Blaise, M. An intermolecular binding mechanism involving multiple LysM domains mediates carbohydrate recognition by an endopeptidase. Acta Crystallogr. Sect. D Boil. Crystallogr. 2015, 71, 592–605. [Google Scholar] [CrossRef] [PubMed]
- Holthöfer, H. Lectin binding sites in kidney. A comparative study of 14 animal species. J. Histochem. Cytochem. 1983, 31, 531–537. [Google Scholar] [CrossRef]
- Rawal, S.; Majumdar, S.; Vohra, H. Activation of MAPK kinase pathway by Gal/GalNAc adherence lectin of E. histolytica: Gateway to host response. Mol. Cell. Biochem. 2005, 268, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-Activated Protein Kinases: A Family of Protein Kinases with Diverse Biological Functions. Microbiol. Mol. Boil. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Cassidy, H.; Radford, R.; Slyne, J.; O’Connell, S.; Slattery, C.; Ryan, M.P.; McMorrow, T. The Role of MAPK in Drug-Induced Kidney Injury. J. Signal Transduct. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Weening, J.J.; Van Guldener, C.; Daha, M.R.; Klar, N.; Van Der Wal, A.; Prins, F.A. The pathophysiology of protein-overload proteinuria. Am. J. Pathol. 1987, 129, 64–73. [Google Scholar]
- Koff, S.A. The point of view of the urologist. In Functional Imaging in Nephro-Urology; Prigent, A., Piepsz, A., Eds.; Taylor & Francis: Brussels, Belgium, 2006; pp. 117–123. [Google Scholar]
- Bonventre, J.V. Mechanisms of Acute Kidney Injury and Repair. In Management of Acute Kidney Problems, 1st ed.; Jörres, A., Ronco, C., Kellum, J.A., Eds.; Springer: Berli/Heidelberg, Germany, 2010; pp. 13–21. [Google Scholar]
- Toblli, J.E.; Bevione, P.; Di Gennaro, F.; Madalena, L.; Cao, G.; Angerosa, M. Understanding the Mechanisms of Proteinuria: Therapeutic Implications. Int. J. Nephrol. 2012, 2012, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brunskill, N.J. Albumin and proximal tubular cells--beyond endocytosis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2000, 15, 1732–1734. [Google Scholar] [CrossRef]
- Miller, M.A.; Zachary, J.F. Mechanisms and Morphology of Cellular Injury, Adaptation, and Death. In Pathologic Basis of Veterinary Disease, 5th ed.; McGavin, M.D., Ed.; Elsevier: St. Louis, MO, USA, 2013; Volume 53, pp. 2–59. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yan, Q.; Jiang, Z.; Yang, S.; Deng, W.; Han, L. A novel homodimeric lectin from Astragalus mongholicus with antifungal activity. Arch. Biochem. Biophys. 2005, 442, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Adamova, L.; Malinovska, L.; Wimmerová, M. New sensitive detection method for lectin hemagglutination using microscopy. Microsc. Res. Tech. 2014, 77, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.C.; Pinto, L.D.S.; Teixeira, E.H.; Nascimento, K.S.; Cavada, B.S.; Silva, A.L.C. BUL: A novel lectin from Bauhinia ungulata L. seeds with fungistatic and antiproliferative activities. Process. Biochem. 2013, 49, 203–209. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Shevchenko, A.; Tomas, H.; Havli, J.; Olsen, J.V.; Mann, M.; Havli\[Sbreve], J. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2006, 1, 2856–2860. [Google Scholar] [CrossRef]
- Rappsilber, J.; Ishihama, Y.; Mann, M. Stop and Go Extraction Tips for Matrix-Assisted Laser Desorption/Ionization, Nanoelectrospray, and LC/MS Sample Pretreatment in Proteomics. Anal. Chem. 2003, 75, 663–670. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Badouin, H.; Gouzy, J.; Grassa, C.J.; Murat, F.; Staton, S.E.; Cottret, L.; Lelandais-Brière, C.; Owens, G.L.; Carrère, S.; Mayjonade, B.; et al. The sunflower genome provides insights into oil metabolism, flowering and Asterid evolution. Nature 2017, 546, 148–152. [Google Scholar] [CrossRef]
- Altschul, S.F.; Wootton, J.C.; Gertz, E.M.; Agarwala, R.; Schäffer, A.A.; Yu, Y. NIH Public Access. Library 2006, 272, 5101–5109. [Google Scholar]
- Altschul, S. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Higgins, D.G. Clustal Omega for making accurate alignments of many protein sequences. Protein Sci. 2018, 27, 135–145. [Google Scholar] [CrossRef] [PubMed]
| Total Protein (mg) | Specific Activity a (HU, mg Protein−1) | Amount of Lectin b (μg mL−1) | Purification Fold c | |
|---|---|---|---|---|
| Crude extract | 90.58 ± 1.74 | 5.66 | 113.22 | 1 |
| F60-80 | 11.38 ± 0.71 | 0.35 | 35.56 | 3.18 |
| Purified LysM lectin | 0.04 ± 0.008 | 0.00204 | 1.02 | 111.00 |
| Minimum Inhibitory Concentration (mM) | |
|---|---|
| Lactose | 3 |
| D-mannose | 21 |
| D-galactose | 21 |
| Fructose | 42 |
| Sucrose | 42 |
| Maltose | 42 |
| N-Acetylglucosamine | 42 |
| Ribose | NI |
| D-glucose | NI |
| D-Xylose | NI |
| Acetyl-galactosamine | NI |
| Description | Max Score | Total Score | Query Coverage | E value | Percent Identity | Accession Number in NCBI Protein Database | |
|---|---|---|---|---|---|---|---|
| epa_locus_7790_iso_1_len_1277 | LysM domain-containing GPI-anchored protein [Helianthus annuus] | 676 | 676 | 96% | 0.0 | 90 | XP_021976452.1 |
| LysM domain-containing GPI-anchored protein [Lactuca sativa] | 629 | 629 | 97% | 0.0 | 85 | XP_023762850.1 | |
| LysM domain-containing GPI-anchored protein [Cynara cardunculus var. scolymus] | 646 | 646 | 99% | 0.0 | 83 | XP_024991685.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balciunaite, G.; Haimi, P.-J.; Mikniene, Z.; Savickas, G.; Ragazinskiene, O.; Juodziukyniene, N.; Baniulis, D.; Pangonyte, D. Identification of Echinacea Purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties. Toxins 2020, 12, 88. https://doi.org/10.3390/toxins12020088
Balciunaite G, Haimi P-J, Mikniene Z, Savickas G, Ragazinskiene O, Juodziukyniene N, Baniulis D, Pangonyte D. Identification of Echinacea Purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties. Toxins. 2020; 12(2):88. https://doi.org/10.3390/toxins12020088
Chicago/Turabian StyleBalciunaite, Gabriele, Perttu-Juhani Haimi, Zoja Mikniene, Girius Savickas, Ona Ragazinskiene, Nomeda Juodziukyniene, Danas Baniulis, and Dalia Pangonyte. 2020. "Identification of Echinacea Purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties" Toxins 12, no. 2: 88. https://doi.org/10.3390/toxins12020088
APA StyleBalciunaite, G., Haimi, P.-J., Mikniene, Z., Savickas, G., Ragazinskiene, O., Juodziukyniene, N., Baniulis, D., & Pangonyte, D. (2020). Identification of Echinacea Purpurea (L.) Moench Root LysM Lectin with Nephrotoxic Properties. Toxins, 12(2), 88. https://doi.org/10.3390/toxins12020088